Cellulose/Grape-Seed-Extract Composite Films with High Transparency and Ultraviolet Shielding Performance Fabricated from Old Cotton Textiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fabrication of Cellulose/GSEs Composite Materials
2.3. Characterization
2.3.1. Wide-Angle X-ray Diffraction (WAXD) of P-OCTs, GSEs, CGSEs 0, CGSEs 0.25, CGSEs 0.5, CGSEs 1.0 and CGSEs 2.0
2.3.2. Ultraviolet and Visible (UV-Vis) Spectra of the Cellulose/GSEs Films
2.3.3. The Surface Hydrophilicity of CGSEs 0, CGSEs 0.25, CGSEs 0.5, CGSEs 1.0 and CGSEs 2.0
2.3.4. Fourier-Transform Infrared (FTIR) Spectra of the P-OCTs, GSEs, CGSEs 0, CGSEs 0.25, CGSEs 0.5, CGSEs 1.0 and CGSEs 2.0
2.3.5. Micro-Morphologies of the Cellulose/GSEs Films
2.3.6. Thermogravimetric Analysis (TGA) of the P-OCTs, GSEs, CGSEs 0, CGSEs 0.25, CGSEs 0.5, CGSEs 1.0 and CGSEs 2.0
2.3.7. Mechanical Tests of CGSEs 0, CGSEs 0.25, CGSEs 0.5, CGSEs 1.0 and CGSEs 2.0
2.3.8. Barrier Properties of CGSEs 0, CGSEs 0.25, CGSEs 0.5, CGSEs 1.0 and CGSEs 2.0
2.3.9. Statistical Analysis
3. Results
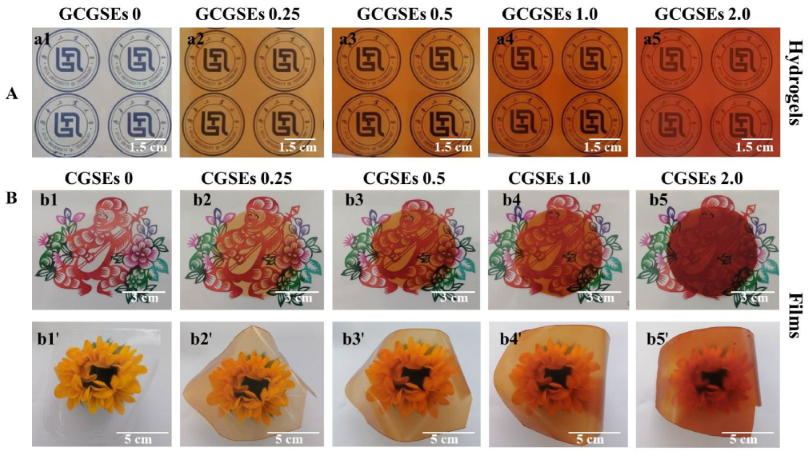
3.1. Transparency of Cellulose/GSEs Hydrogels and Films
3.2. Ultraviolet and Visible (UV-Vis) Spectra of Cellulose/GSEs Composite Films
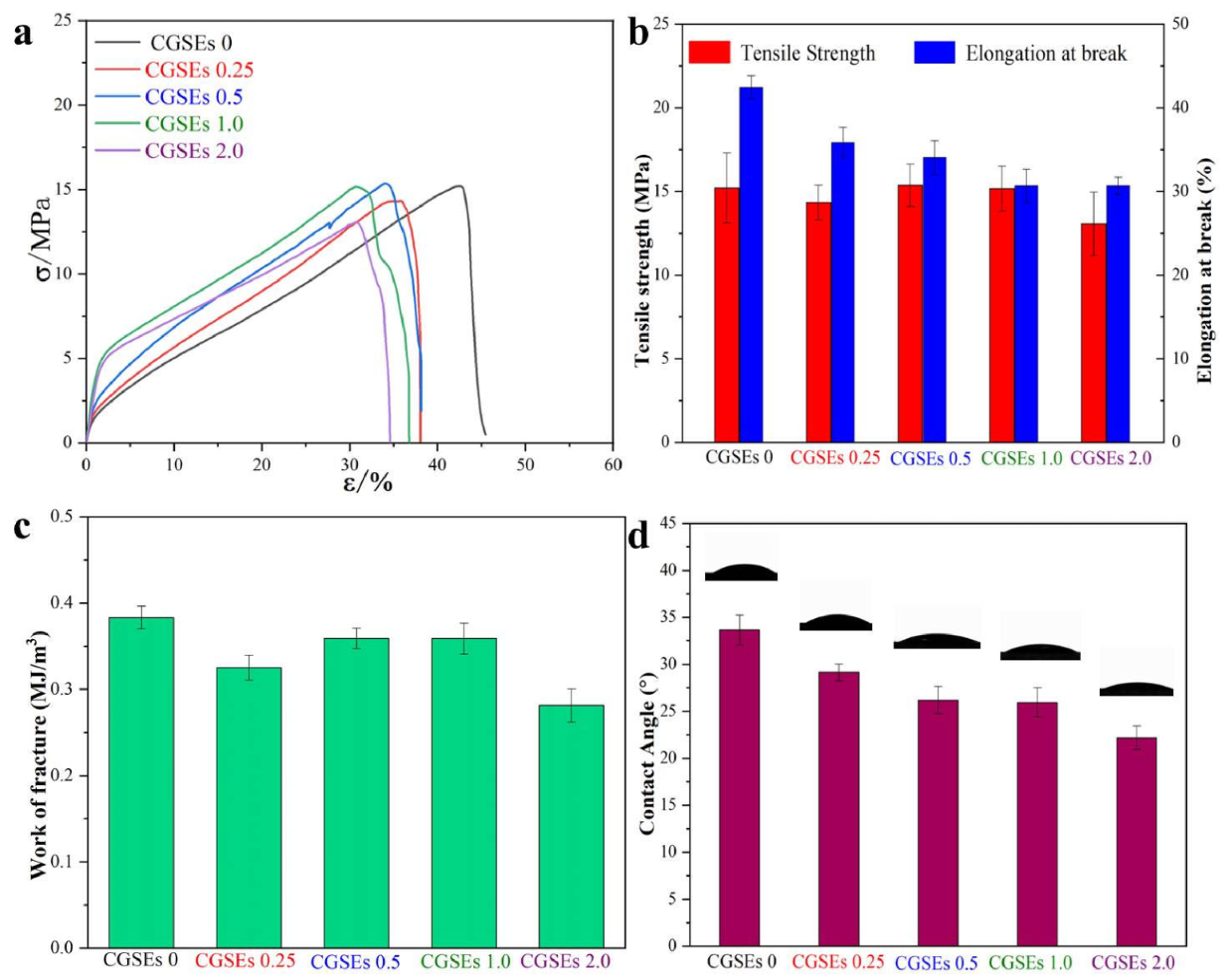
3.3. Mechanical and Hydrophilicity Performances of CGSEs 0, CGSEs 0.25, CGSEs 0.5, CGSEs 1.0 and CGSEs 2.0 Films
3.4. TGA, FTIR and XRD of Cellulose/GSEs Hybrid Films
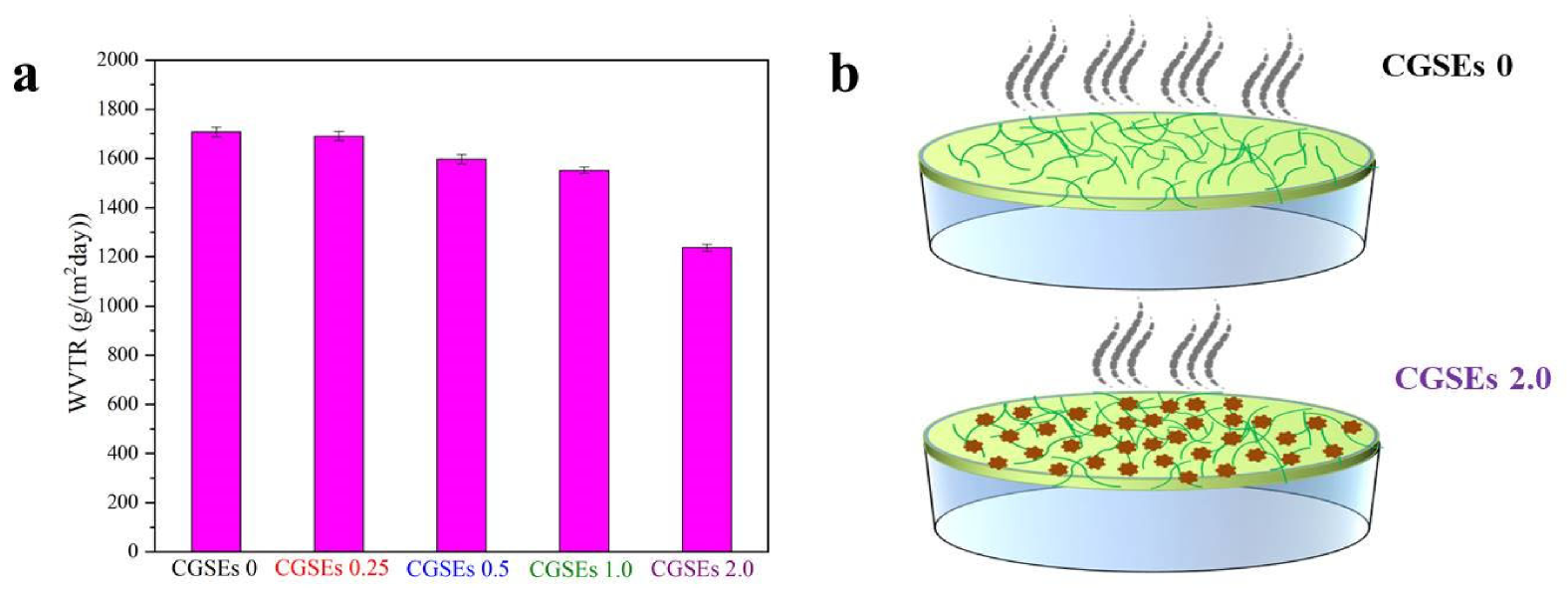
3.5. Barrier Property of the Cellulose/GSEs Film
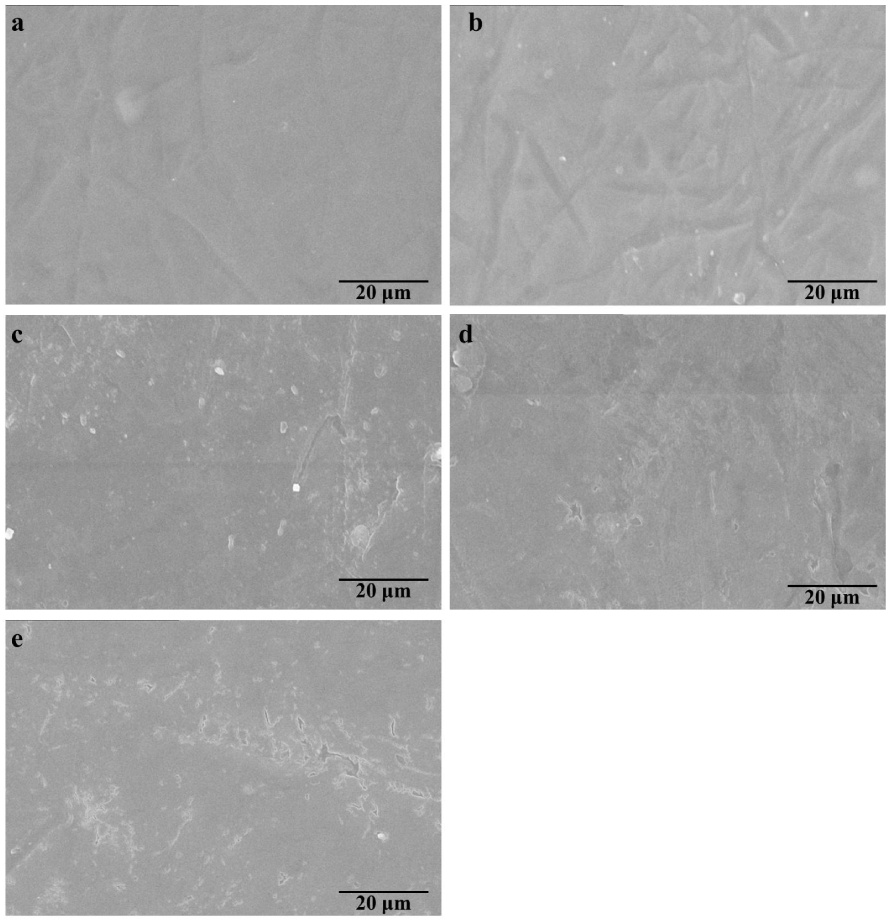
3.6. Micro-Morphologies of the Cellulose/GSEs Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, G.; Ji, X.; Xu, Z.; Ji, X. Transparent cellulose-based bio-hybrid films with enhanced anti-ultraviolet, antioxidant and antibacterial performance. Carbohydr. Polym. 2022, 298, 120118. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Hou, L.; Wu, P. Hydrogen-Bonding Affords Sustainable Plastics with Ultrahigh Robustness and Water-Assisted Arbitrarily Shape Engineering. Adv. Mater. 2022, 34, e2201065. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Chen, C.; Yao, Y.; Li, J.; He, S.; Zhou, Y.; Li, T.; Pan, X.; Yao, Y.; Hu, L. A strong, biodegradable and recyclable lignocellulosic bioplastic. Nat. Sustain. 