Aliphatic Polybenzimidazoles: Synthesis, Characterization and High-Temperature Shape-Memory Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. PBI Synthesis
2.3. Analytical Methods
3. Results and Discussion
3.1. Synthesis and Characterization of PBIs
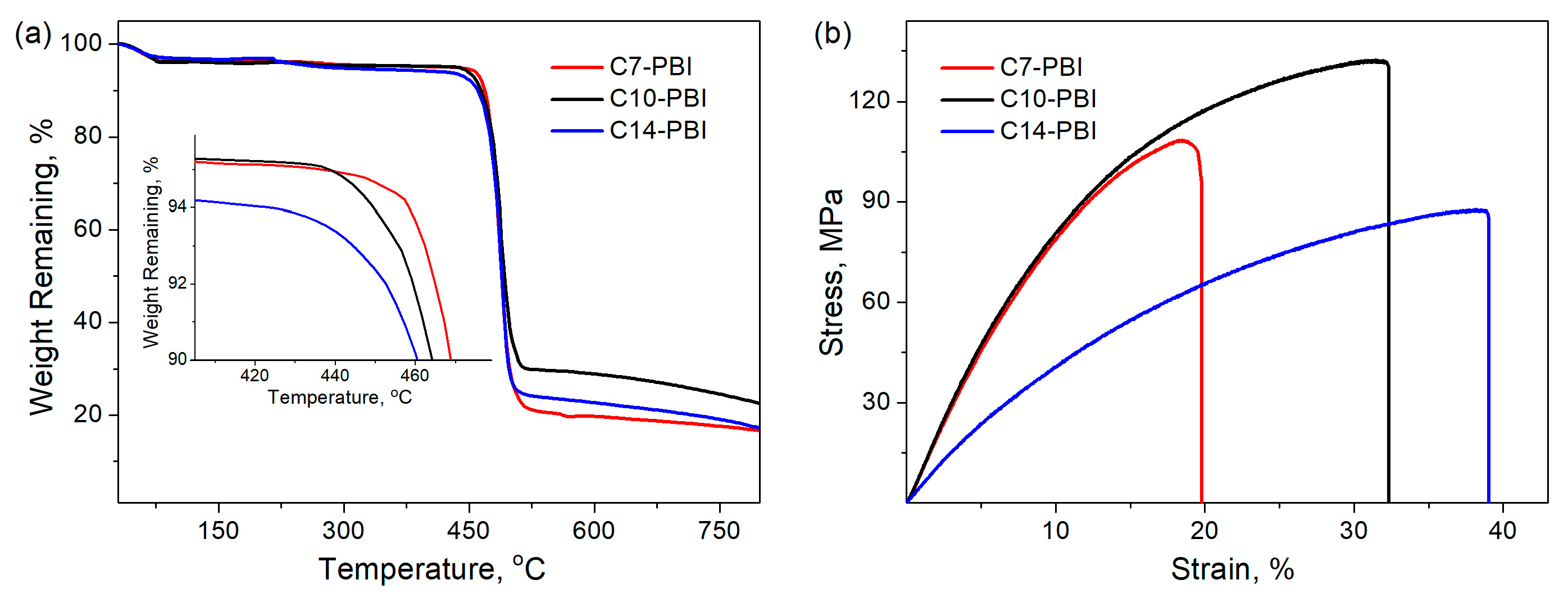
3.2. Thermal and Mechanical Properties
3.3. Dynamic Mechanical Properties of PBIs
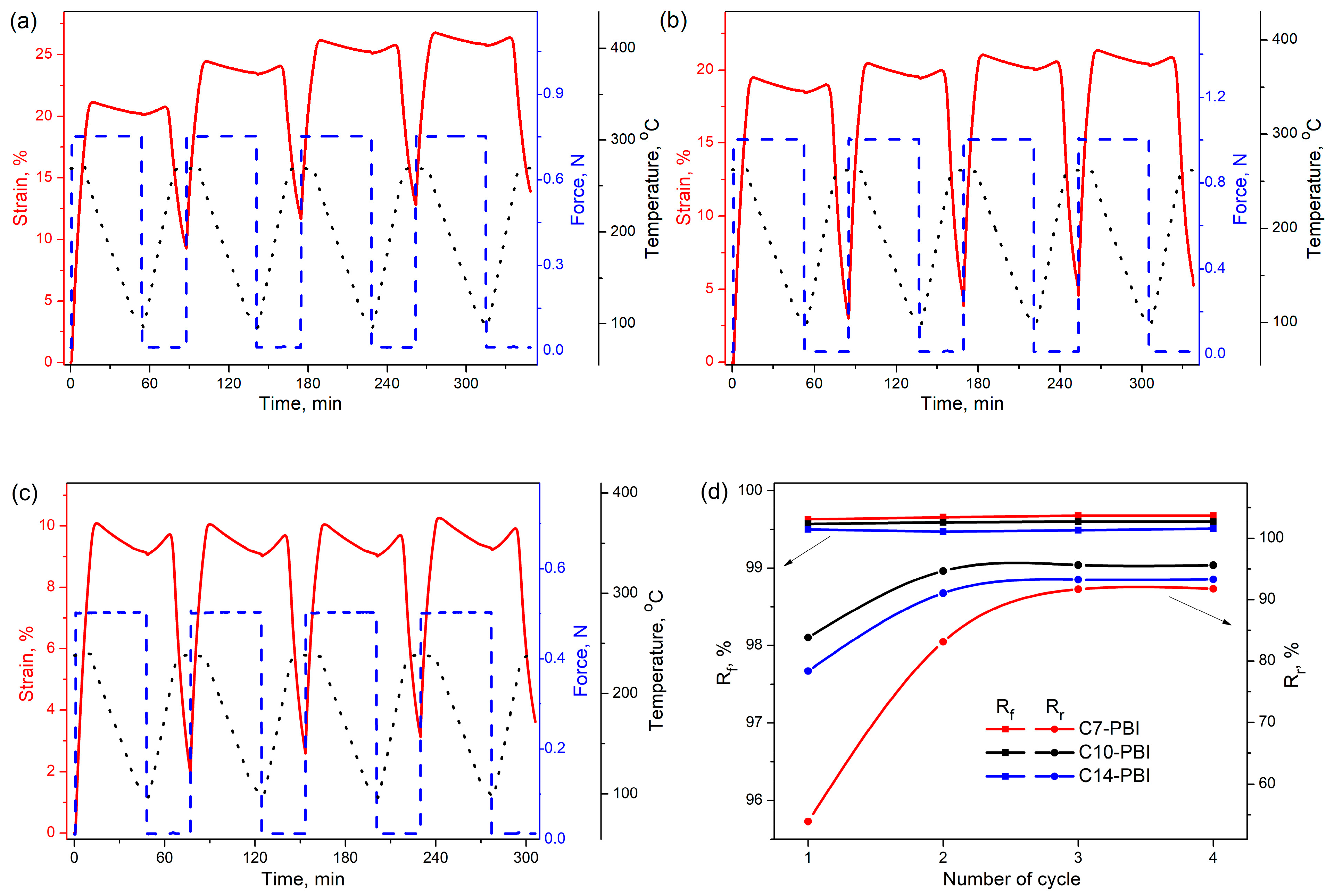
3.4. Shape-Memory Performance of PBIs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High Temperature Proton Exchange Membranes Based on Polybenzimidazoles for Fuel Cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Aili, D.; Yang, J.; Jankova, K.; Henkensmeier, D.; Li, Q. From Polybenzimidazoles to Polybenzimidazoliums and Polybenzimidazolides. J. Mater. Chem. A 2020, 8, 12854–12886. [Google Scholar] [CrossRef]
- Iqbal, H.M.S.; Bhowmik, S.; Benedictus, R. Performance Evaluation of Polybenzimidazole under High-Energy Radiation Environment. J. Thermophys. Heat Transf. 2016, 30, 825–830. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Burdukovskii, V.F.; Mognonov, D.M. Preparation of Aromatic Polyamidines and Their Transformation in Polybenzimidazoles. Express Polym. Lett. 2014, 8, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Harilal, H.; Shukla, A.; Chandra Ghosh, P.; Jana, T. Copolymers of Pyridine-Bridged Polybenzimidazole for the Use in High Temperature PEM Fuel Cell. Eur. Polym. J. 2022, 177, 111445. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Matveev, Z.A.; Nikishina, A.N.; Burdukovskii, V.F. Polybenzimidazole-Based Thiol-Ene Photosensitive Composition for DLP 3D Printing. Mendeleev Commun. 2022, 32, 813–815. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Ampah, J.D.; Wilberforce, T.; Afrane, S.; Nutakor, C. Research Progress, Trends, and Current State of Development on PEMFC-New Insights from a Bibliometric Analysis and Characteristics of Two Decades of Research Output. Membranes 2022, 12, 1103. [Google Scholar] [CrossRef]
