Impact of In-Process Crystallinity of Biodegradable Scaffolds Fabricated by Material Extrusion on the Micro- and Nanosurface Topography, Viability, Proliferation, and Differentiation of Human Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Processing Parameters
2.2. Fabrication of Scaffolds, Tensile Strength, and Module of Elasticity Testing
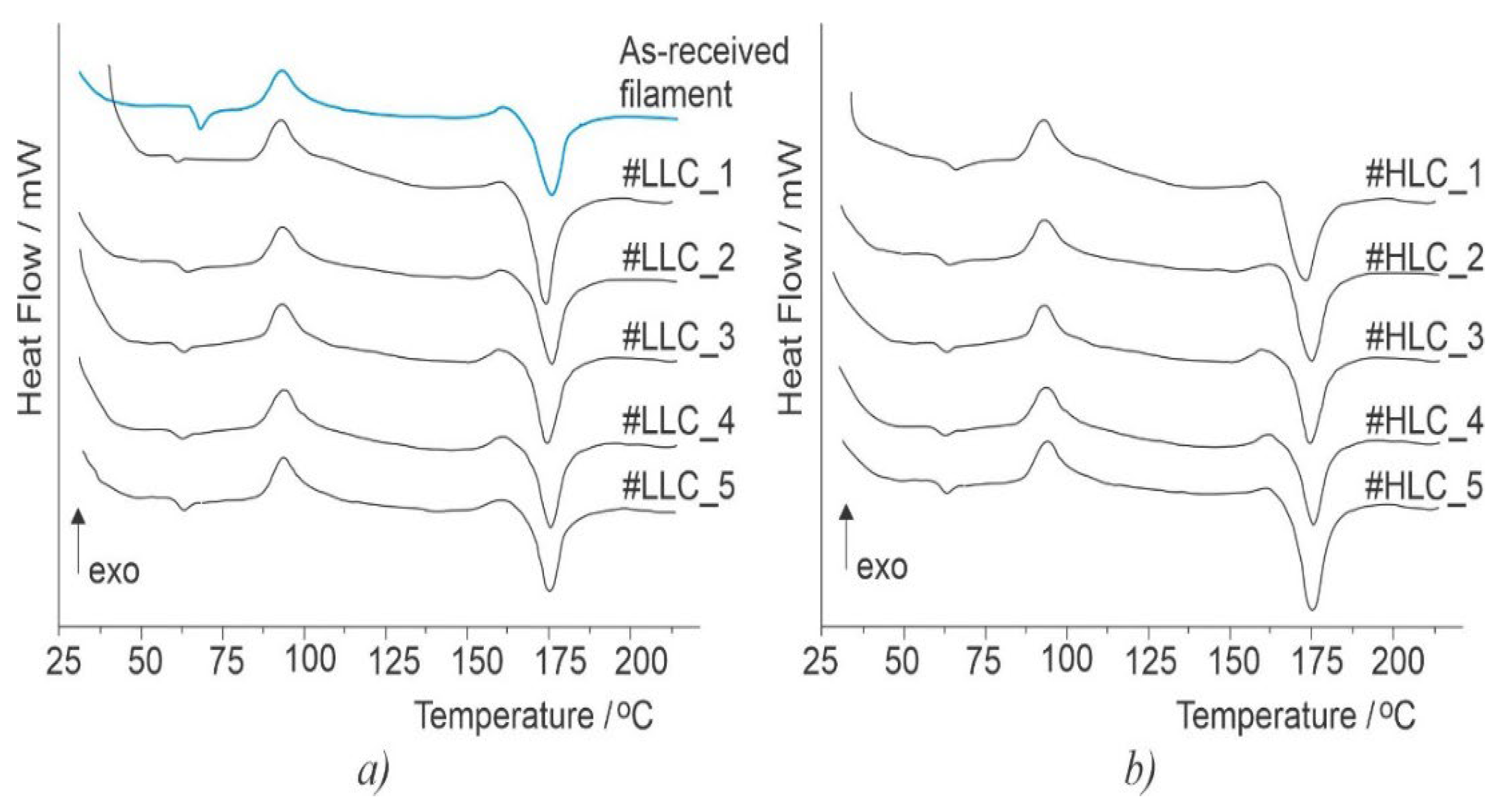
2.3. Differential Scanning Calorimetry Analysis
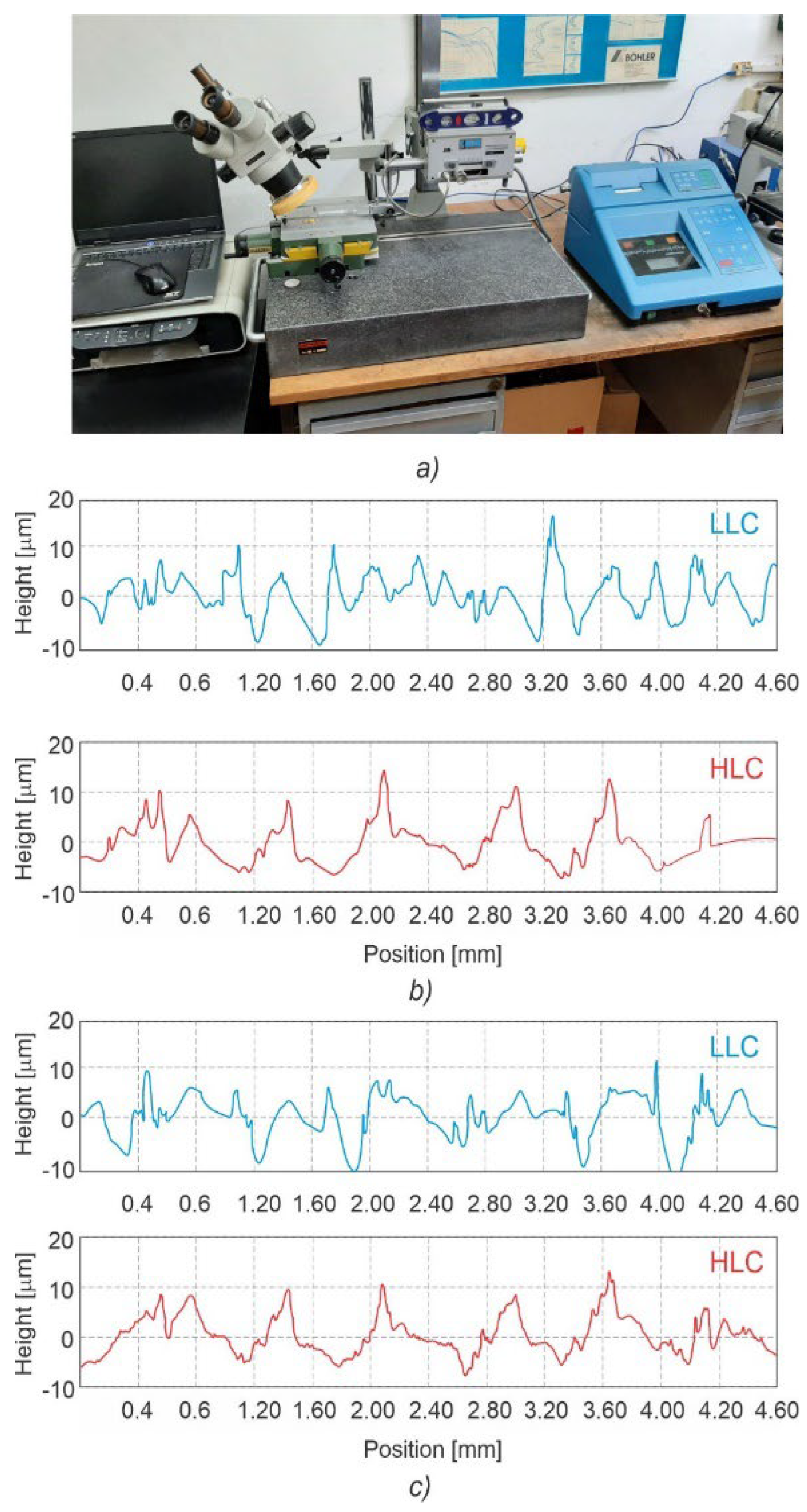
2.4. Surface Characterization
2.5. Cell Viability Tests and Biochemical Analyses
3. Results and Discussion
3.1. Surface Characterization
3.2. Crystallinity Content, Tensile Strength, and Module of Elasticity
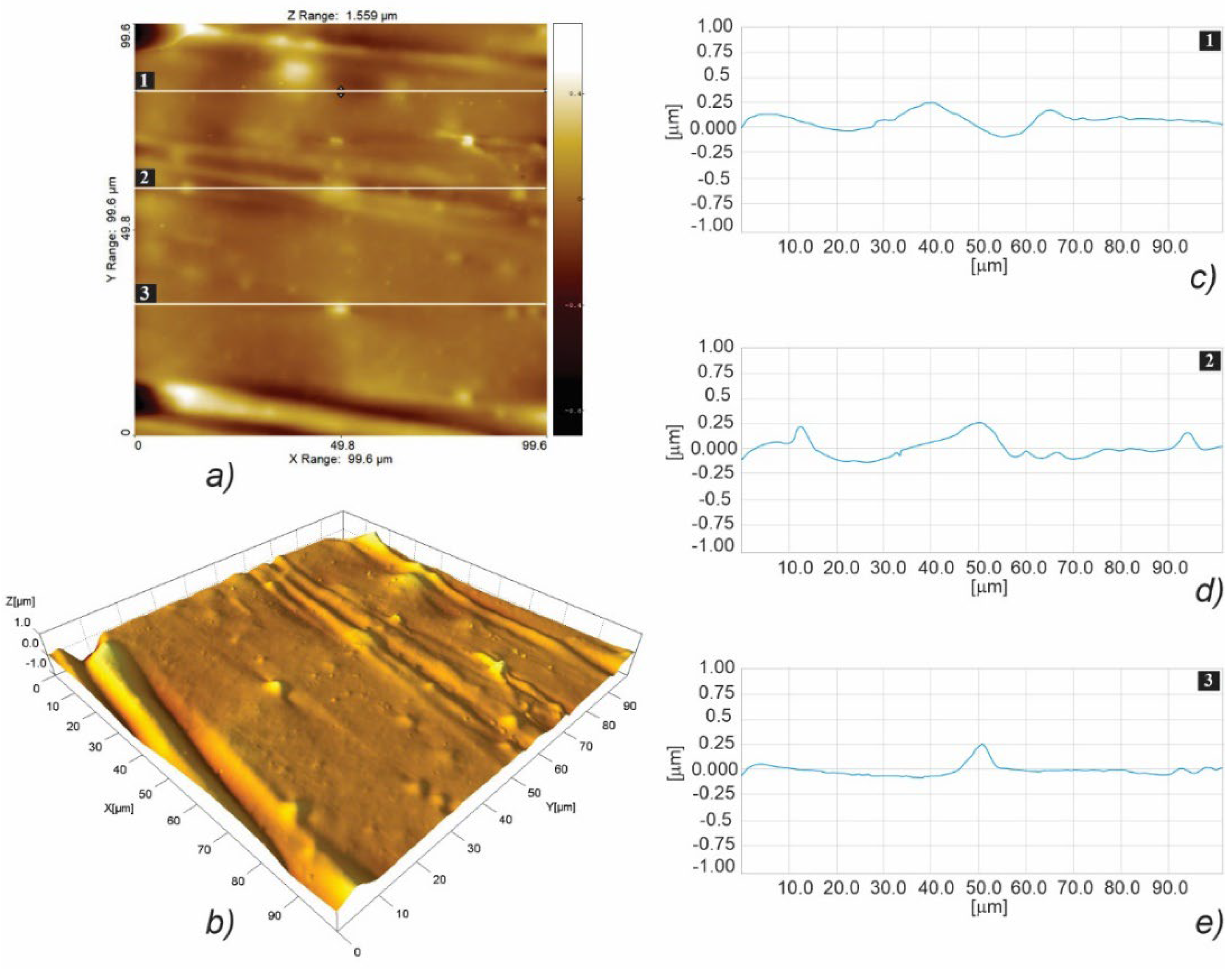
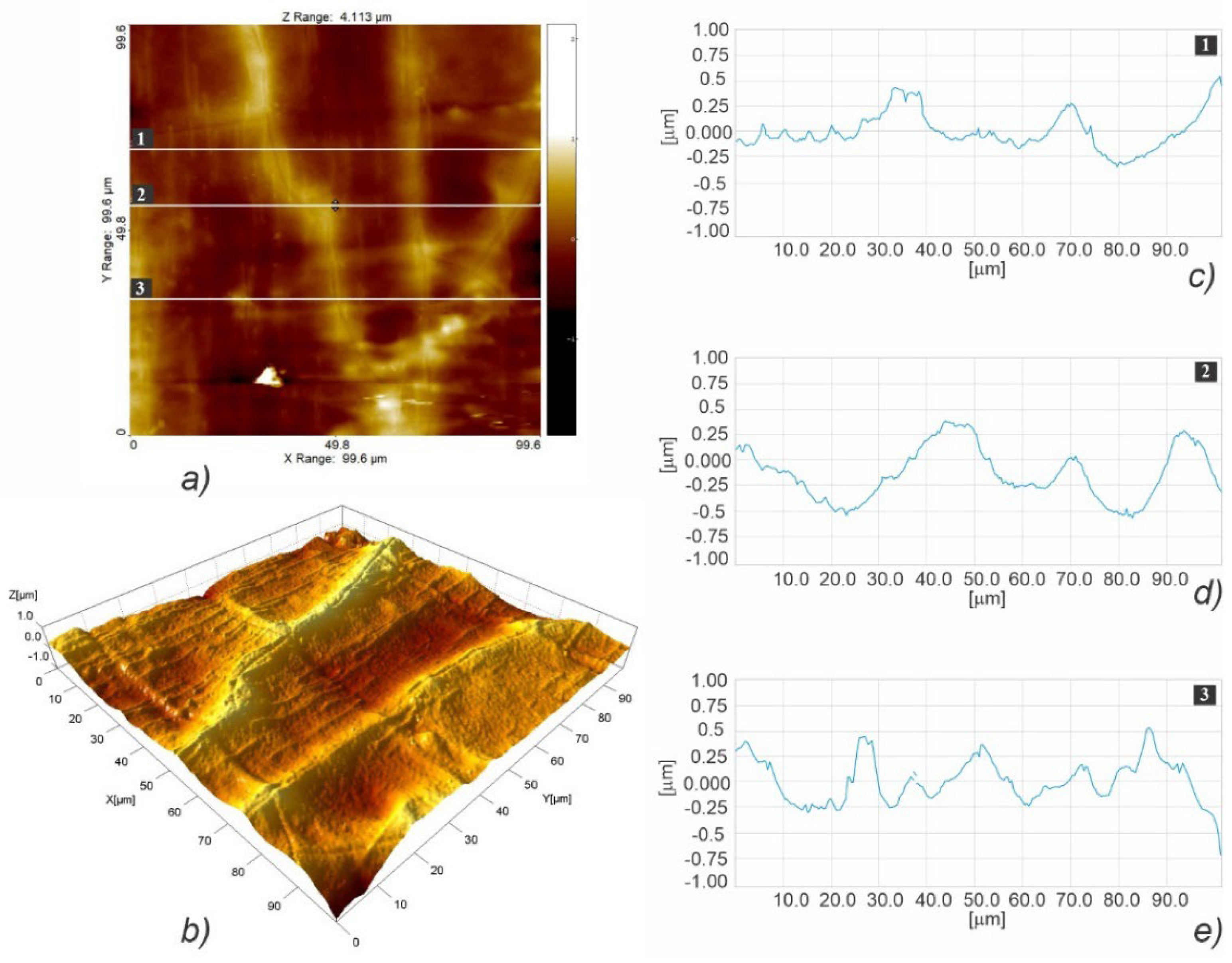
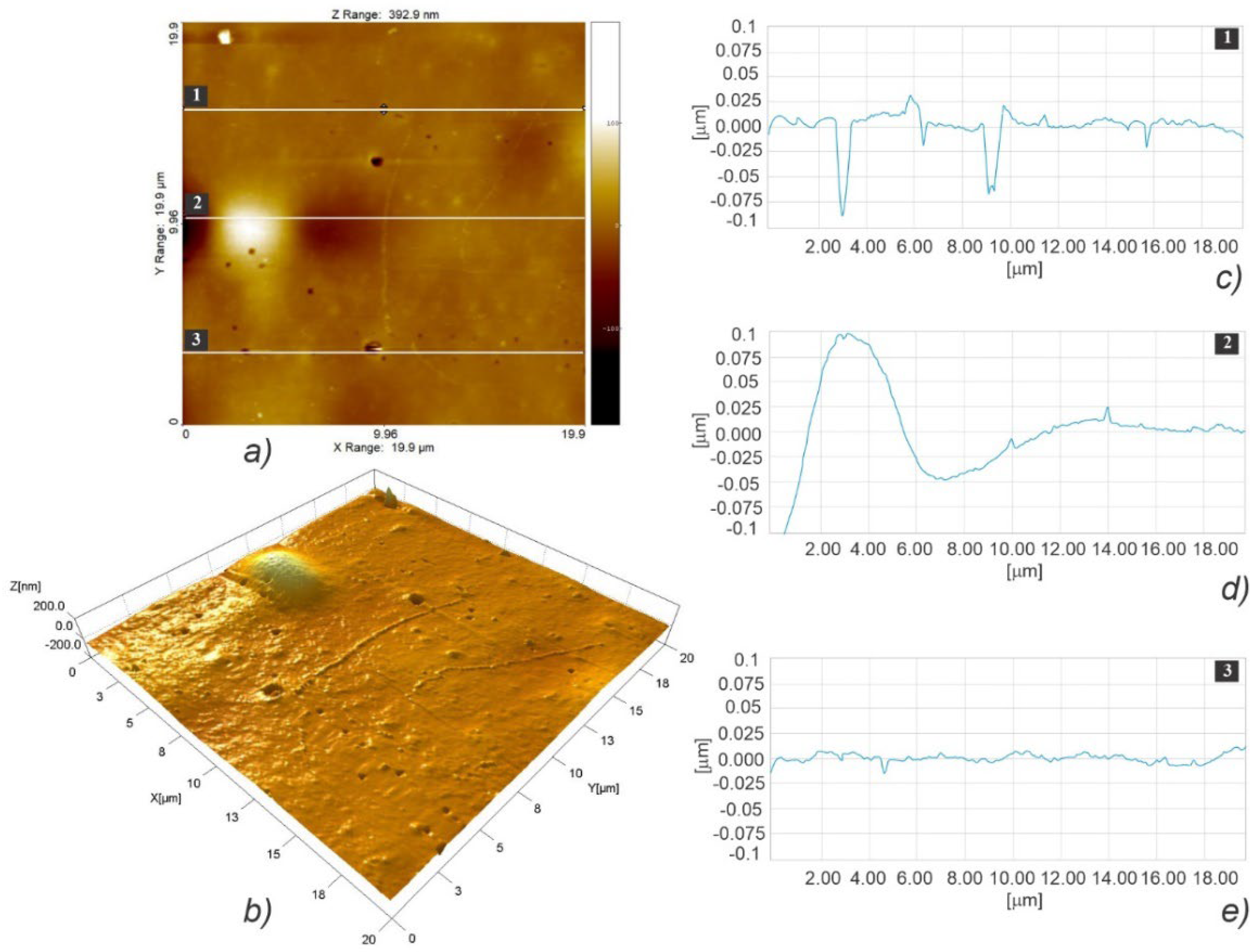
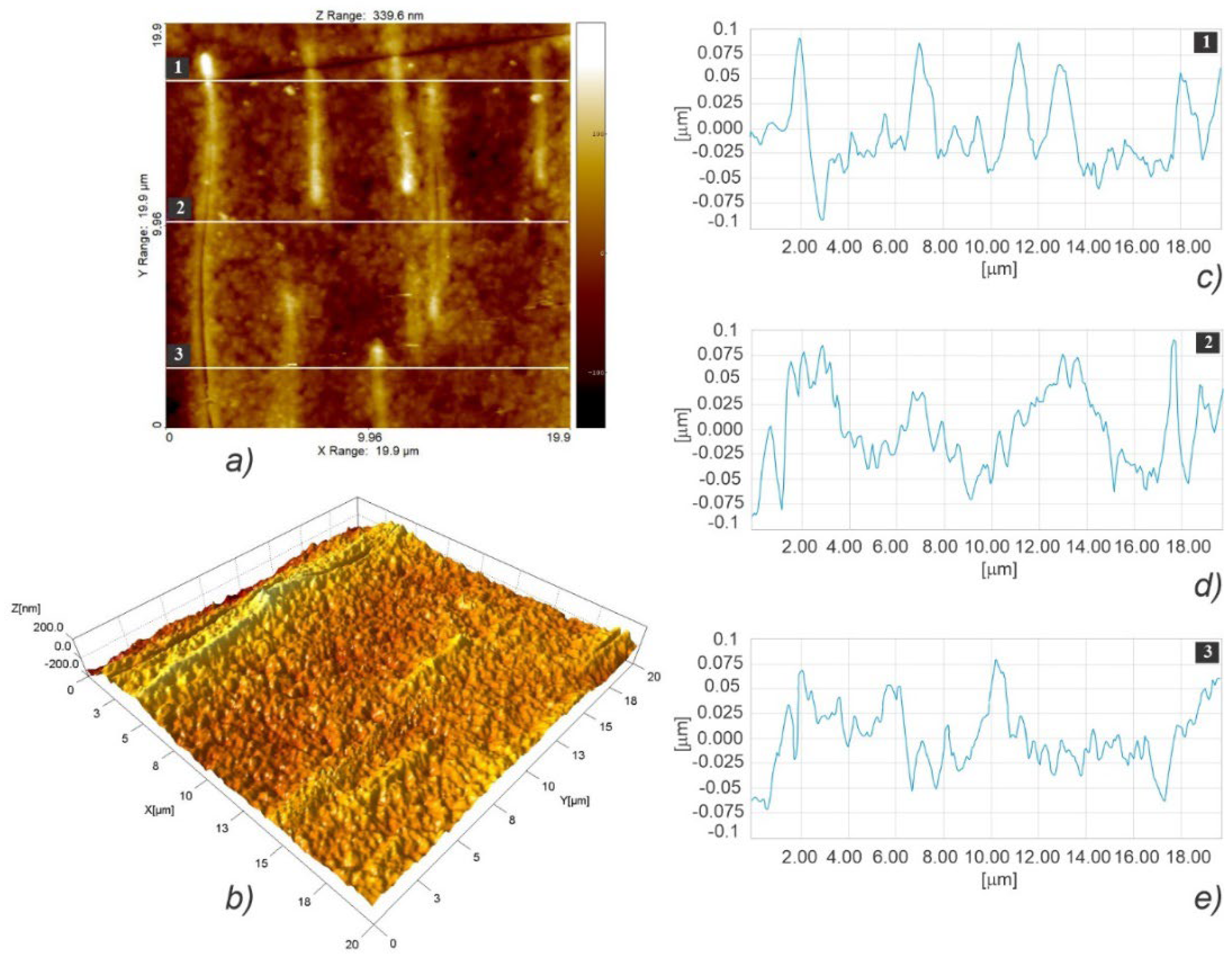
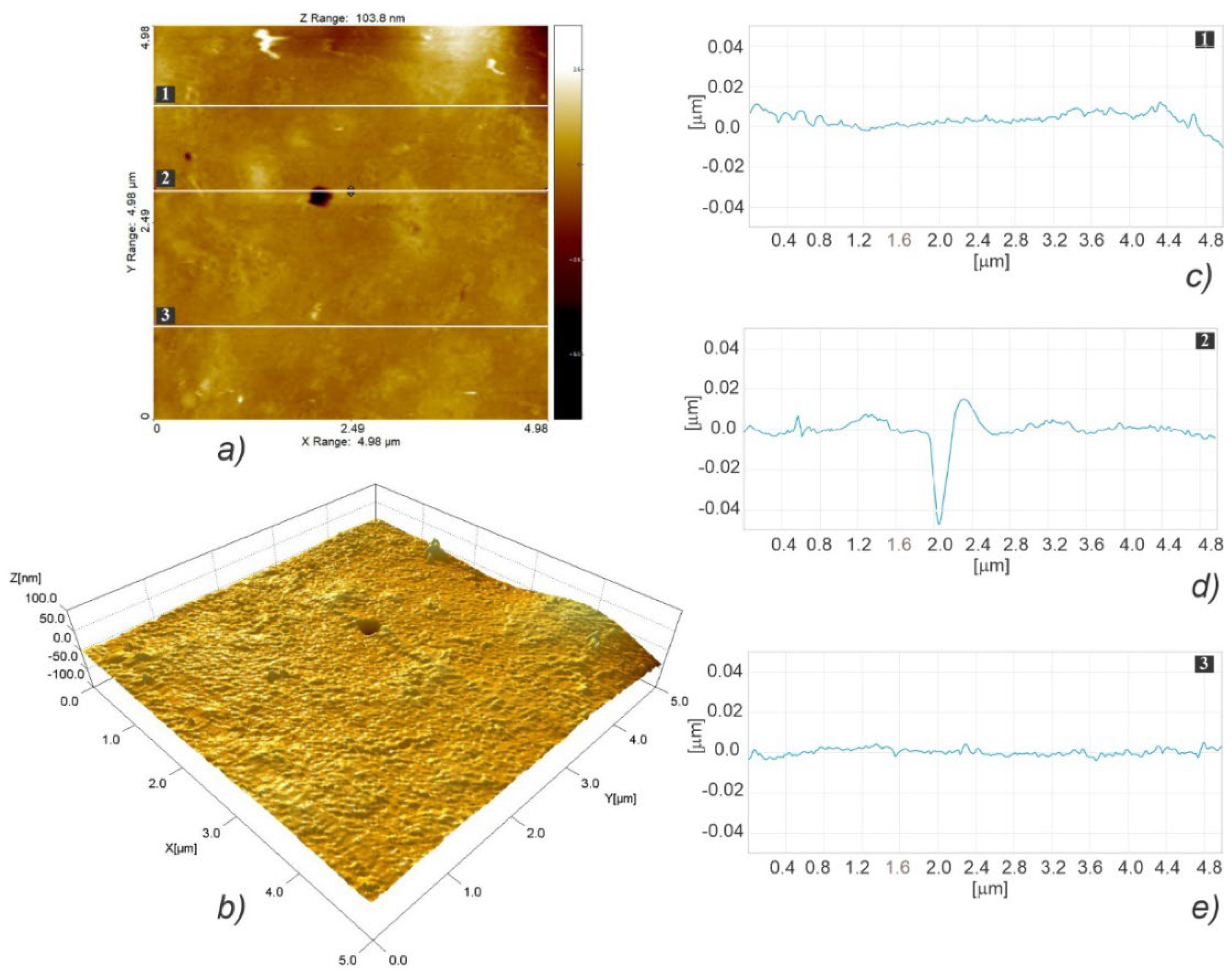
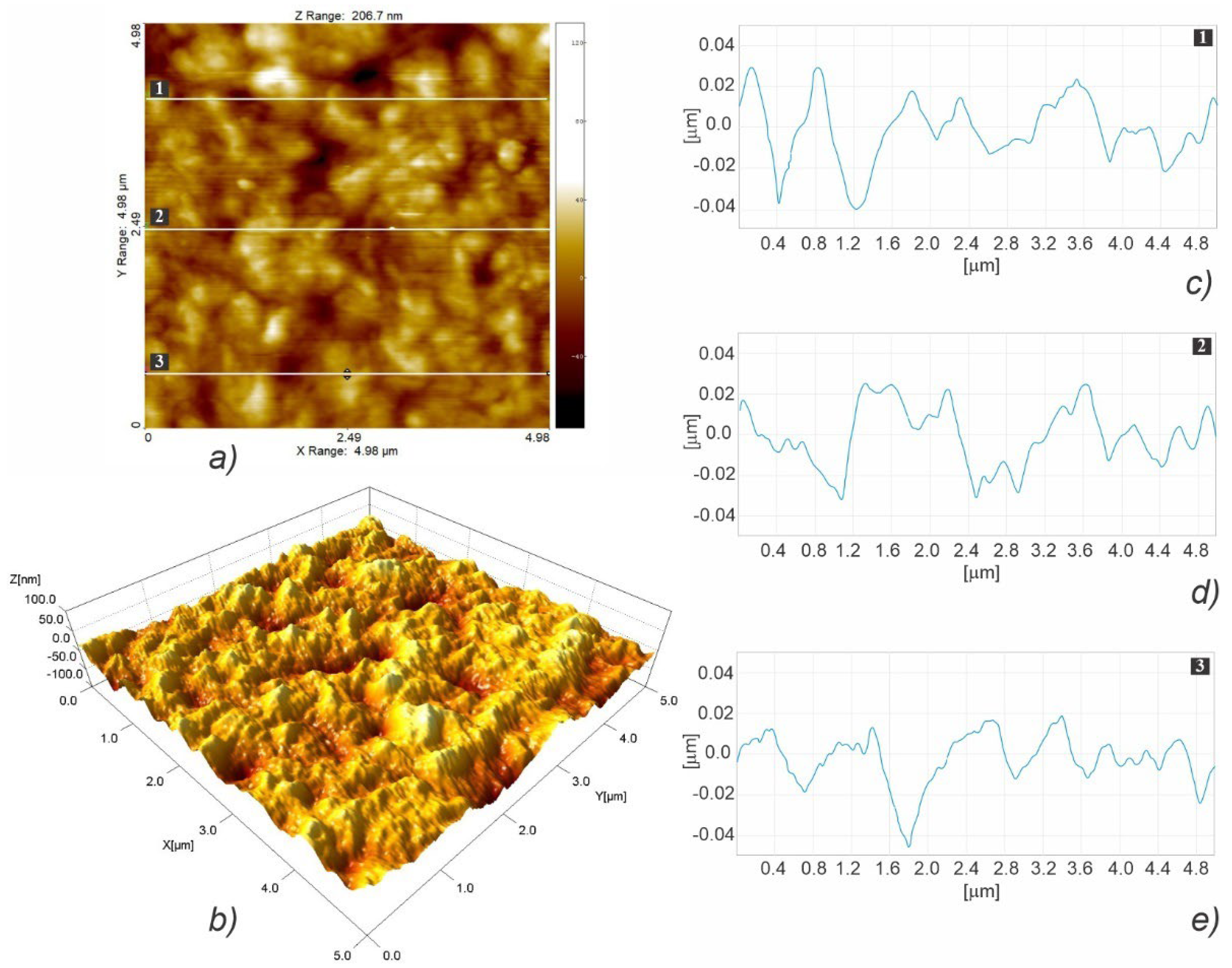
3.3. Micro- and Nanosurface Topography Analysis Results
3.3.1. Microsurface Topography
3.3.2. Nanosurface Topography
3.4. Contact Angle Measurement
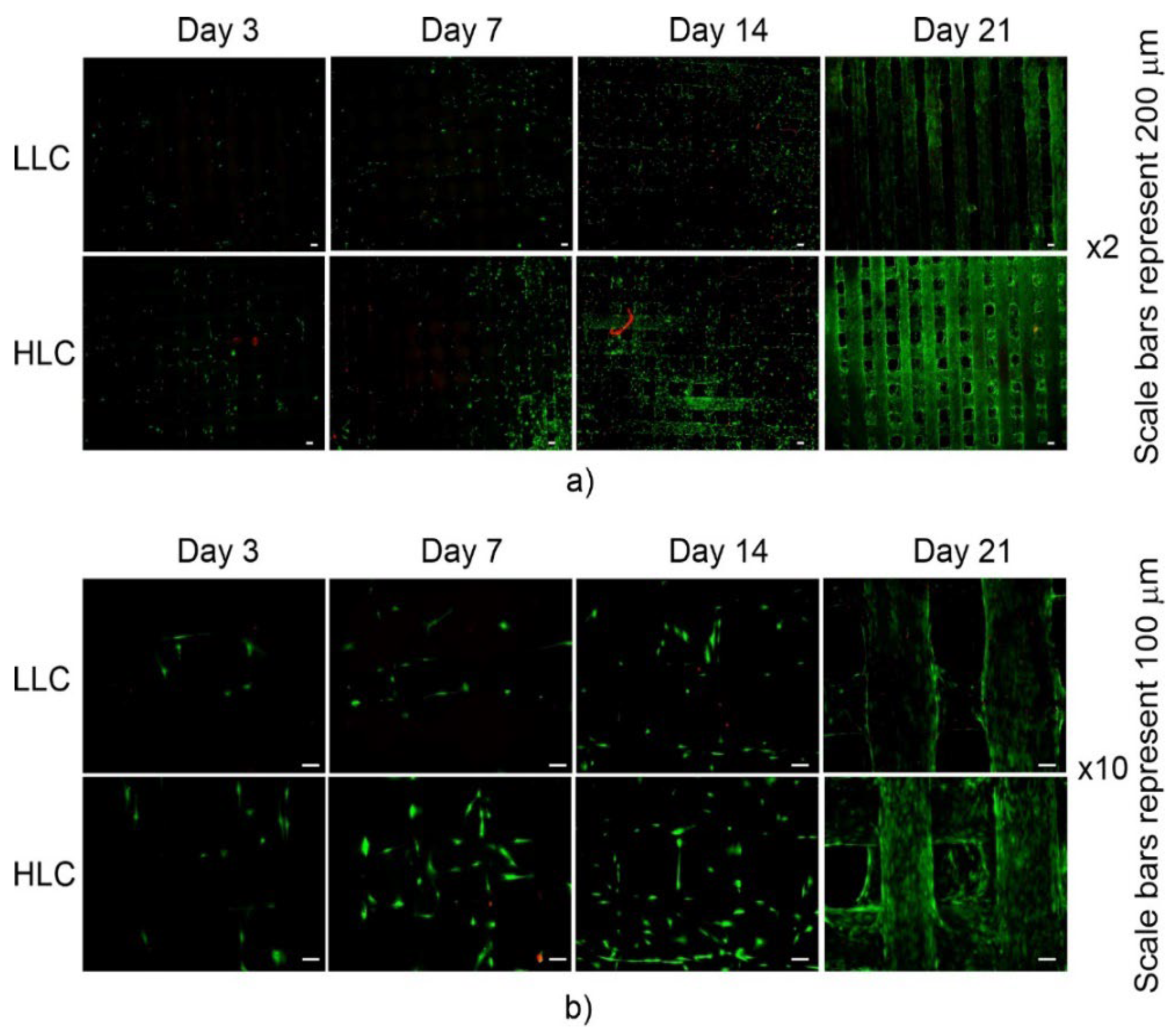
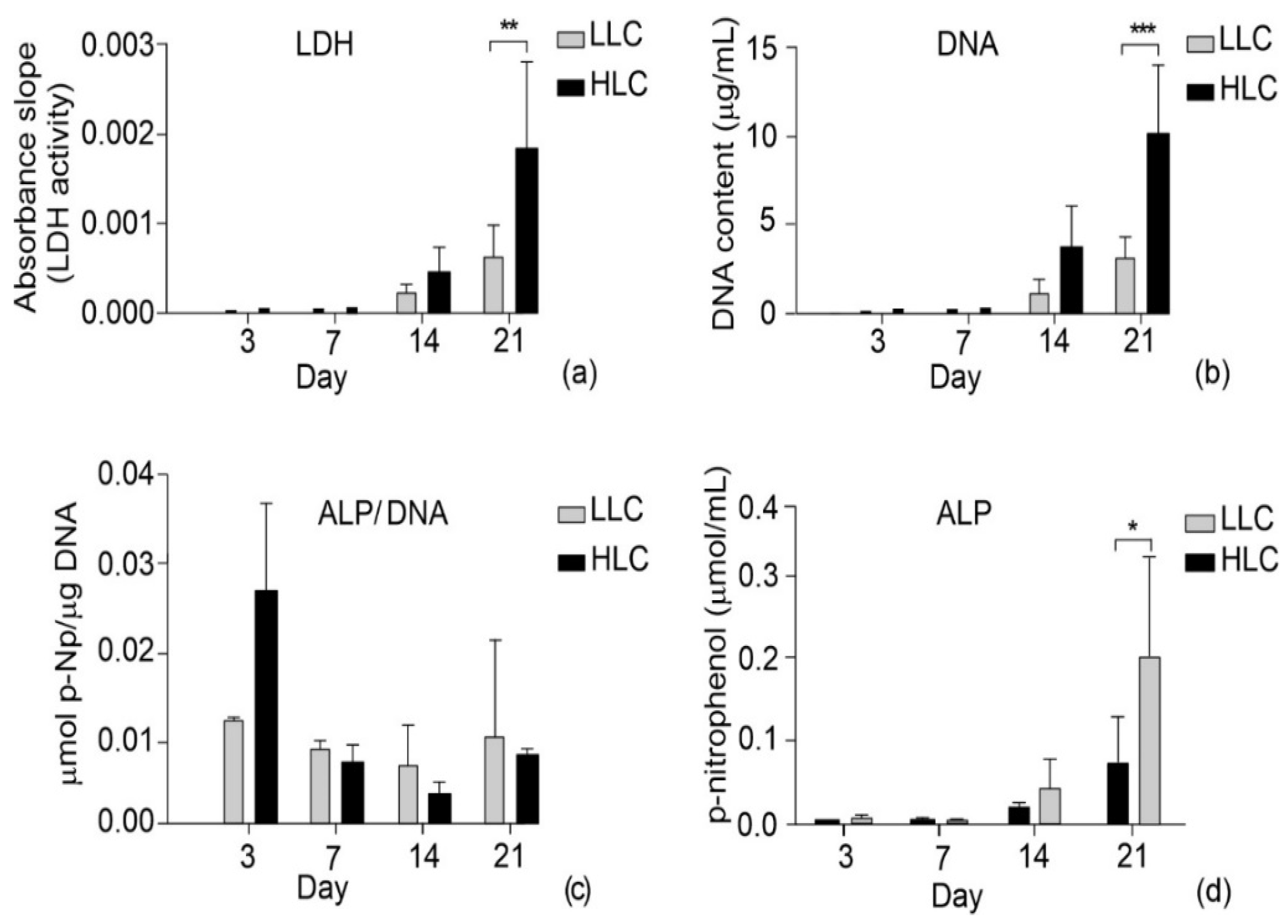
3.5. Biological Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Negi, S.; Dhiman, S.; Sharma, R.K. Basics and Applications of Rapid Prototyping Medical Models. Rapid Prototyp. J. 2014, 20, 256–267. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone Tissue Engineering Scaffolding: Computer-Aided Scaffolding Techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [PubMed]
- Weems, A.C.; Pérez-Madrigal, M.M.; Arno, M.C.; Dove, A.P. 3D Printing for the Clinic: Examining Contemporary Polymeric Biomaterials and Their Clinical Utility. Biomacromolecules 2020, 21, 1037–1059. [Google Scholar] [CrossRef] [PubMed]
- Drummer, D.; Cifuentes-Cuéllar, S.; Rietzel, D. Suitability of PLA/TCP for Fused Deposition Modeling. Rapid Prototyp. J. 2012, 18, 500–507. [Google Scholar] [CrossRef]