2021, 4, 627–635. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Khakalo, A.; Liimatainen, H. Anti-oxidative and UV-absorbing biohybrid film of cellulose nanofibrils and tannin extract. Food Hydrocoll. 2019, 92, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.R.; Zheng, R.; Tang, J.; Sun, H.J.; Wang, J. A mini-review on building insulation materials from perspective of plastic pollution: Current issues and natural fibres as a possible solution. J. Hazard. Mater. 2022, 438, 129449. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhong, Y.; Wu, S.; Wei, P.; Huang, J.; Xu, D.; Zhang, L.; Ye, Q.; Cai, J. Biocompatible and biodegradable super-toughness regenerated cellulose via water molecule-assisted molding. Chem. Eng. J. 2021, 417, 129229. [Google Scholar] [CrossRef]
- Shim, W.J.; Kim, S.K.; Lee, J.; Eo, S.; Kim, J.S.; Sun, C. Toward a long-term monitoring program for seawater plastic pollution in the north Pacific Ocean: Review and global comparison. Environ. Pollut. 2022, 311, 119911. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L. Lignin as a Wood-Inspired Binder Enabled Strong, Water Stable, and Biodegradable Paper for Plastic Replacement. Adv. Funct. Mater. 2019, 30, 1906307. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [Green Version]
- Omeyer, L.C.M.; Duncan, E.M.; Aiemsomboon, K.; Beaumont, N.; Bureekul, S.; Cao, B.; Carrasco, L.R.; Chavanich, S.; Clark, J.R.; Cordova, M.R.; et al. Priorities to inform research on marine plastic pollution in Southeast Asia. Sci. Total. Environ. 2022, 841, 156704. [Google Scholar] [CrossRef]
- Bangar, S.P.; Kajla, P.; Ghosh, T. Valorization of wheat straw in food packaging: A source of cellulose. Int. J. Biol. Macromol. 2023, 227, 762–776. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2022, 344 Pt B, 126195. [Google Scholar] [CrossRef]
- Culaba, A.B.; Mayol, A.P.; San Juan, J.L.G.; Ubando, A.T.; Bandala, A.A.; Concepcion, R.S., II; Alipio, M.; Chen, W.H.; Show, P.L.; Chang, J.S. Design of biorefineries towards carbon neutrality: A critical review. Bioresour. Technol. 2023, 369, 128256. [Google Scholar] [CrossRef]
- Gan, M.J.; Niu, Y.Q.; Qu, X.J.; Zhou, C.H. Lignin to value-added chemicals and advanced materials: Extraction, degradation, and functionalization. Green Chem. 2022, 24, 7705–7750. [Google Scholar] [CrossRef]