- Lysova, A.A.; Ponomarev, I.I.; Skupov, K.M.; Vtyurina, E.S.; Lysov, K.A.; Yaroslavtsev, A.B. Effect of Organo-Silanes Structure on the Properties of Silane-Crosslinked Membranes Based on Cardo Polybenzimidazole PBI-O-PhT. Membranes 2022, 12, 1078. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Modestov, A.D.; Lysova, A.A.; Ponomarev, I.I.; Vtyurina, E.S. Cardo Polybenzimidazole (PBI-O-PhT) Based Membrane Reinforced with m-Polybenzimidazole Electrospun Nanofiber Mat for HT-PEM Fuel Cell Applications. Membranes 2022, 12, 956. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compañ, V. Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Proton Exchange Membrane (PEM) Fuel Cell Applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Sana, B.; Das, A.; Jana, T. Cross-Linked Polybenzimidazoles as Alkaline Stable Anion Exchange Membranes. ACS Appl. Energy Mater. 2022, 5, 3626–3637. [Google Scholar] [CrossRef]
- Mukherjee, N.; Das, A.; Jana, T. Poly(N-Vinyl Triazole-b-N-Vinyl Imidazole) Grafted on MWCNTs as Nanofillers to Improve Proton Conducting Membranes. ACS Appl. Nano Mater. 2022, 6, 544–557. [Google Scholar] [CrossRef]
- Basu, O.; Das, A.; Jana, T.; Das, S.K. Design of Flexible Metal–Organic Framework-Based Superprotonic Conductors and Their Fabrication with a Polymer into Proton Exchange Membranes. ACS Appl. Energy Mater. 2022. [Google Scholar] [CrossRef]
- Zhou, P.K.; Song, K.Y.; Zong, L.L.; Yang, Z.C.; Li, H.H.; Chen, Z.R. Enhanced Ternary Memory Performances with High-Temperature Tolerance in AIE@PBI Composites by Tuning the Azobenzol Substituents on Tetraphenylethylene. Mater. Today Chem. 2022, 25, 100941. [Google Scholar] [CrossRef]