- Wang, L.; Gramlich, W.M.; Gardner, D.J. Improving the Impact Strength of Poly(Lactic Acid) (PLA) in Fused Layer Modeling (FLM). Polymer 2017, 114, 242–248. [Google Scholar] [CrossRef]
- Levenhagen, N.P.; Dadmun, M.D. Bimodal Molecular Weight Samples Improve the Isotropy of 3D Printed Polymeric Samples. Polymer 2017, 122, 232–241. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Song, W.; Yee, K.; Lee, K.-Y.; Tagarielli, V.L. Measurements of the Mechanical Response of Unidirectional 3D-Printed PLA. Mater. Des. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Cuiffo, M.A.; Snyder, J.; Elliott, A.M.; Romero, N.; Kannan, S.; Halada, G.P. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Appl. Sci. 2017, 7, 579. [Google Scholar] [CrossRef]
- Luzanin, O.; Movrin, D.; Stathopoulos, V.; Pandis, P.; Radusin, T.; Guduric, V. Impact of Processing Parameters on Tensile Strength, in-Process Crystallinity and Mesostructure in FDM-Fabricated PLA Specimens. Rapid Prototyp. J. 2019, 25, 1398–1410. [Google Scholar] [CrossRef]
- Vanaei, H.; Shirinbayan, M.; Deligant, M.; Raissi, K.; Fitoussi, J.; Khelladi, S.; Tcharkhtchi, A. Influence of Process Parameters on Thermal and Mechanical Properties of Polylactic Acid Fabricated by Fused Filament Fabrication. Polym. Eng. Sci. 2020, 60, 1822–1831. [Google Scholar] [CrossRef]
- Park, A.; Cima, L.G. In Vitro Cell Response to Differences in Poly-L-Lactide Crystallinity. J. Biomed. Mater. Res. 1996, 31, 17–30. [Google Scholar] [CrossRef]
- Sarasua, J.R.; López-Rodríguez, N.; Zuza, E.; Petisco, S.; Castro, B.; del Olmo, M.; Palomares, T.; Alonso-Varona, A. Crystallinity Assessment and in Vitro Cytotoxicity of Polylactide Scaffolds for Biomedical Applications. J. Mater. Sci. Mater. Med. 2011, 22, 2513–2523. [Google Scholar] [CrossRef]
