Prussian Blue Analogue-Derived Fe-Doped CoS2 Nanoparticles Confined in Bayberry-like N-Doped Carbon Spheres as Anodes for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe-CoS2/NC Spheres
2.2. Material Characterization
2.3. Electrochemical Measurements
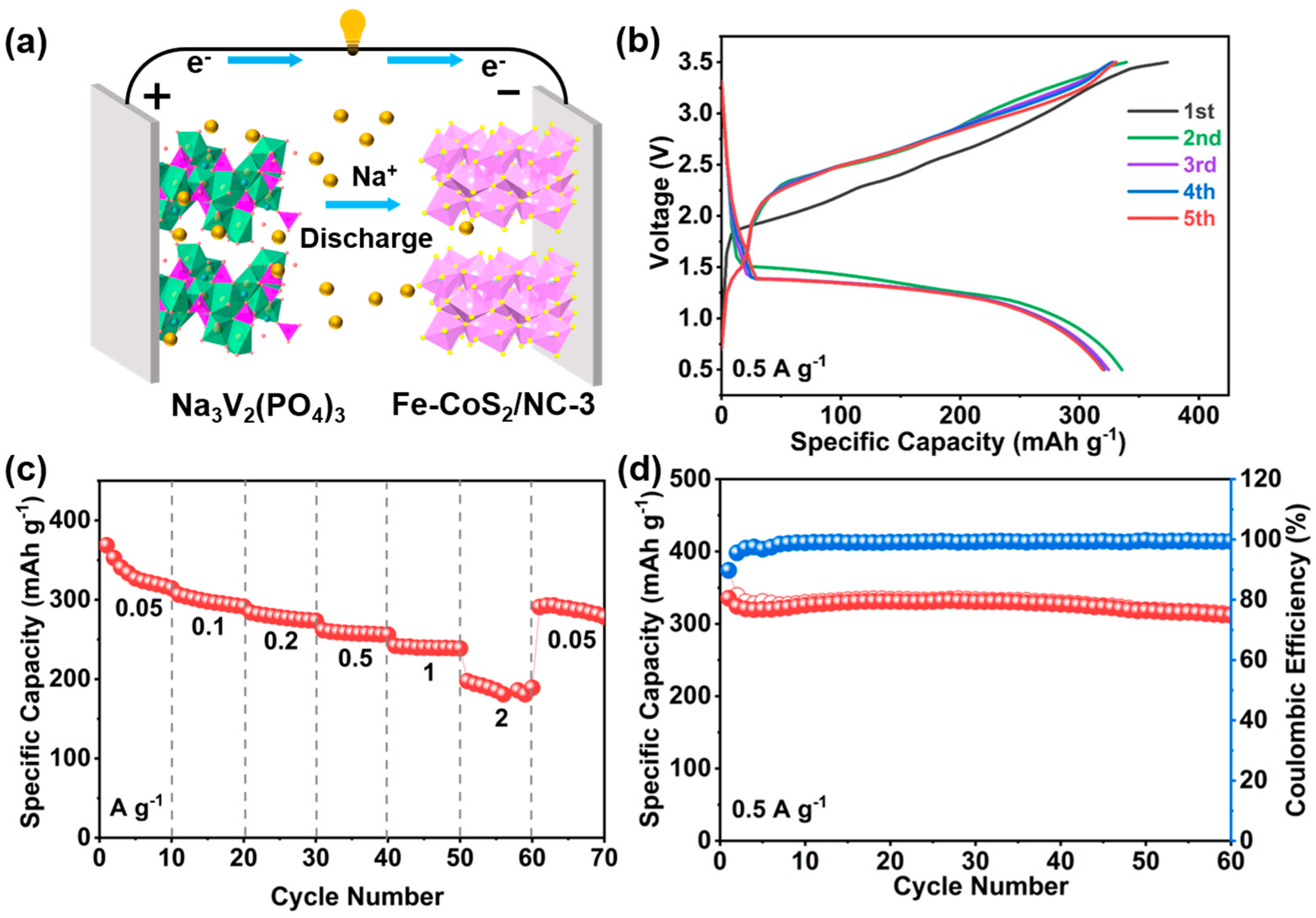
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, H.; Zheng, M.; Hu, Q.; Chi, Y.; Xu, B.; Zhang, S.; Xue, H.; Pang, H. Derivatives of coordination compounds for rechargeable batteries. J. Mater. Chem. A 2018, 6, 13999–14024. [Google Scholar] [CrossRef]
- Khan, T.; Garg, A.K.; Gupta, A.; Madan, A.K.; Jain, P.K. Comprehensive review on latest advances on rechargeable batteries. J. Energy Storage 2023, 57, 106204. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, H.S.; Li, Y.; Tan, D.H.S.; Zhang, M.; Zhao, E.; Meng, Y.S. Sodium-ion batteries paving the way for grid energy storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- Goikolea, E.; Palomares, V.; Wang, S.; Larramendi, I.R.; Guo, X.; Wang, G.; Rojo, T. Na-ion batteries-approaching old and new challenges. Adv. Energy Mater. 2020, 10, 2002055. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. EnergyChem 2019, 1, 100012. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Zhao, D.; Yang, H.; Ai, X.; Cao, S.; Chen, Z.; Cao, Y. Recent progress in rechargeable sodium-ion batteries: Toward high-power applications. Small 2019, 15, e1805427. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, F.; Bai, Y.; Li, Y.; Gong, Y.; Feng, X.; Li, Y.; Wang, X.; Wu, C. Advances in carbon materials for sodium and potassium storage. Adv. Funct. Mater. 2022, 32, 2203117. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, R.; Mu, D.; Tan, G.; Gao, H.; Li, N.; Chen, R.; Wu, F. Progress in electrolyte and interface of hard carbon and graphite anode for sodium-ion battery. Carbon Energy 2022, 4, 458–479. [Google Scholar] [CrossRef]
- Song, K.; Liu, C.; Mi, L.; Chou, S.; Chen, W.; Shen, C. Recent progress on the alloy-based anode for sodium-ion batteries and potassium-ion batteries. Small 2021, 17, e1903194. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Cheng, Y.J.; Zhu, J.; Xia, Y.; Müller-Buschbaum, P. A Chronicle Review of nonsilicon (Sn, Sb, Ge)-based lithium/sodium-ion battery alloying anodes. Small Methods 2020, 4, 2000218. [Google Scholar] [CrossRef]
- Fang, Y.; Luan, D.; Lou, X.W.D. Recent advances on mixed metal sulfides for advanced sodium-ion batteries. Adv. Mater. 2020, 32, e2002976. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, B.; Guo, X.; Li, W.; Ma, W.; Wu, X.; Pang, H. MOF-derived metal sulfides for electrochemical energy applications. Energy Storage Mater. 2022, 51, 840–872. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Li, X.; Gu, F.; Zhang, L.; Liu, H.; Xia, Q.; Li, Q.; Ye, W.; Ge, C.; et al. Reacquainting the electrochemical conversion mechanism of FeS2 sodium-ion batteries by operando magnetometry. J. Am. Chem. Soc. 2021, 143, 12800–12808. [Google Scholar] [CrossRef]