- Liu, D.; Hu, G.; Huang, T.; Yu, B.; Yu, H.; Zhu, M. Elastic Polybenzimidazole Nanofiber Aerogel for Thermal Insulation and High-Temperature Oil Adsorption. J. Mater. Sci. 2022, 57, 12125–12137. [Google Scholar] [CrossRef]
- Akovantseva, A.A.; Aksenova, N.A.; Zarkhina, T.S.; Krotova, L.I.; Minaev, N.V.; Rybaltovskii, A.O.; Kholkhoev, B.C.; Farion, I.A.; Yusupov, V.I.; Burdukovskii, V.F.; et al. Preparation and Optical Properties of Composite Materials Based on Polybenzimidazole and Silver Nanoparticles. Russ. J. Appl. Chem. 2017, 90, 84–90. [Google Scholar] [CrossRef]
- Akovantseva, A.; Kotova, S.; Gromov, O.; Burdukovskii, V.; Kholkhoev, B.; Timashev, P.; Yusupov, V.; Rybaltovsky, A. Formation of Luminescent States in Polybenzimidazole-Based Films. J. Polym. Sci. 2020, 58, 2926–2935. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Gorenskaya, E.N.; Bal’zhinov, S.A.; Farion, I.A.; Batorova, G.N.; Nomoev, A.V.; Timashev, P.S.; Radnaev, B.R.; Chailakhyan, R.K.; Fedorov, V.E.; et al. Functional Composites Based on Polybenzimidazole and Graphite Nanoplates. Russ. J. Appl. Chem. 2016, 89, 780–786. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape Memory Polymers and Their Composites in Aerospace Applications: A Review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Bhanushali, H.; Amrutkar, S.; Mestry, S.; Mhaske, S.T. Shape Memory Polymer Nanocomposite: A Review on Structure–Property Relationship; Springer: Berlin/Heidelberg, Germany, 2022; Volume 79, ISBN 0123456789. [Google Scholar]
- Dayyoub, T.; Maksimkin, A.V.; Filippova, O.V.; Tcherdyntsev, V.V.; Telyshev, D.V. Shape Memory Polymers as Smart Materials: A Review. Polymers 2022, 14, 3511. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Noroozi, R.; Zolfagharian, A.; Bodaghi, M. 4D Printing of Shape Memory Polymer Composites: A Review on Fabrication Techniques, Applications, and Future Perspectives. J. Manuf. Process. 2022, 81, 759–797. