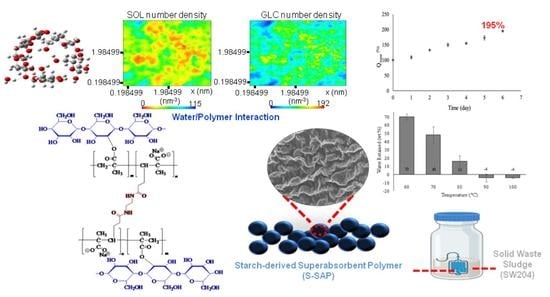
Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water–Polymer Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Starch-Derived Superabsorbent Polymer (S-SAP)
2.3. Characterization
2.4. Density Functional Theory
2.5. Molecular Dynamics
2.6. Absorption Properties
2.7. Swelling Properties
3. Results and Discussion
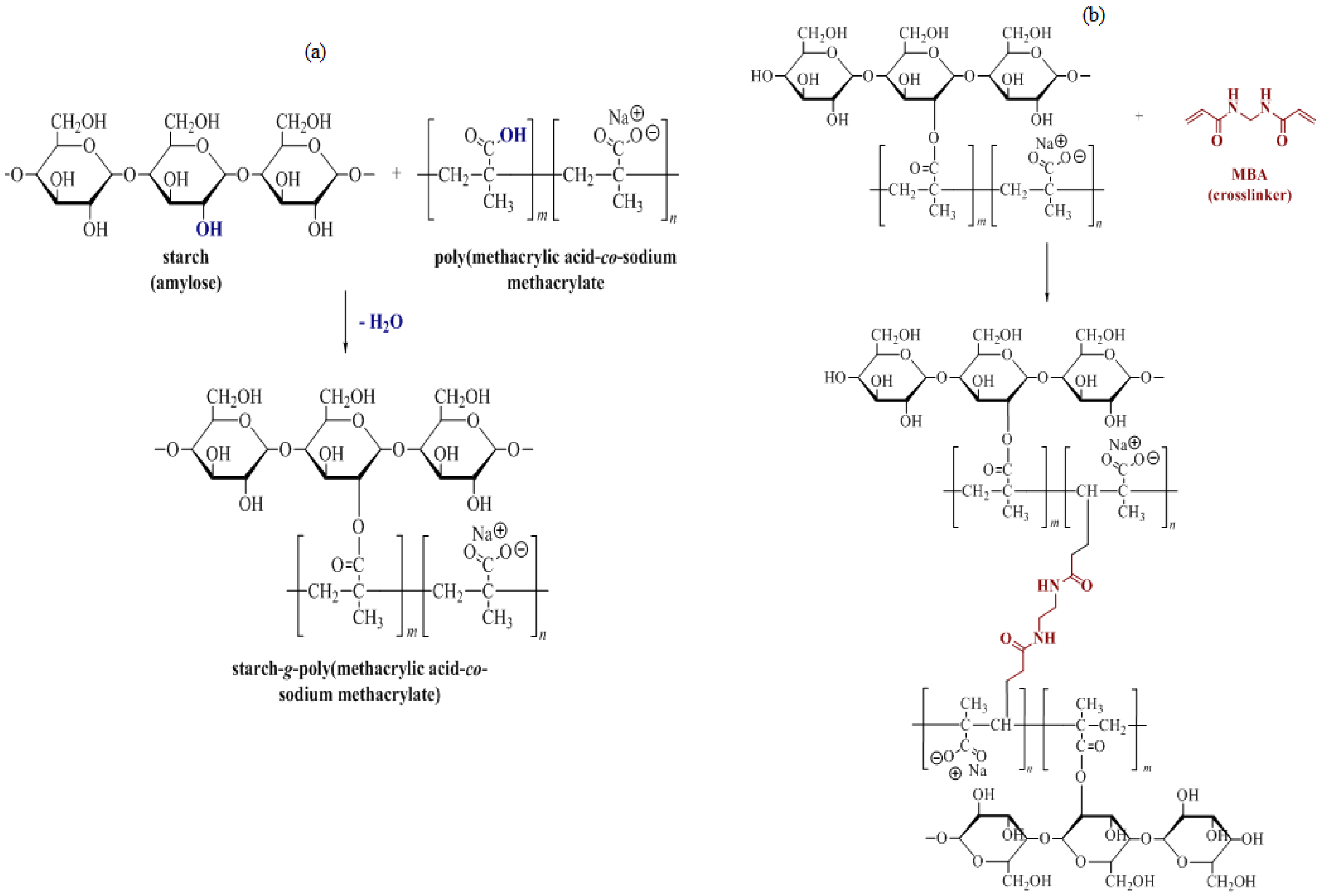
3.1. Synthesis of Starch-Derived Superabsorbent Polymer (S-SAP)
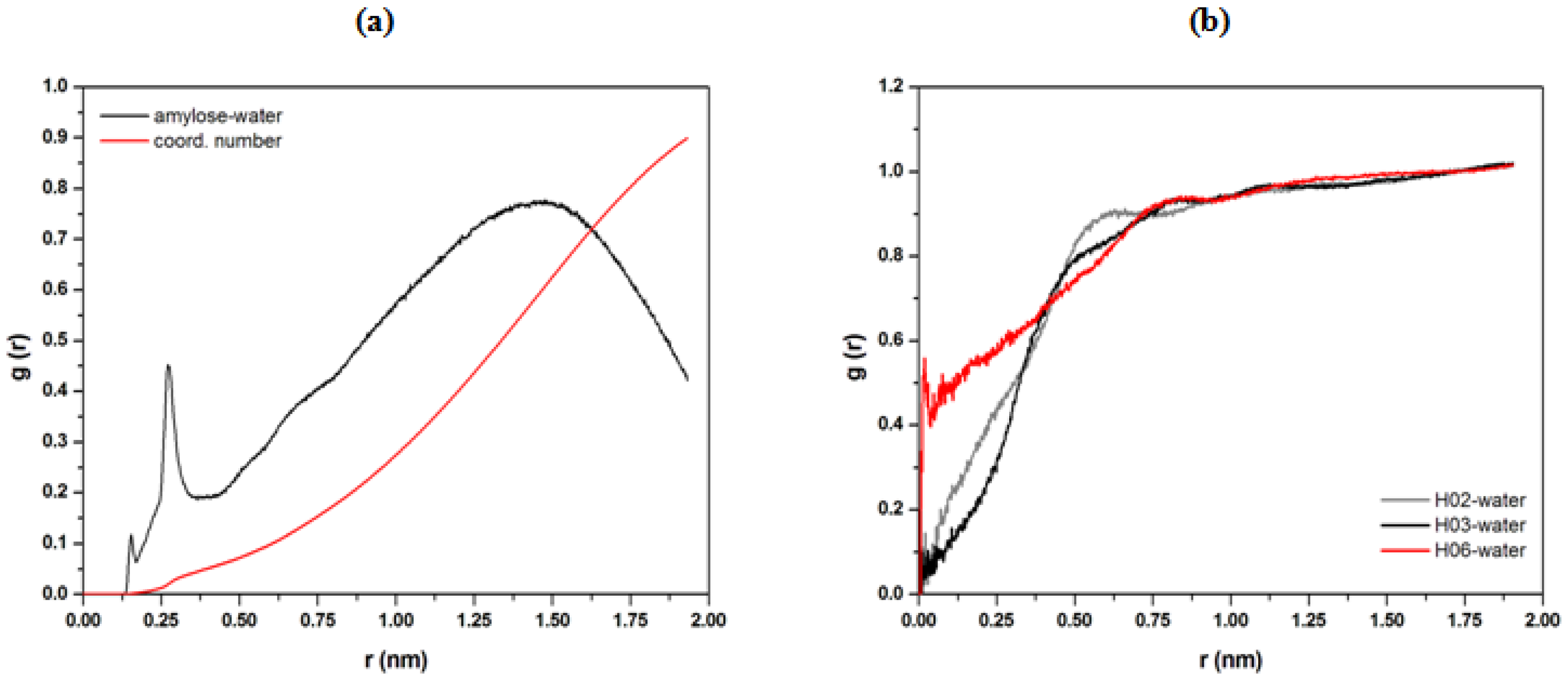
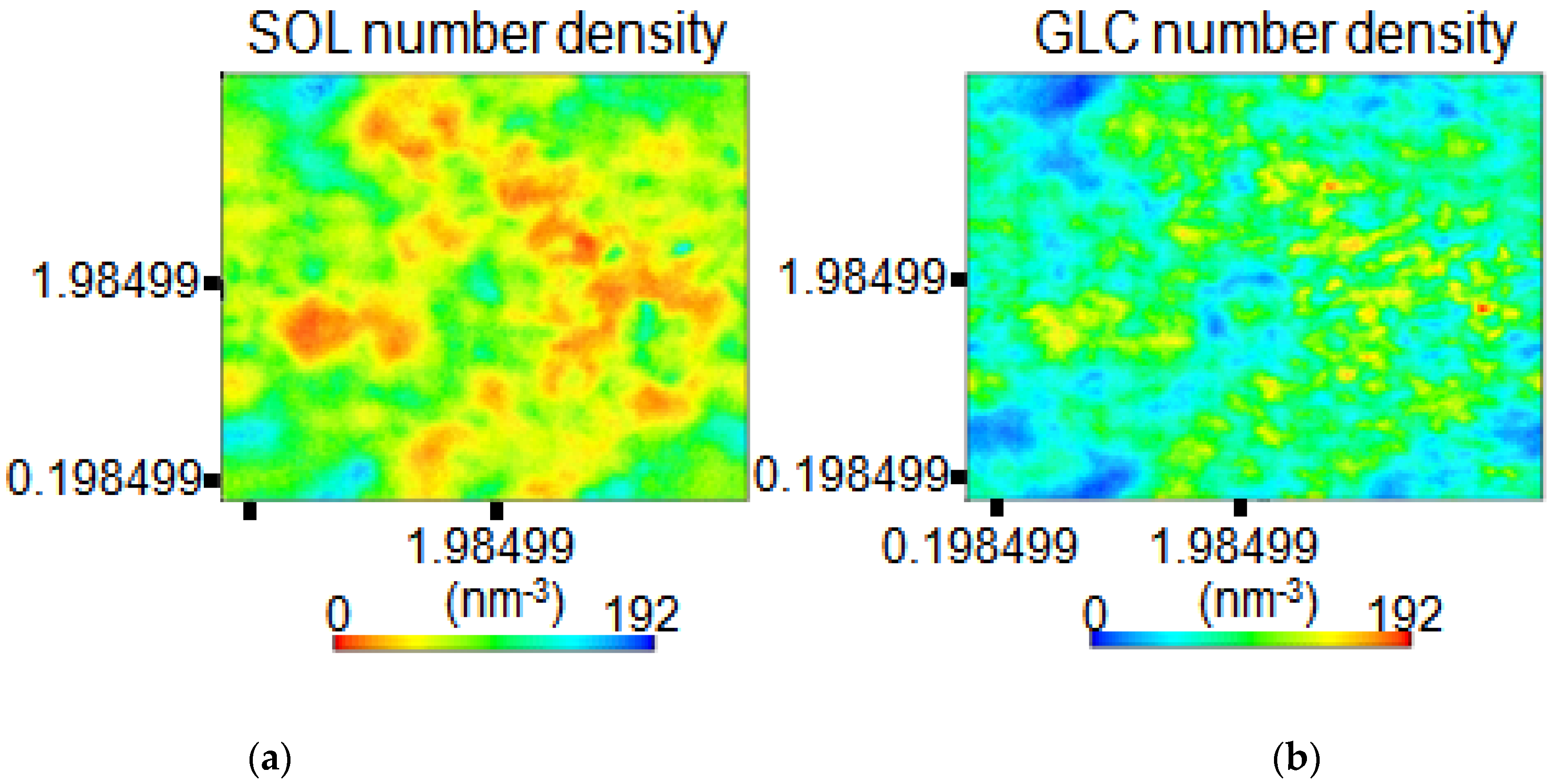
3.2. Determination on Water–Polymer Interaction of Starch Backbone
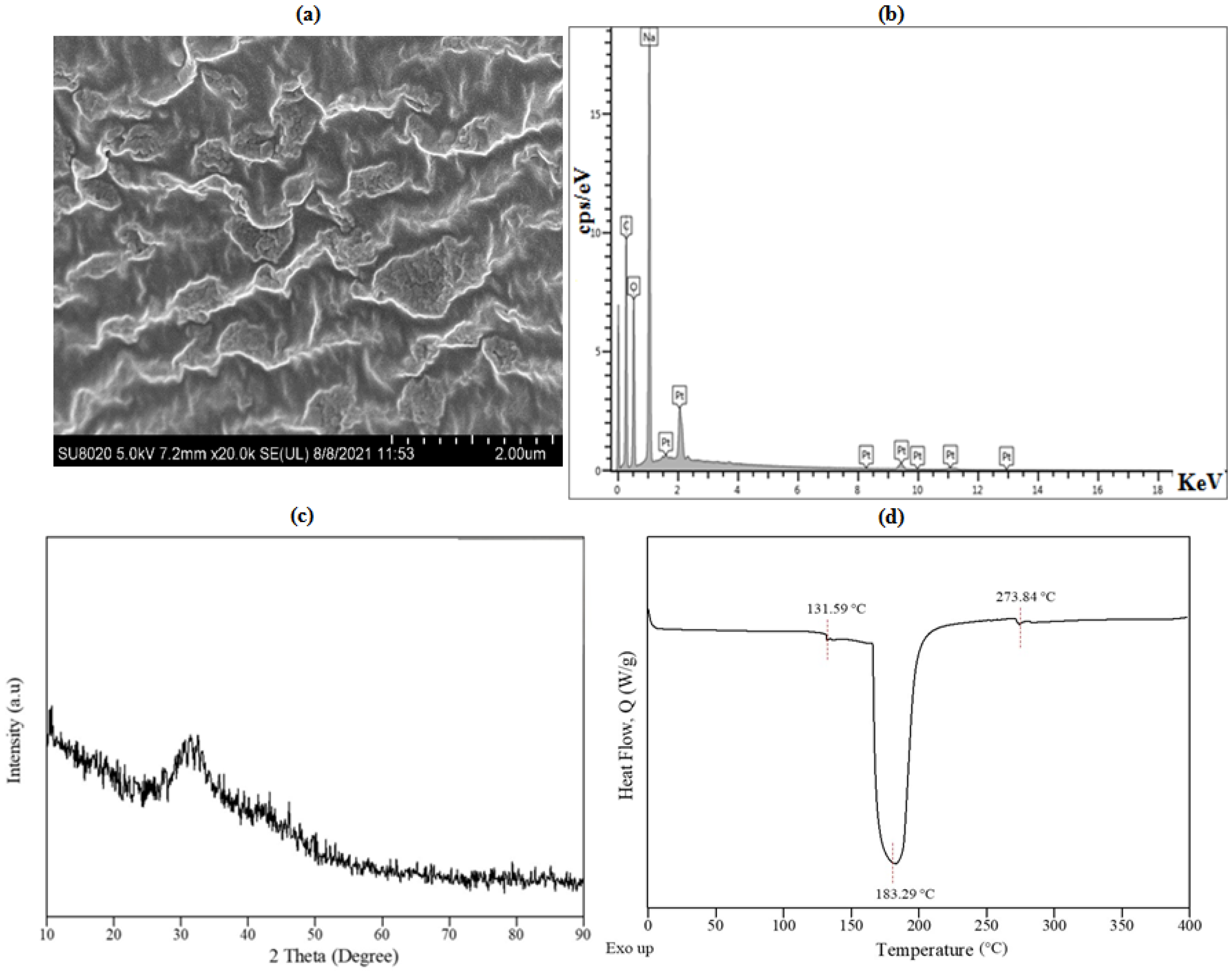
3.3. Physiochemical Properties of S-SAP
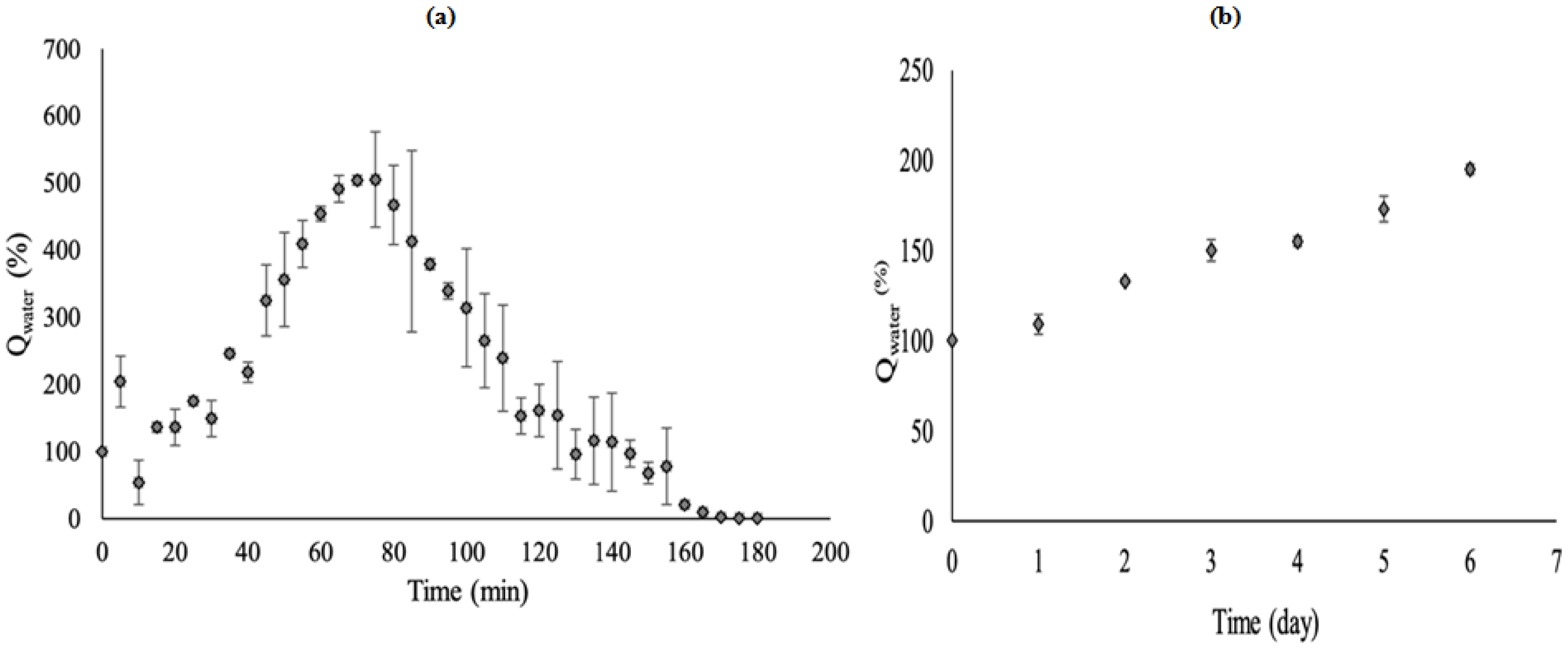
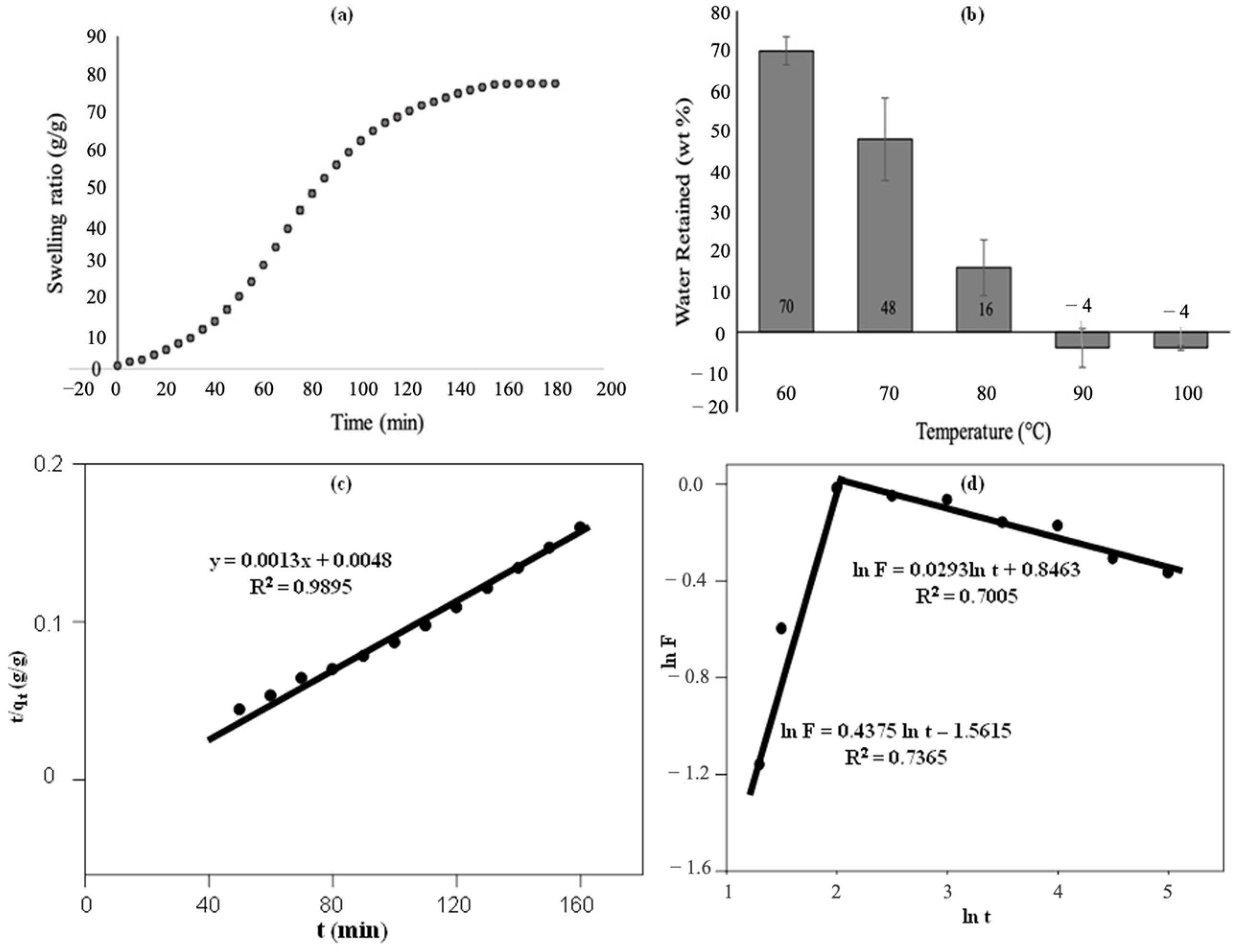
3.4. The Performance of S-SAP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Del Río-Gamero, B.; Perez-Baez, S.O.; Gómez-Gotor, A. Calculation of greenhouse gas emissions in Canary Islands wastewater treatment plants. Desalin. Water Treat 2020, 197, 101–111. [Google Scholar] [CrossRef]
- Farzaneh, G.; Khorasani, N.; Ghodousi, J.; Panahi, M. Assessment of surface and groundwater resources quality close to municipal solid waste landfill using multiple indicators and multivariate statistical methods. Int. J. Environ. Res. 2021, 15, 383–394. [Google Scholar] [CrossRef]
- Lin, Y.T.; Zhuang, G.L.; Wey, M.Y.; Tseng, H.H. The viable fabrication of gas separation membrane used by reclaimed rubber from waste tires. Polymers 2020, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef] [PubMed]