- Cui, H.; Sinko, P.J. The Role of Crystallinity on Differential Attachment/Proliferation of Osteoblasts and Fibroblasts on Poly (Caprolactone-Co-Glycolide) Polymeric Surfaces. Front. Mater. Sci. 2012, 6, 47–59. [Google Scholar] [CrossRef]
- Washburn, N.R.; Yamada, K.M.; Simon, C.G.; Kennedy, S.B.; Amis, E.J. High-Throughput Investigation of Osteoblast Response to Polymer Crystallinity: Influence of Nanometer-Scale Roughness on Proliferation. Biomaterials 2004, 25, 1215–1224. [Google Scholar] [CrossRef]
- Salgado, A.J. Influence of Molecular Weight and Cristallinity of Poly(L-Lactic Acid) on the Adhesion and Proliferation of Human Osteoblast Like Cells. Mater. Sci. Forum 2006, 514–516, 1020–1024. [Google Scholar] [CrossRef]
- Yi, Q.; Wen, X.; Li, L.; He, B.; Wu, Y.; Nie, Y.; Gu, Z. The Polymeric Crystallinity Effect on the Responses of Bone Marrow Stromal Cells. E-Polymers 2009, 9, 517–526. [Google Scholar] [CrossRef]
- Goreham, R.V.; Mierczynska, A.; Smith, L.E.; Sedev, R.; Vasilev, K. Small Surface Nanotopography Encourages Fibroblast and Osteoblast Cell Adhesion. RSC Adv. 2013, 3, 10309. [Google Scholar] [CrossRef]
- de Peppo, G.M.; Agheli, H.; Karlsson, C.; Ekstrom, K.; Brisby, H.; Lenneras, M.; Gustafsson, S.; Sjövall, P.; Johansson, A.; Olsson, E.; et al. Osteogenic Response of Human Mesenchymal Stem Cells to Well-Defined Nanoscale Topography in Vitro. Int. J. Nanomed. 2014, 9, 2499. [Google Scholar] [CrossRef]
- Onesto, V.; Cancedda, L.; Coluccio, M.L.; Nanni, M.; Pesce, M.; Malara, N.; Cesarelli, M.; Di Fabrizio, E.; Amato, F.; Gentile, F. Nano-Topography Enhances Communication in Neural Cells Networks. Sci. Rep. 2017, 7, 9841. [Google Scholar] [CrossRef]
- Veiko, V.; Karlagina, Y.; Itina, T.; Kuznetsova, D.; Elagin, V.; Zagaynova, E.; Chernenko, G.; Egorova, E.; Zernitskaia, C.; Manokhin, S.; et al. Laser-Assisted Fabrication and in Vitro Verification of Functionalized Surface for Cells Biointegration. Opt. Laser Technol. 2021, 138, 106871. [Google Scholar] [CrossRef]