- Ge, M.; Liu, S.; Li, J.; Li, M.; Li, S.; James, T.D.; Chen, Z. Luminescent materials derived from biomass resources. Coord. Chem. Rev. 2023, 477, 214951. [Google Scholar] [CrossRef]
- Carreira, M.C.A.; Oliveira, M.C.; Fernandes, A.C. One-pot sustainable synthesis of valuable nitrogen compounds from biomass resources. Mol. Catal. 2022, 518, 112094. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.D.; Patel, A.K.; Puri, M. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour. Technol. 2022, 344 Pt B, 126415. [Google Scholar] [CrossRef]
- Shen, X.; Sun, R. Recent advances in lignocellulose prior-fractionation for biomaterials, biochemicals, and bioenergy. Carbohydr. Polym. 2021, 261, 117884. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dai, G.; Liu, Y.; Fan, W.; Yang, K.; Li, Z. A reusable, biomass-derived, and pH-responsive collagen fiber based oil absorbent material for effective separation of oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127906. [Google Scholar] [CrossRef]
- Mary, S.K.; Koshy, R.R.; Arunima, R.; Thomas, S.; Pothen, L.A. A review of recent advances in starch-based materials: Bionanocomposites, pH sensitive films, aerogels and carbon dots. Carbohydr. Polym. Technol. Appl. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Hou, X.; Wang, H.; Shi, Y.; Yue, Z. Recent advances of antibacterial starch-based materials. Carbohydr. Polym. 2023, 302, 120392. [Google Scholar] [CrossRef] [PubMed]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Vinodh, R.; Sasikumar, Y.; Kim, H.-J.; Atchudan, R.; Yi, M. Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: A critical review. J. Ind. Eng. Chem. 2021, 104, 155–171. [Google Scholar] [CrossRef]
- Bi, S.; Li, F.; Qin, D.; Wang, M.; Yuan, S.; Cheng, X.; Chen, X. Construction of chitin functional materials based on a “green” alkali/urea solvent and their applications in biomedicine: Recent advance. Appl. Mater. Today 2021, 23, 101030. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-based materials and their adsorptive removal efficiency for dyes: A review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef]