- Dogaris, I.; Ammar, E.; Philippidis, G.P. Prospects of integrating algae technologies into landfill leachate treatment. World J. Microbiol. Biotechnol. 2020, 36, 39. [Google Scholar] [CrossRef]
- Kajiwara, N.; Noma, Y.; Tamiya, M.; Teranishi, T.; Kato, Y.; Ito, Y.; Sakai, S. Destruction of decabromodiphenyl ether during incineration of plastic television housing waste at commercial-scale industrial waste incineration plants. J. Environ. Chem. Eng. 2021, 9, 105172. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, X. Detoxification, solidification and recycling of municipal solid waste incineration fly ash: A review. J. Chem. Eng. 2021, 420, 130349. [Google Scholar] [CrossRef]
- Liang, Y.; Donghai, X.; Peng, F.; Botian, H.; Yang, G.; Shuzhong, W. Municipal sewage sludge incineration and its air pollution control. J. Clean. Prod. 2021, 295, 126456. [Google Scholar] [CrossRef]
- Vattanapuripakorn, W.; Khannam, K.; Sonsupap, S.; Tongsantia, U.; Sarasamkan, J.; Bubphachot, B. Treatment of flue gas from an infectious waste incinerator using the ozone system. Environ. Nat. Resour. J. 2021, 19, 348–357. [Google Scholar] [CrossRef]
- Aldalbahi, A.; El-Naggar, M.; Khattab, T.; Abdelrahman, M.; Rahaman, M.; Alrehaili, A.; El-Newehy, M. Development of Green and Sustainable Cellulose Acetate/Graphene Oxide Nanocomposite Films as Efficient Adsorbents for Wastewater Treatment. Polymers 2020, 12, 2501. [Google Scholar] [CrossRef]