- Malinauskas, M.; Rekštytė, S.; Lukoševičius, L.; Butkus, S.; Balčiūnas, E.; Pečiukaitytė, M.; Baltriukienė, D.; Bukelskienė, V.; Butkevičius, A.; Kucevičius, P.; et al. 3D Microporous Scaffolds Manufactured via Combination of Fused Filament Fabrication and Direct Laser Writing Ablation. Micromachines 2014, 5, 839–858. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chatterjee, K. Surface Functionalization of 3D Printed Polymer Scaffolds to Augment Stem Cell Response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Wang, P.; Yin, H.-M.; Li, X.; Liu, W.; Chu, Y.-X.; Wang, Y.; Wang, Y.; Xu, J.-Z.; Li, Z.-M.; Li, J.-H. Simultaneously Constructing Nanotopographical and Chemical Cues in 3D-Printed Polylactic Acid Scaffolds to Promote Bone Regeneration. Mater. Sci. Eng. C 2021, 118, 111457. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M.J.; Salmeron-Sanchez, M. The Influence of Nanotopography on Cell Behaviour through Interactions with the Extracellular Matrix–A Review. Bioact. Mater. 2022, 15, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the Biomimetic Behavior of Scaffolds for Regenerative Medicine through Surface Modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Alam, F.; Varadarajan, K.M.; Kumar, S. 3D Printed Polylactic Acid Nanocomposite Scaffolds for Tissue Engineering Applications. Polym. Test. 2020, 81, 106203. [Google Scholar] [CrossRef]
- Baptista, R.; Guedes, M. Morphological and Mechanical Characterization of 3D Printed PLA Scaffolds with Controlled Porosity for Trabecular Bone Tissue Replacement. Mater. Sci. Eng. C 2021, 118, 111528. [Google Scholar] [CrossRef]
- Ahmed, J.; Zhang, J.X.; Song, Z.; Varshney, K. Thermal Properties of Polylactides Effect of Molecular Mass and Nature of Lactide Isomer. J. Therm. Anal. Calorim. 2009, 95, 957–964. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Zhu, Y.; Goh, C.; Shrestha, A. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci. 2021, 21, 2000365. [Google Scholar] [CrossRef]