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape Memory Polymers: Past, Present and Future Developments. Prog. Polym. Sci. 2015, 49, 3–33. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-Memory Polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Bardakova, K.N.; Kholkhoev, B.C.; Farion, I.A.; Epifanov, E.O.; Korkunova, O.S.; Efremov, Y.M.; Minaev, N.V.; Solovieva, A.B.; Timashev, P.S.; Burdukovskii, V.F. 4D Printing of Shape-Memory Semi-Interpenetrating Polymer Networks Based On Aromatic Heterochain Polymers. Adv. Mater. Technol. 2022, 7, 2100790. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Shalygina, T.A.; Matveev, Z.A.; Kurbatov, R.V.; Farion, I.A.; Voronina, S.Y.; Burdukovskii, V.F. High Temperature Shape Memory Aliphatic Polybenzimidazole. Polymer 2022, 245, 124676. [Google Scholar] [CrossRef]
- Yang, Z.; Song, F.; Wang, Q.; Wang, T. Shape Memory Induced Structural Evolution of High Performance Copolyimides. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3858–3867. [Google Scholar] [CrossRef]
- Iwakura, Y.; Uno, K.; Imai, Y. Polybenzimidazoles. II Polyalkylenebenzimidazoles. Die Makromol. Chemie 1964, 77, 33–40. [Google Scholar] [CrossRef]
- Tsur, Y.; Levine, H.H.; Levy, M. Effects of Structure on Properties of Some New Aromatic-Aliphatic Polybenzimidazoles. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 1515–1529. [Google Scholar] [CrossRef]
- Ueda, M.; Sato, M.; Mochizuki, A. Poly(Benzimidazole) Synthesis by Direct Reaction of Diacids and Tetramine. Macromolecules 1985, 18, 2723–2726. [Google Scholar] [CrossRef]
- Bhavsar, R.; Nahire, S.; Kale, M.; Patil, S.; Aher, P.; Bhavsar, R.; Kharul, U. Polybenzimidazoles Based on 3,3′-Diaminobenzidine and Aliphatic Dicarboxylic Acids: Synthesis and Evaluation of Physicochemical Properties toward Their Applicability as Proton Exchange and Gas Separation Membrane Material. J. Appl. Polym. Sci. 2011, 120, 1090–1099. [Google Scholar] [CrossRef]