- Cai, Y.H.; Burkhardt, C.J.; Schäfer, A.I. Renewable energy powered membrane technology: Impact of osmotic backwash on organic fouling during solar irradiance fluctuation. J. Membr. Sci. 2022, 647, 120286. [Google Scholar] [CrossRef]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. J. Chem. Eng. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Khan, S.B.; Irfan, S.; Lam, S.S.; Sun, X.; Chen, S. 3D printed nanofiltration membrane technology for waste water distillation. J. Water Process. Eng. 2022, 49, 102958. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. J. Chem. Eng. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Elmi, A.; AlOlayan, M. Sewage sludge land application: Balancing act between agronomic benefits and environmental concerns. J. Clean. Prod. 2020, 250, 119512. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Haliru, B.S. Superabsorbent polymer hydrogels for sustainable agriculture: A review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P.A. Superabsorbent polymers in agriculture and other applications: A review. Polym. Plast. Technol. Eng. 2020, 59, 341–356. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Raffa, P.; Picchioni, F.; Koning, C.E. Superabsorbent polymers: From long-established, microplastics generating systems, to sustainable, biodegradable and future proof alternatives. Prog. Polym. Sci. 2022, 125, 101475. [Google Scholar] [CrossRef]
- El Idrissi, A.; El Gharrak, A.; Achagri, G.; Essamlali, Y.; Amadine, O.; Akil, A.; Zahouily, M. Synthesis of urea-containing sodium alginate-g-poly (acrylic acid-co-acrylamide) superabsorbent-fertilizer hydrogel reinforced with carboxylated cellulose nanocrystals for efficient water and nitrogen utilization. J. Environ. Chem. Eng. 2022, 10, 108282. [Google Scholar] [CrossRef]