- Maia-Pinto, M.O.C.; Brochado, A.C.B.; Teixeira, B.N.; Sartoretto, S.C.; Uzeda, M.J.; Alves, A.T.N.N.; Alves, G.G.; Calasans-Maia, M.D.; Thiré, R.M.S.M. Biomimetic Mineralization on 3D Printed PLA Scaffolds: On the Response of Human Primary Osteoblasts Spheroids and In Vivo Implantation. Polymers 2020, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, C.; Coppola, B.; Barra, G.; Di Maio, L.; Incarnato, L.; Lafdi, K. Effect of Porosity and Crystallinity on 3D Printed PLA Properties. Polymers 2019, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; van Hooy-Corstjens, C.S.J.; Harings, J.A.W. Correlating Molecular and Crystallization Dynamics to Macroscopic Fusion and Thermodynamic Stability in Fused Deposition Modeling; a Model Study on Polylactides. Polymers 2018, 142, 348–355. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Huwiler, C.; Drobek, T.; Vörös, J.; Spencer, N.D. Systematic Study of Osteoblast Response to Nanotopography by Means of Nanoparticle-Density Gradients. Biomaterials 2007, 28, 5000–5006. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Wilkinson, C. Topographical Control of Cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Kołbuk, D.; Ciechomska, M.; Jeznach, O.; Sajkiewicz, P. Effect of Crystallinity and Related Surface Properties on Gene Expression of Primary Fibroblasts. RSC Adv. 2022, 12, 4016–4028. [Google Scholar] [CrossRef]
- Sun, L.; Danoux, C.B.; Wang, Q.; Pereira, D.; Barata, D.; Zhang, J.; LaPointe, V.; Truckenmüller, R.; Bao, C.; Xu, X.; et al. Independent Effects of the Chemical and Microstructural Surface Properties of Polymer/Ceramic Composites on Proliferation and Osteogenic Differentiation of Human MSCs. Acta Biomater. 2016, 42, 364–377. [Google Scholar] [CrossRef]