- Sadan, M.K.; Kim, H.; Kim, C.; Cho, G.B.; Cho, K.K.; Ahn, J.H.; Ahn, H.J. Ultrahigh-rate nickel monosulfide anodes for sodium/potassium-ion storage. Nanoscale 2021, 13, 10447–10454. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Shi, X.; Yang, Z.; Li, L.; Chou, S.L. Developing high-performance metal selenides for sodium-ion batteries. Adv. Funct. Mater. 2022, 32, 2208093. [Google Scholar] [CrossRef]
- Luo, M.; Yu, H.; Hu, F.; Liu, T.; Cheng, X.; Zheng, R.; Bai, Y.; Shui, M.; Shu, J. Metal selenides for high performance sodium ion batteries. Chem. Eng. J. 2020, 380, 122557. [Google Scholar] [CrossRef]
- Qi, S.; Wu, D.; Dong, Y.; Liao, J.; Foster, C.W.; O’Dwyer, C.; Feng, Y.; Liu, C.; Ma, J. Cobalt-based electrode materials for sodium-ion batteries. Chem. Eng. J. 2019, 370, 185–207. [Google Scholar] [CrossRef]
- Fang, Y.; Guan, B.Y.; Luan, D.; Lou, X.W.D. Synthesis of CuS@CoS2 double-shelled nanoboxes with enhanced sodium storage properties. Angew. Chem. Int. Ed. 2019, 58, 7739–7743. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Ma, H.; Tan, Z.; Wang, Y.; Liu, Q.; Li, M.; Chen, Y.; Wang, D. One pot synthesis and capacitive sodium storage properties of rGO confined CoS2 anode materials. J. Alloys Compd. 2020, 813, 151598. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Ciucci, F.; Huang, B.; Kim, J.K. Rationally designed nanostructured metal chalcogenides for advanced sodium-ion batteries. Energy Storage Mater. 2021, 34, 582–628. [Google Scholar] [CrossRef]
- Anh Tran, V.; Huu Do, H.; Duy Cam Ha, T.; Hyun Ahn, S.; Kim, M.G.; Young Kim, S.; Lee, S.W. Metal-organic framework for lithium and sodium-ion batteries: Progress and perspectivez. Fuel 2022, 319, 123856. [Google Scholar] [CrossRef]
- Xiao, L.; Ji, F.; Zhang, J.; Chen, X.; Fang, Y. Doping regulation in Polyanionic compounds for advanced sodium-ion batteries. Small 2023, 19, e2205732. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Kim, D.H.; Nam, K.H.; Park, C.-M. Robust nanocube framework CoS2-based composites as high-performance anodes for Li- and Na-ion batteries. Compos. Part B Eng. 2022, 231, 109592. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, Z.; Li, D.; Ullah, S.; Hai, Y.; Xin, H.; Liao, W.; Yang, B.; Fan, H.; Xu, J.; et al. NiS2@CoS2 nanocrystals encapsulated in N-doped carbon nanocubes for high performance lithium/sodium ion batteries. Energy Storage Mater. 2018, 11, 67–74. [Google Scholar] [CrossRef]
- Lian, Y.; Xin, W.; Zhang, M.; Li, Y.; Yang, L.; Guo, Y.; Xu, S. Low-content Ni-doped CoS2 embedded within N, P-codoped biomass-derived carbon spheres for enhanced lithium/sodium storage. J. Mater. Sci. 2019, 54, 8504–8514. [Google Scholar] [CrossRef]