- Cho, H.; Henkensmeier, D.; Brela, M.; Michalak, A.; Jang, J.H.; Lee, K.Y. Anion Conducting Methylated Aliphatic PBI and Its Calculated Properties. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 256–265. [Google Scholar] [CrossRef]
- Adrova, N.A.; Koton, M.M.; Dubnova, A.M.; Moskvina, Y.M.; Pokrovskii, Y.I.; Fedorova, Y.F. Synthesis and Properties of Polybenzimidazoles Containing Aliphatic Units in the Main Chain. Polym. Sci. U.S.S.R. 1965, 7, 335–337. [Google Scholar] [CrossRef]
- Kumar, B.S.; Sana, B.; Unnikrishnan, G.; Jana, T.; Kumar, K.S.S. Polybenzimidazole Co-Polymers: Their Synthesis, Morphology and High Temperature Fuel Cell Membrane Properties. Polym. Chem. 2020, 11, 1043–1054. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Bardakova, K.N.; Epifanov, E.O.; Matveev, Z.A.; Shalygina, T.A.; Atutov, E.B.; Voronina, S.Y.; Timashev, P.S.; Burdukovskii, V.F. A Photosensitive Composition Based on an Aromatic Polyamide for LCD 4D Printing of Shape Memory Mechanically Robust Materials. Chem. Eng. J. 2023, 454, 140423. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Bai, Y.; Wang, T. AO-Resistant Shape Memory Polyimide/Silica Composites with Excellent Thermal Stability and Mechanical Properties. RSC Adv. 2015, 5, 72971–72980. [Google Scholar] [CrossRef]
- Tan, W.; Lv, J.; Li, R.; Hu, J.; Zeng, K.; Yang, G. Bio-Based Adenine-Containing Copolyimides with High Switching Temperatures and High-Strain Storage. Mol. Syst. Des. Eng. 2022, 7, 986–995. [Google Scholar] [CrossRef]
- Leykin, A.Y.; Fomenkov, A.I.; Galpern, E.G.; Stankevich, I.V.; Rusanov, A.L. Some Aspects of Polybenzimidazoles’ Synthesis in P2O5 Containing Condensation Media. Polymer 2010, 51, 4053–4057. [Google Scholar] [CrossRef]