- Pedrosa, C.R.; Arl, D.; Grysan, P.; Khan, I.; Durrieu, S.; Krishnamoorthy, S.; Durrieu, M.-C. Controlled Nanoscale Topographies for Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2019, 11, 8858–8866. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A Review of Old Concepts with New Standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
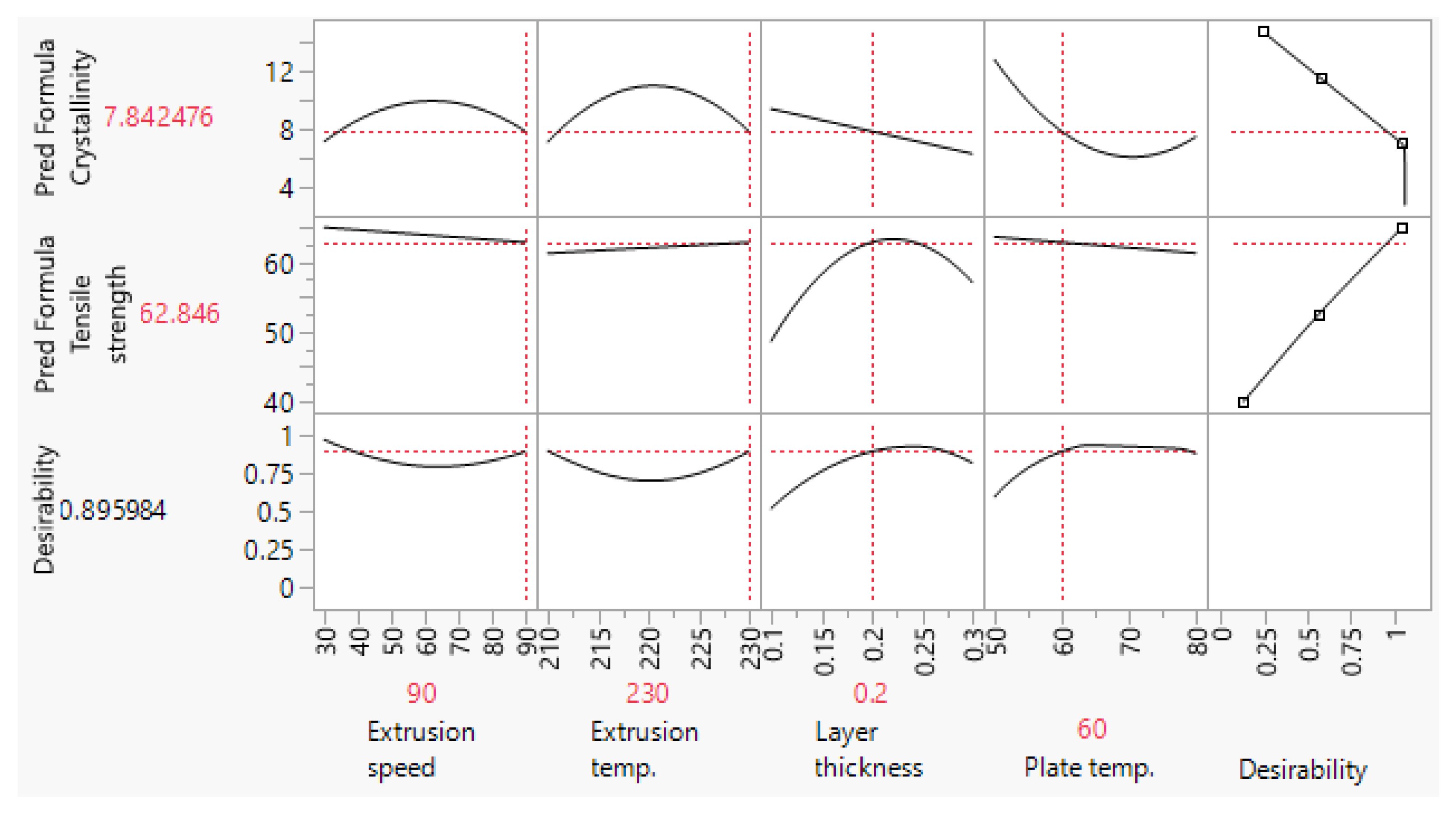

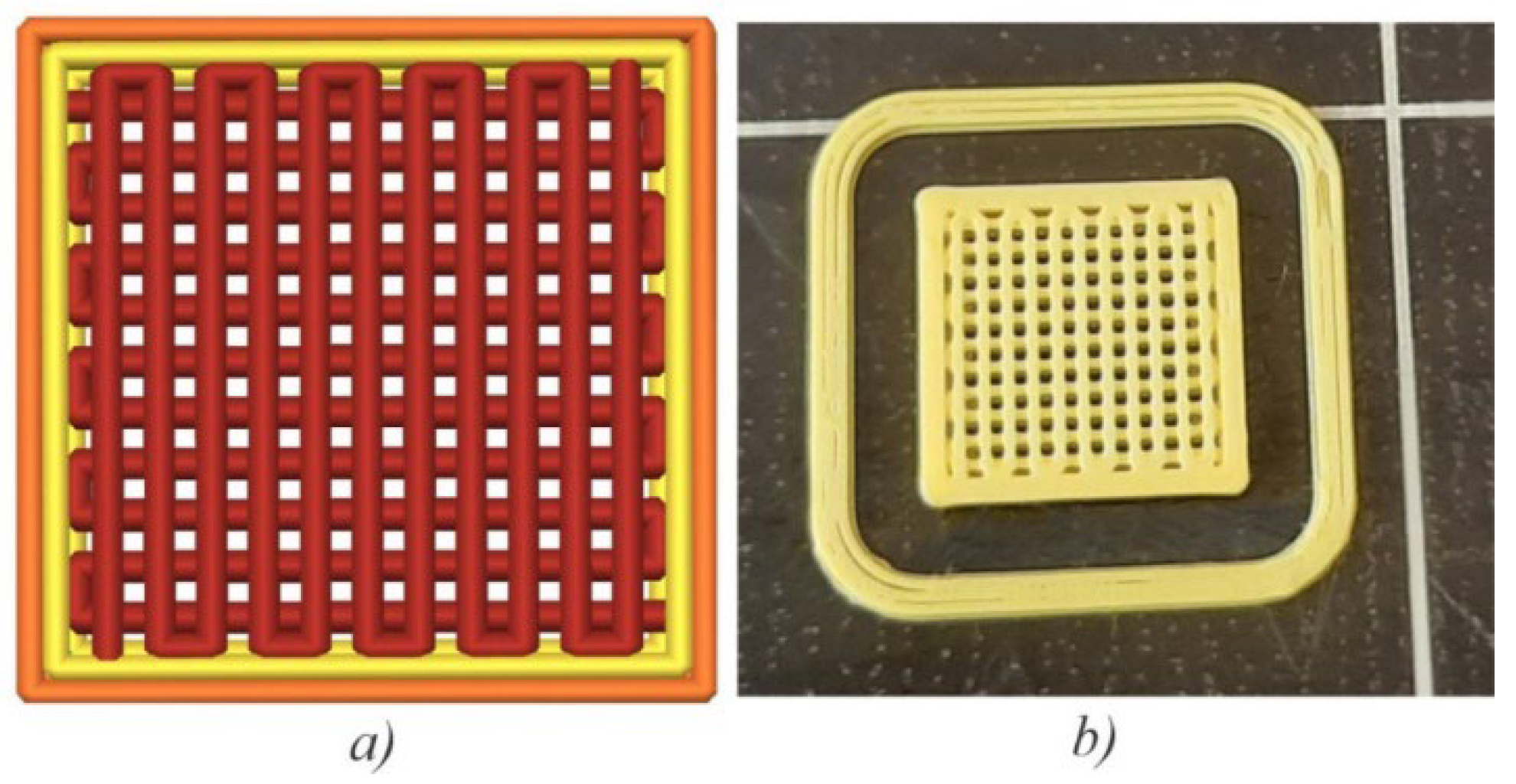



| Scaffold Plate Type | FFF Processing Parameter | |||
|---|---|---|---|---|
| Extrusion Speed (mm/s) | Extruder Temperature (°C) | Layer Thickness (mm) | Build Plate Temperature (°C) | |
| Low-level cryst. (LLC) | 90 | 230 | 0.2 | 60 |
| High-level cryst. (HLC) | 30 | 220 | 0.2 | 80 |
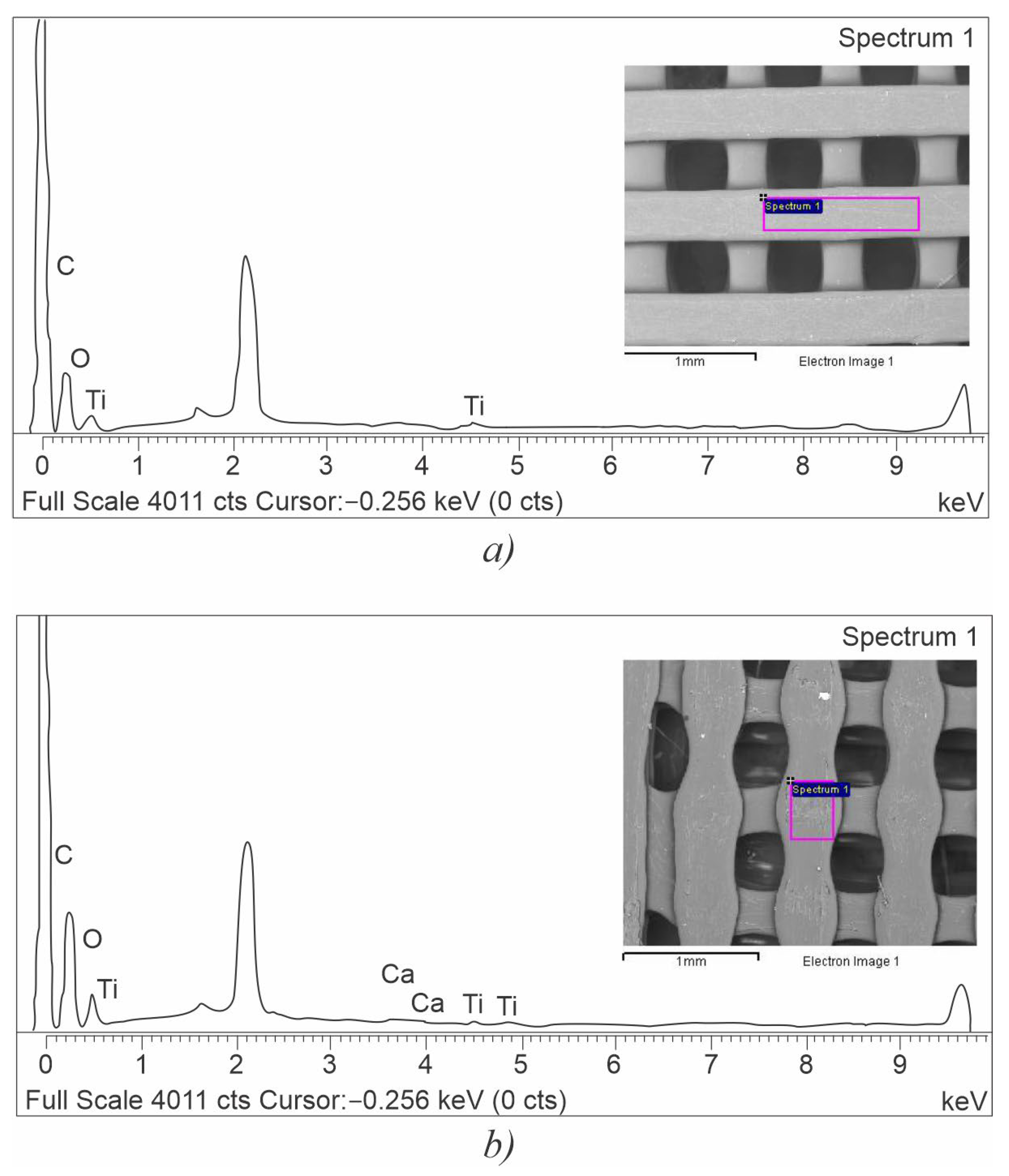
| Scaffold Plate Type | C [wt.%] | O [wt.%] | Ti [wt.%] | Ca [wt.%] |
|---|---|---|---|---|
| LLC | 62.02 ± 1.28 | 37.05 ± 1.29 | 0.63 ± 0.15 | 0.30 ± 0.11 |
| HLC | 62.62 ± 2.00 | 35.73 ± 2.02 | 1.65 ± 0.32 | - |
| LLCSpecimen Number | Crystallization Enthalpy ΔHc (J/g) | Recrystallization Enthalpy ΔHr (J/g) | Melting Enthalpy ΔHm (J/g) | Crystallinity Xc (%) |
|---|---|---|---|---|
| #1 | 29.73 | 4.10 | 42.21 | 9.01 |
| #2 | 27.78 | 3.47 | 40.21 | 9.63 |
| #3 | 22.79 | 3.89 | 38.58 | 12.79 |
| #4 | 25.55 | 5.49 | 37.14 | 6.56 |
| #5 | 26.24 | 4.58 | 40.01 | 9.90 |
| HLC Specimen Number | Crystallization Enthalpy ΔHc (J/g) | Recrystallization Enthalpy ΔHr (J/g) | Melting Enthalpy ΔHm (J/g) | Crystallinity Xc (%) |
|---|---|---|---|---|
| #1 | 18.78 | 4.40 | 36.24 | 14.04 |
| #2 | 21.07 | 4.17 | 41.76 | 17.77 |
| #3 | 18.255 | 3.29 | 37.054 | 16.68 |
| #4 | 20.56 | 3.98 | 40.016 | 16.64 |
| #5 | 20.17 | 3.67 | 42.05 | 19.58 |
| Force (N) | 511.8 | 497.4 | 505.8 | 511.8 | 480.7 | 535.7 | 513.0 | 524.9 |
| Tensile Stress (MPa) | 63.97 | 62.17 | 63.22 | 63.97 | 60.08 | 66.96 | 64.12 | 65.61 |