- Yun, T.; Tao, Y.; Li, Q.; Cheng, Y.; Lu, J.; Lv, Y.; Du, J.; Wang, H. Superhydrophobic modification of cellulosic paper-based materials: Fabrication, properties, and versatile applications. Carbohydr. Polym. 2023, 305, 120570. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Bangar, S.P.; Esua, O.J.; Nickhil, C.; Whiteside, W.S. Microcrystalline cellulose for active food packaging applications: A review. Food Packag. Shelf Life 2023, 36, 101048. [Google Scholar] [CrossRef]
- Wong, L.C.; Leh, C.P.; Goh, C.F. Designing cellulose hydrogels from non-woody biomass. Carbohydr. Polym. 2021, 264, 118036. [Google Scholar] [CrossRef]
- Xia, G.; Wan, J.; Zhang, J.; Zhang, X.; Xu, L.; Wu, J.; He, J.; Zhang, J. Cellulose-based films prepared directly from waste newspapers via an ionic liquid. Carbohydr. Polym. 2016, 151, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Zhou, Q.; Xu, Z.; Zhang, J.; Zhang, J.; Wang, J.; You, J.; Wang, Y.; Nawaz, H. Transparent cellulose/aramid nanofibers films with improved mechanical and ultraviolet shielding performance from waste cotton textiles by in-situ fabrication. Carbohydr. Polym. 2021, 273, 118569. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, N.; Wan, J.; Xia, G.; Yu, J.; He, J.; Zhang, J. Directly Converting Agricultural Straw into All-Biomass Nanocomposite Films Reinforced with Additional in Situ-Retained Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2017, 5, 5127–5133. [Google Scholar] [CrossRef]
- Xian, L.; Li, Z.; Tang, A.X.; Qin, Y.M.; Li, Q.Y.; Liu, H.B.; Liu, Y.Y. A novel neutral and thermophilic endoxylanase from Streptomyces ipomoeae efficiently produced xylobiose from agricultural and forestry residues. Bioresour. Technol. 2019, 285, 121293. [Google Scholar] [CrossRef]
- Janaswamy, S.; Yadav, M.P.; Hoque, M.; Bhattarai, S.; Ahmed, S. Cellulosic fraction from agricultural biomass as a viable alternative for plastics and plastic products. Ind. Crops Prod. 2022, 179, 114692. [Google Scholar] [CrossRef]
- Wang, C.; Mei, J.; Zhang, L. High-added-value biomass-derived composites by chemically coupling post-consumer plastics with agricultural and forestry wastes. J. Clean. Prod. 2021, 284, 124768. [Google Scholar] [CrossRef]