- Kowalski, G.; Ptaszek, P.; Kuterasiński, L. Swelling of hydrogels based on carboxymethylated starch and poly(acrylic acid): Nonlinear rheological approach. Polymers 2020, 12, 2564. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef] [PubMed]
- Meshram, I.; Kanade, V.; Nandanwar, N.; Ingle, P. Super-absorbent polymer: A review on the characteristics and application. Int. J. Adv. Res. Chem. Sci. 2020, 7, 8–21. [Google Scholar]
- Wanyan, Q.; Qiu, Y.; Xie, W.; Wu, D. Tuning degradation and mechanical properties of Poly (l-lactic acid) with biomass-Derived Poly (l-malic acid). J. Polym. Environ. 2020, 28, 884–891. [Google Scholar] [CrossRef]
- Sand, A.; Vyas, A. Superabsorbent polymer based on guar gum-graft-acrylamide: Synthesis and characterization. J. Polym. Res. 2020, 27, 43. [Google Scholar] [CrossRef]
- Menceloğlu, Y.; Menceloğlu, Y.Z.; Seven, S.A. Triblock superabsorbent polymer nanocomposites with enhanced water retention capacities and rheological characteristics. ACS Omega 2022, 7, 20486–20494. [Google Scholar] [CrossRef]
- Metin, C.; Alparslan, Y.; Baygar, T.; Baygar, T. Physicochemical, microstructural and thermal characterization of chitosan from blue crab shell waste and its bioactivity characteristics. J. Polym. Environ. 2019, 27, 2552–2561. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Synthesis and applications of biopolymer composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef]