| Force (N) | 502.1 | 480.5 | 525.3 | 500.8 | 510.7 | 550.2 | 507.8 | 518.6 |
| Tensile Stress (MPa) | 62.76 | 60.10 | 65.66 | 62.6 | 63.84 | 68.77 | 63.47 | 64.82 |
| Specimen No. | Yield Tensile Force (0.20%) (N) | Yield Stress (0.20%) (MPa) | Yield Strain (0.20%) | Young’s Modulus (GPa) |
|---|---|---|---|---|
| #1 LLC | 1027.4 | 26.195 | 1.981 | 1.322 |
| #2 LLC | 1132.9 | 28.695 | 2.052 | 1.398 |
| #3 LLC | 1292.5 | 32.737 | 2.054 | 1.593 |
| #4 HLC | 1142.0 | 28.972 | 1.802 | 1.607 |
| #5 HLC | 1191.3 | 29.964 | 1.911 | 1.567 |
| #6 HLC | 1123.1 | 28.278 | 1.937 | 1.460 |
| Scaffold Plate Type | Root Mean Square (Rq ± SD) [μm] (n = 6) | Surface Skewness (Rsk ± SD) (n = 6) | Surface Kurtosis (Rku ± SD) (n = 6) | Mean Width (Rsm ± SD) [μm] (n = 6) | Root Mean Square Slope (Rdq ± SD) (n = 6) |
|---|---|---|---|---|---|
| LLC | 3.507 ± 0.278 | 0.677 ± 0.596 | 3.960 ± 0.573 | 364.25 ± 64.39 | 0.146 ± 0.02 |
| HLC | 3.646 ± 0.785 | 0.967 ± 0.210 | 3.768 ± 0.746 | 510.95 ± 100.79 | 0.145 ± 0.037 |
| Scaffold Type | Root Mean Square (Sq ± SD) [nm] (n = 3) | Surface Skewness (Ssk ± SD) (n = 3) | Surface Kurtosis (Sku ± SD) (n = 3) |
|---|---|---|---|
| LLC | 115.13 ± 43.59 | 0.453 ± 1.017 | 9.048 ± 3.765 |
| HLC | 225.30 ± 57.35 | 0.465 ± 0.055 | 3.281 ± 0.513 |
| Scaffold Type | Fastest Decay Autocorrelation Length (Scl37 ± SD) [nm] (n = 3) | Texture Aspect Ratio (Str37 ± SD) (n = 3) | Density of Summits (Sds ± SD) [1/μm2] (n = 3) |
|---|---|---|---|
| LLC | 4687.5 ± 2067 | 0.484 ± 0.089 | 0.124 ± 0.036 |
| HLC | 6510.4 ± 451 | 0.350 ± 0.099 | 0.257 ± 0.053 |
| Scaffold Type | RMS Surf. Slope (Sdq ± SD) (n = 3) | Developed Surf. Area Ratio (Sdr ± SD) (n = 3) | Arith. Mean Summit Curv. (Ssc ± SD) [1/nm] (n = 3) |
|---|---|---|---|
| LLC | 0.0513 ± 0.007 | 0.133 ± 0.036 | 131 × 10−6 ± 41 × 10−6 |
| HLC | 0.106 ± 0.016 | 0.571 ± 0.161 | 348 × 10−6 ± 43 × 10−6 |
| Scaffold Type | Sample No. | Contact Angle (Sdq ± SD) [°] (n = 5) |