- Rhofita, E.I.; Rachmat, R.; Meyer, M.; Montastruc, L. Mapping analysis of biomass residue valorization as the future green energy generation in Indonesia. J. Clean. Prod. 2022, 354, 131667. [Google Scholar] [CrossRef]
- Deep Singh, A.; Gajera, B.; Sarma, A.K. Appraising the availability of biomass residues in India and their bioenergy potential. Waste Manag. 2022, 152, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, Q.; Wang, L.; Xia, G.; Ji, X.; Zhang, J.; Zhang, J.; Nawaz, H.; Wang, J.; Peng, J. Transparent Cellulose-Based Films Prepared from Used Disposable Paper Cups via an Ionic Liquid. Polymers 2021, 13, 4209. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Han, W.; Xu, Z.; Zhang, J.; Kong, F.; Zhang, J.; Zhang, X.; Jia, F. Complete recycling and valorization of waste textiles for value-added transparent films via an ionic liquid. J. Environ. Chem. Eng. 2021, 9, 106182. [Google Scholar] [CrossRef]
- Xia, G.; Zhou, Q.; Xu, Z.; Zhang, J.; Ji, X.; Zhang, J.; Nawaz, H.; Wang, J.; Peng, J. Cellulose-Based Films with Ultraviolet Shielding Performance Prepared Directly from Waste Corrugated Pulp. Polymers 2021, 13, 3359. [Google Scholar] [CrossRef]
- Fan, P.; Yuan, Y.; Ren, J.; Yuan, B.; He, Q.; Xia, G.; Chen, F.; Song, R. Facile and green fabrication of cellulosed based aerogels for lampblack filtration from waste newspaper. Carbohydr. Polym. 2017, 162, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Han, S.; Li, J.; Sun, Q. Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr. Polym. 2015, 123, 150–156. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Ji, X.; Peng, J.; Nawaz, H.; Xia, G.; Ji, X.; Zhang, J.; Zhang, J. Fabrication and Characterization of Transparent and Uniform Cellulose/Polyethylene Composite Films from Used Disposable Paper Cups by the “One-Pot Method”. Polymers 2022, 14, 1070. [Google Scholar] [CrossRef]
- Xia, G.; Ji, X.; Peng, J.; Ji, X. Cellulose/Poly(meta-phenylene isophthalamide) Light-Management Films with High Antiultraviolet and Tunable Haze Performances. ACS Appl. Polym. Mater. 2022, 4, 8407–8417. [Google Scholar] [CrossRef]
- Ramchandani, A.G.; Chettiyar, R.S.; Pakhale, S.S. Evaluation of antioxidant and anti-initiating activities of crude polyphenolic extracts from seedless and seeded Indian grapes. Food Chem. 2010, 119, 298–305. [Google Scholar] [CrossRef]