- Shimazu, A.; Miyazaki, T.; Ikeda, K. Interpretation of D-Spacing Determined by Wide Angle X-Ray Scattering in 6FDA-Based Polyimide by Molecular Modeling. J. Memb. Sci. 2000, 166, 113–118. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Cao, B. Effects of the Side Groups of the Spirobichroman-Based Diamines on the Chain Packing and Gas Separation Properties of the Polyimides. J. Memb. Sci. 2017, 530, 176–184. [Google Scholar] [CrossRef]
- McHattie, J.S.; Koros, W.J.; Paul, D.R. Gas Transport Properties of Polysulphones: 3. Comparison of Tetramethyl-Substituted Bisphenols. Polymer 1992, 33, 1701–1711. [Google Scholar] [CrossRef]
- Sannigrahi, A.; Ghosh, S.; Lalnuntluanga, J.; Jana, T. How the Monomer Concentration of Polymerization Influences Various Properties of Polybenzimidazole: A Case Study with Poly(4,4′-Diphenylether-5,5′-Bibenzimidazole). J. Appl. Polym. Sci. 2009, 111, 2194–2203. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, D.; Qiu, X.; Zhang, W.; Liu, Y.; Zhang, S.; Zhang, F.; Hu, Y.; Leng, J. Shape Memory Polymers with High and Low Temperature Resistant Properties. Sci. Rep. 2015, 5, 14137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Wang, S.; Jin, S.; Wang, Y.; Yao, J.; Zhao, X.; Chen, C. Construction of High-Performance, High-Temperature Shape Memory Polyimides Bearing Pyridine and Trifluoromethyl Group. Polymer 2020, 210, 122972. [Google Scholar] [CrossRef]
- Satheesh Kumar, B.; Sana, B.; Unnikrishnan, G.; Jana, T.; Santhosh Kumar, K.S. Nano-Ordered Aromatic/Alicyclic Polybenzimidazole Blend Membranes. React. Funct. Polym. 2020, 146, 104312. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, D.; Qiu, X.; Zhang, W.; Zhang, F.; Liu, L.; Liu, Y.; Zhang, S.; Hu, Y.; Leng, J. Shape-Memory Polymers with Adjustable High Glass Transition Temperatures. Macromolecules 2015, 48, 3582–3589. [Google Scholar] [CrossRef]