- Wei, R.; Dong, Y.; Zhang, Y.; Kang, X.; Sheng, X.; Zhang, J. Hollow cubic MnS-CoS2-NC@NC designed by two kinds of nitrogen-doped carbon strategy for sodium ion batteries with ultraordinary rate and cycling performance. Nano Res. 2021, 15, 3273–3282. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.; Yan, Y.; Li, H.; Zhou, W.; Gu, T.; Shen, X.; Lu, C.; Zhao, Y.; Zhang, Y.; et al. Optimized Co-S bonds energy and confinement effect of hollow MXene@CoS2/NC for enhanced sodium storage kinetics and stability. Chem. Eng. J. 2022, 450, 137922. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Kong, X.; Song, Y.; Zhao, Y. Three-dimensional porous N-doped graphite carbon with embedded CoS2 nanoparticles as advanced anode for sodium-ion batteries. Appl. Surf. Sci. 2022, 603, 154481. [Google Scholar] [CrossRef]
- Liu, J.; Xie, J.; Dong, H.; Wei, H.; Sun, C.; Yang, J.; Geng, H. Iron doping of NiSe2 nanosheets to accelerate reaction kinetics in sodium-ion half/full batteries. Sci. China Mater. 2022, 66, 69–78. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, F.; Long, G.; Fan, S.; Zheng, Y.; Ye, W.; Li, Q.; Wang, X.; Li, H.; et al. Fe, N co-doped amorphous carbon as efficient electrode materials for fast and stable Na/K-storage. Electrochim. Acta 2021, 396, 139265. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Zhang, Q.; Fu, F.; Tang, Y.; Guo, J.; Shao, M.; Wang, H. Iron-doped cauliflower-like rutile TiO2 with superior sodium storage properties. ACS Appl. Mater. Inter. 2017, 9, 6093–6103. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, X.; Wang, J.; Yu, Q.; Qian, X.; Chen, L.; Dan, Y. Fe-doped CoP flower-like microstructure on carbon membrane as integrated electrode with enhanced sodium ion storage. Chem. Eur. J. 2020, 26, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Indra, A. Prussian blue- and prussian blue analogue-derived materials: Progress and prospects for electrochemical energy conversion. Mater. Today Energy 2020, 16, 100404. [Google Scholar] [CrossRef]
- Li, W.; Han, C.; Wang, W.; Xia, Q.; Chou, S.; Gu, Q.; Johannessen, B.; Liu, H.; Dou, S. Stress distortion restraint to boost the sodium ion storage performance of a novel binary hexacyanoferrate. Adv. Energy Mater. 2019, 10, 1903006. [Google Scholar] [CrossRef]
- Du, G.; Pang, H. Recent advancements in Prussian blue analogues: Preparation and application in batteries. Energy Storage Mater. 2021, 36, 387–408. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, X.; Jin, L.; Chen, P.; Tao, J.; Zhou, J.; Yao, Z. Regulating pyrolysis strategy to construct CNTs-linked porous cubic prussian blue analogue derivatives for lightweight and broadband microwave absorption. Chem. Eng. J. 2022, 430, 132879. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Z.; Yan, X.; Xu, M.; Chen, Y.; Li, J.; Li, J.; Zhang, K.; Lai, Y. Pomegranate-like microclusters organized by ultrafine Co nanoparticles@nitrogen-doped carbon subunits as sulfur hosts for long-life lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 14178–14187. [Google Scholar] [CrossRef]