- Gomez-Mejia, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Ferreira, I.; Barros, L. Valorisation of black mulberry and grape seeds: Chemical characterization and bioactive potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef]
- Ao, X.; Kim, I.H. Effects of grape seed extract on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult. Sci. 2020, 99, 2078–2086. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, M. From grape seed extracts to extremely stable strain sensors with freezing tolerance, drying resistance and anti-oxidation properties. Mater. Today Commun. 2022, 33, 104551. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benitez, J.J.; Tedeschi, G.; Porras-Vazquez, J.M.; Heredia, A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-blocking, and high barrier cellulose-based bioplastics with naringin as active food packaging materials. Int. J. Biol. Macromol. 2022, 209 Pt B, 1985–1994. [Google Scholar] [CrossRef]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Water vapor transport properties of regenerated cellulose and nanofibrillated cellulose films. J. Membr. Sci. 2015, 493, 46–57. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Lan, W.; Qin, W.; Liu, Y. Preparation and properties of polylactic acid-tea polyphenol-chitosan composite membranes. Int. J. Biol. Macromol. 2018, 117, 632–639. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Xu, Z.; Xia, X.; Wei, Z.; Zhang, J.; Xia, G.; Ji, X. Cellulose/Grape-Seed-Extract Composite Films with High Transparency and Ultraviolet Shielding Performance Fabricated from Old Cotton Textiles. Polymers 2023, 15, 1451. https://doi.org/10.3390/polym15061451
Ji X, Xu Z, Xia X, Wei Z, Zhang J, Xia G, Ji X. Cellulose/Grape-Seed-Extract Composite Films with High Transparency and Ultraviolet Shielding Performance Fabricated from Old Cotton Textiles. Polymers. 2023; 15(6):1451. https://doi.org/10.3390/polym15061451
Chicago/Turabian StyleJi, Xiaoqian, Zhen Xu, Xinqun Xia, Zhaoning Wei, Jun Zhang, Guangmei Xia, and Xingxiang Ji. 2023. "Cellulose/Grape-Seed-Extract Composite Films with High Transparency and Ultraviolet Shielding Performance Fabricated from Old Cotton Textiles" Polymers 15, no. 6: 1451. https://doi.org/10.3390/polym15061451