- Merline, J.D.; Reghunadhan, N.C.P.; Gouri, C.; Bandyopadhyay, G.G.; Ninan, K.N. Polyether Polyurethanes: Synthesis, Characterization, and Thermoresponsive Shape Memory Properties. J. Appl. Polym. Sci. 2008, 107, 4082–4092. [Google Scholar] [CrossRef]
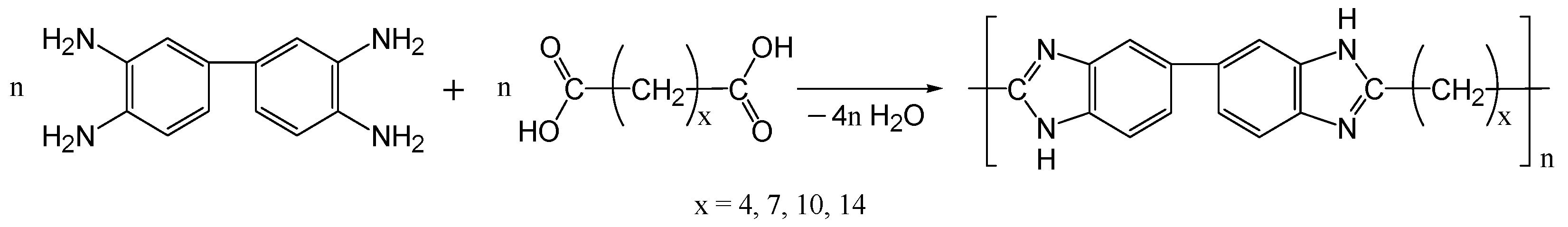
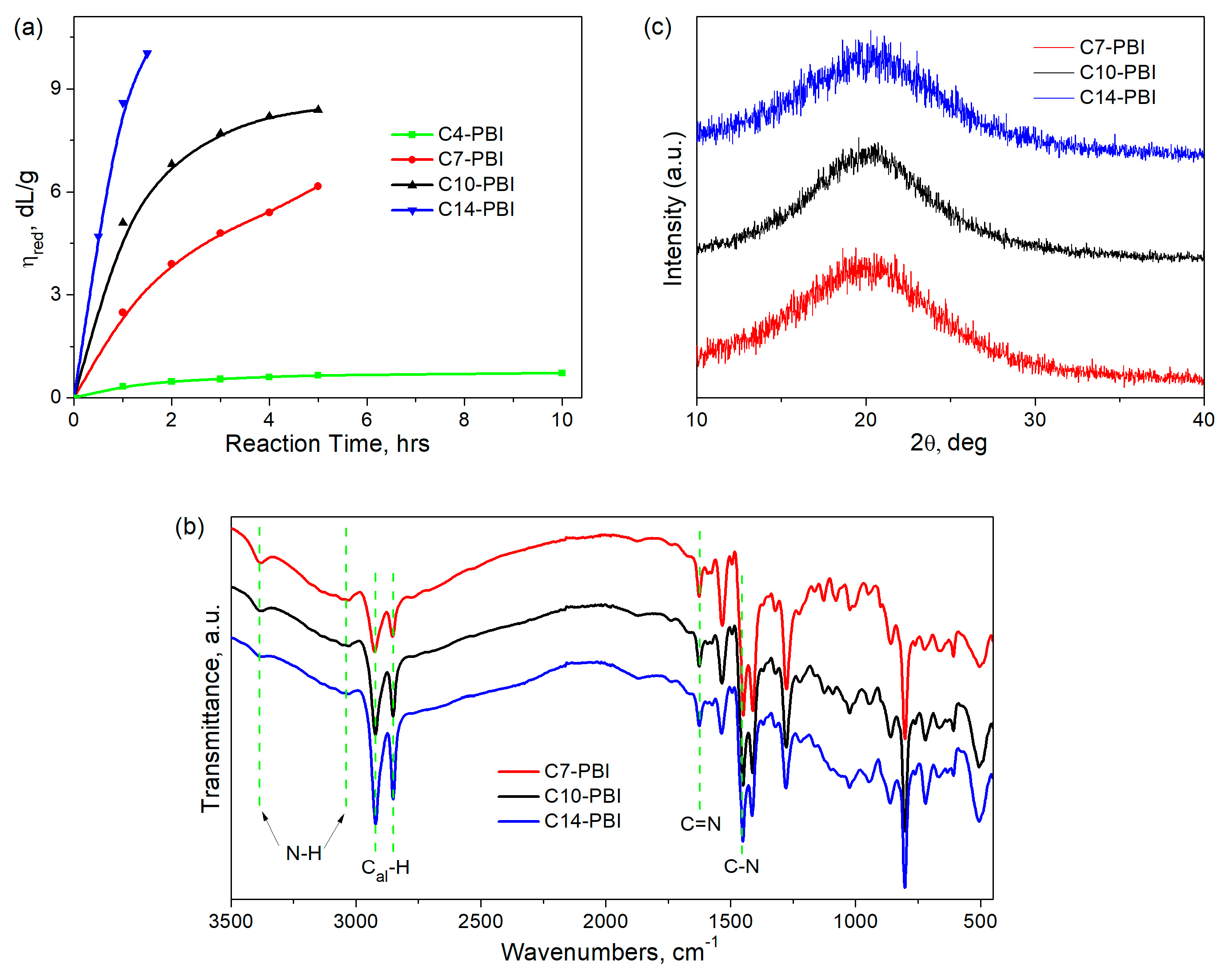



| Polymer | Dicarboxylic Acid Used | Reaction Conditions | ηred *, dL/g | Film-Forming Ability | ||
|---|---|---|---|---|---|---|
| Temperature, °C | Duration, h | Monomer Concentration, mol/L | ||||
| C4-PBI | HOOC–(CH2)4–COOH adipic acid | 120 | 10 | 0.2 | 0.72 | No |
| C7-PBI | HOOC–(CH2)7–COOH azelaic acid | 5 | 0.2 | 6.17 | Yes | |
| C10-PBI | HOOC–(CH2)10–COOH dodecanedioic acid | 5 | 0.2 | 8.39 | Yes | |
| C14-PBI | HOOC–(CH2)14–COOH hexadecanedioic acid | 1.5 | 0.13 | 10.03 | Yes | |
| Sample | TGA | Mechanical Test | ||
|---|---|---|---|---|
| T10%, °C 1 | Char Yield, % 2 | Tensile Strength, MPa | Elongation at Break, % | |
| C7-PBI | 470 | 17 | 95.5 ± 6.2 | 19.8 ± 1.9 |
| C10-PBI | 464 | 23 | 129.3 ± 7.1 | 32.3 ± 2.6 |
| C14-PBI | 460 | 17 | 83.9 ± 5.3 | 39.1 ± 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholkhoev, B.C.; Matveev, Z.A.; Bardakova, K.N.; Timashev, P.S.; Burdukovskii, V.F. Aliphatic Polybenzimidazoles: Synthesis, Characterization and High-Temperature Shape-Memory Performance. Polymers 2023, 15, 1399. https://doi.org/10.3390/polym15061399
Kholkhoev BC, Matveev ZA, Bardakova KN, Timashev PS, Burdukovskii VF. Aliphatic Polybenzimidazoles: Synthesis, Characterization and High-Temperature Shape-Memory Performance. Polymers. 2023; 15(6):1399. https://doi.org/10.3390/polym15061399
Chicago/Turabian StyleKholkhoev, Bato Ch., Zakhar A. Matveev, Kseniia N. Bardakova, Peter S. Timashev, and Vitaliy F. Burdukovskii. 2023. "Aliphatic Polybenzimidazoles: Synthesis, Characterization and High-Temperature Shape-Memory Performance" Polymers 15, no. 6: 1399. https://doi.org/10.3390/polym15061399