|---|---|---|
| LLC | #1 | 96.68 ± 0.658 |
| #2 | 91.60 ± 0.639 | |
| #3 | 94.67 ± 0.321 | |
| HLC | #1 | 91.25 ± 0.557 |
| #2 | 97.84 ± 0.204 | |
| #3 | 95.65 ± 0.464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lužanin, O.; Gudurić, V.; Bernhardt, A.; Movrin, D.; Damjanović-Vasilić, L.; Terek, P.; Ostojić, G.; Stankovski, S. Impact of In-Process Crystallinity of Biodegradable Scaffolds Fabricated by Material Extrusion on the Micro- and Nanosurface Topography, Viability, Proliferation, and Differentiation of Human Mesenchymal Stromal Cells. Polymers 2023, 15, 1468. https://doi.org/10.3390/polym15061468
Lužanin O, Gudurić V, Bernhardt A, Movrin D, Damjanović-Vasilić L, Terek P, Ostojić G, Stankovski S. Impact of In-Process Crystallinity of Biodegradable Scaffolds Fabricated by Material Extrusion on the Micro- and Nanosurface Topography, Viability, Proliferation, and Differentiation of Human Mesenchymal Stromal Cells. Polymers. 2023; 15(6):1468. https://doi.org/10.3390/polym15061468
Chicago/Turabian StyleLužanin, Ognjan, Vera Gudurić, Anne Bernhardt, Dejan Movrin, Ljiljana Damjanović-Vasilić, Pal Terek, Gordana Ostojić, and Stevan Stankovski. 2023. "Impact of In-Process Crystallinity of Biodegradable Scaffolds Fabricated by Material Extrusion on the Micro- and Nanosurface Topography, Viability, Proliferation, and Differentiation of Human Mesenchymal Stromal Cells" Polymers 15, no. 6: 1468. https://doi.org/10.3390/polym15061468
APA StyleLužanin, O., Gudurić, V., Bernhardt, A., Movrin, D., Damjanović-Vasilić, L., Terek, P., Ostojić, G., & Stankovski, S. (2023). Impact of In-Process Crystallinity of Biodegradable Scaffolds Fabricated by Material Extrusion on the Micro- and Nanosurface Topography, Viability, Proliferation, and Differentiation of Human Mesenchymal Stromal Cells. Polymers, 15(6), 1468. https://doi.org/10.3390/polym15061468