- Bachra, Y.; Grouli, A.; Damiri, F.; Talbi, M.; Berrada, M. A novel superabsorbent polymer from crosslinked carboxymethyl tragacanth gum with glutaraldehyde: Synthesis, characterization, and swelling properties. Int. J. Biomater. 2021, 2021, 5008833. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Sanders, J.M.; Misra, M.; Mustard, T.J.; Giesen, D.J.; Zhang, T.; Shelley, J.; Halls, M.D. Characterizing moisture uptake and plasticization effects of water on amorphous amylose starch models using molecular dynamics methods. Carbohydr. Polym. 2021, 15, 117161. [Google Scholar] [CrossRef]
- Zhiguang, C.; Junrong, H.; Huayin, P.; Keipper, W. The effects of temperature on starch molecular conformation and hydrogen bonding. Starch 2022, 74, 2100288. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Pieta, I.S.; Nowakowski, R.; Przekora, A. Effect of gelation temperature on the molecular structure and physicochemical properties of the curdlan matrix: Spectroscopic and microscopic analyses. Int. J. Mol. Sci. 2020, 21, 6154. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kong, L.; Du, B.; Xu, B. Morphological and physicochemical characterization of starches isolated from chestnuts cultivated in different regions of China. Int. J. Biol. Macromol. 2019, 130, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lin, X.; Yu, J. Preparation and characterization of oxidized starch-chitosan complexes for adsorption of procyanidins. LWT 2020, 117, 108610. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Zhang, J.; He, Z.; Wang, Y.; Wang, J.; Zhang, J. Hygroscopic hydrophobic coatings from cellulose: Manipulation of the aggregation morphology of water. J. Chem. Eng. 2022, 441, 136016. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Xu, H.; Liang, Y.; Zheng, B. Understanding the digestibility of rice starch-gallic acid complexes formed by high pressure homogenization. Int. J. Biol. Macromol. 2019, 134, 856–863. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, H.; Ai, L.; Xiong, W. Insight into protein-starch ratio on the gelatinization and retrogradation characteristics of reconstituted rice flour. Int. J. Biol. Macromol. 2020, 146, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, F.; Zi, H.; Zhou, Y.; Liu, H.; Xiao, J. Development and characterization of a hydroxypropyl starch/zein bilayer edible film. Int. J. Biol. Macromol. 2019, 141, 1175–1182. [Google Scholar] [CrossRef]
- Ding, N.; Zhao, B.; Han, X.; Li, C.; Gu, Z.; Li, Z. Starch-binding domain modulates the specificity of maltopentaose production at moderate temperatures. J. Agric. Food Chem. 2022, 70, 9057–9065. [Google Scholar] [CrossRef]
- Marvi, P.K.; Amjad-Iranagh, S.; Halladj, R. Molecular Dynamics Assessment of Doxorubicin Adsorption on Surface-Modified Boron Nitride Nanotubes (BNNTs). J. Phys. Chem. B 2021, 125, 13168–13180. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chao, C.; Yu, J.; Copeland, L.; Huang, Y.; Wang, S. Mechanisms underlying the formation of Amylose–Lauric Acid− β-Lactoglobulin complexes: Experimental and molecular dynamics studies. J. Agric. Food Chem. 2022, 70, 10635–10643. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bie, P.; Tong, X.; Zhang, B.; Fu, X.; Huang, Q. Complexation between high-amylose starch and binary aroma compounds of decanal and thymol: Cooperativity or competition? J. Agric. Food Chem. 2021, 69, 11665–11675. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Guo, D.; Lu, R.; Mao, Y.; Ou, H. Porous boronate imprinted microsphere prepared based on new RAFT functioned cellulose nanocrystalline with multiple H-bonding at the emulsion droplet interface for highly specific separation of Naringin. J. Chem. Eng. 2023, 452, 139294. [Google Scholar] [CrossRef]
- Siemers, M.; Lazaratos, M.; Karathanou, K.; Guerra, F.; Brown, L.S.; Bondar, A.N. Bridge: A graph-based algorithm to analyze dynamic H-bond networks in membrane proteins. J. Chem. Theory Comput. 2019, 15, 6781–6798. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Sekharan, S.; Manna, U.; Sahoo, L. Transformation of non-water sorbing fly ash to a water sorbing material for drought management. Sci. Rep. 2020, 10, 18664. [Google Scholar] [CrossRef] [PubMed]
- Todica, M.; Nagy, E.M.; Niculaescu, C.; Stan, O.; Cioica, N.; Pop, C.V. XRD investigation of some thermal degraded starch- based materials. J. Spectrosc. 2016, 2016, 9605312. [Google Scholar] [CrossRef]
- Gao, L.; Luo, H.; Wang, Q.; Hu, G.; Xiong, Y. Synergistic effect of hydrogen bonds and chemical bonds to construct a starch-based water-absorbing/retaining hydrogel composite reinforced with cellulose and poly (ethylene glycol). ACS Omega 2021, 6, 35039–35049. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Synthesis and characterization superabsorbent polymers made of starch, acrylic acid, acrylamide, poly (Vinyl alcohol), 2-hydroxyethyl methacrylate, 2-acrylamido-2-methylpropane sulfonic acid. Int. J. Mol. Sci. 2021, 22, 4325. [Google Scholar] [CrossRef]
- Kalkan, B.; Orakdogen, N. Anionically modified N-(alkyl) acrylamide-based semi-IPN hybrid gels reinforced with SiO2 for enhanced on–off switching and responsive properties. Soft Matter. 2022, 18, 4582–4603. [Google Scholar] [CrossRef]





| Parameters | Description |
|---|---|
| Code | SW204 |
| Origin | Pasir Gudang, Johor |
| Condition | Sludges containing one or several metals |
| Source | Polymer production plant |
| Crystallinity | Highly amorphous |
| Moisture contents | 60–70 wt.% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matmin, J.; Ibrahim, S.I.; Mohd Hatta, M.H.; Ricky Marzuki, R.; Jumbri, K.; Nik Malek, N.A.N. Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water–Polymer Interaction. Polymers 2023, 15, 1471. https://doi.org/10.3390/polym15061471
Matmin J, Ibrahim SI, Mohd Hatta MH, Ricky Marzuki R, Jumbri K, Nik Malek NAN. Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water–Polymer Interaction. Polymers. 2023; 15(6):1471. https://doi.org/10.3390/polym15061471
Chicago/Turabian StyleMatmin, Juan, Salizatul Ilyana Ibrahim, Mohd Hayrie Mohd Hatta, Raidah Ricky Marzuki, Khairulazhar Jumbri, and Nik Ahmad Nizam Nik Malek. 2023. "Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water–Polymer Interaction" Polymers 15, no. 6: 1471. https://doi.org/10.3390/polym15061471
APA StyleMatmin, J., Ibrahim, S. I., Mohd Hatta, M. H., Ricky Marzuki, R., Jumbri, K., & Nik Malek, N. A. N. (2023). Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water–Polymer Interaction. Polymers, 15(6), 1471. https://doi.org/10.3390/polym15061471