- Wang, J.-G.; Liu, H.; Sun, H.; Hua, W.; Wang, H.; Liu, X.; Wei, B. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Dassanayake, A.C.; Wickramaratne, N.P.; Hossain, M.A.; Perera, V.S.; Jeskey, J.; Huang, S.D.; Shen, H.; Jaroniec, M. Prussian blue-assisted one-pot synthesis of nitrogen-doped mesoporous graphitic carbon spheres for supercapacitors. J. Mater. Chem. A 2019, 7, 22092–22102. [Google Scholar] [CrossRef]
- Chou, H.Y.; Weng, C.C.; Lai, J.Y.; Lin, S.Y.; Tsai, H.C. Design of an Interpenetrating Polymeric Network Hydrogel made of calcium-alginate from a thermos-sensitive pluronic template as a thermal-ionic reversible wound dressing. Polymers 2020, 12, 2138. [Google Scholar] [CrossRef]
- Song, L.; Wang, T.; Li, L.; Wu, C.; He, J. Zn3[Fe(CN)6]2 derived Fe/Fe5C2@N-doped carbon as a highly effective oxygen reduction reaction catalyst for zinc-air battery. Appl. Catal. B Environ. 2019, 244, 197–205. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Bresser, D.; Ji, Y.; Geiger, D.; Kaiser, U.; Streb, C.; Varzi, A.; Passerini, S. Cobalt disulfide nanoparticles embedded in porous carbonaceous micro-polyhedrons interlinked by carbon nanotubes for superior lithium and sodium storage. ACS Nano 2018, 12, 7220–7231. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Chen, J.; Lu, M.; Liu, J.; Zhang, X.; Lin, X.; Wu, Y.; Zeb, A.; Reddy, R.C.K.; Xu, Z. A metal-organic framework approach to engineer ZnO/Co3ZnC/N-doped carbon composite as anode material for boosting lithium storage. J. Alloys Compd. 2022, 923, 166436. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Ge, X.; Deng, Y.P.; Jiang, G.; Li, H.; Sun, G.; Liu, W.; Zheng, Y.; Dou, H.; et al. Self-assembly of colloidal MOFs derived yolk-shelled microcages as flexible air cathode for rechargeable Zn-air batteries. Nano Energy 2021, 89, 106314. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Zheng, Y.; Zhang, L.; Jiao, T.; Peng, Q.; Liu, Z. Facile preparation of self-assembled Ni/Co phosphates composite spheres with highly efficient HER electrocatalytic performances. Appl. Surf. Sci. 2020, 509, 145383. [Google Scholar] [CrossRef]
- Yang, P.; Li, E.; Xiao, F.; Zhou, P.; Wang, Y.; Tang, W.; He, P.; Jia, B. Nanostructure Fe-Co-B/bacterial cellulose based carbon nanofibers: An extremely efficient electrocatalyst toward oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 12953–12963. [Google Scholar] [CrossRef]
- Xu, S.; Qi, Y.; Lu, Y.; Sun, S.; Liu, Y.; Jiang, D. Fe-doped CoP holey nanosheets as bifunctional electrocatalysts for efficient hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2021, 46, 26391–26401. [Google Scholar] [CrossRef]
- Xiang, J.; Guo, Z.; Yi, Z.; Zhang, Y.; Yuan, L.; Cheng, Z.; Shen, Y.; Huang, Y. Facile synthesis of sulfurized polyacrylonitrile composite as cathode for high-rate lithium-sulfur batteries. J. Energy Storage 2020, 49, 161–165. [Google Scholar] [CrossRef]
- Fan, M.P.; Chen, Y.C.; Chen, Y.M.; Huang, Z.X.; Wu, W.L.; Wang, P.; Ke, X.; Sun, S.H.; Shi, Z.C. NiS2 nanosheet arrays on stainless steel foil as binder-free anode for high-power sodium-ion batteries. Rare Metals 2022, 41, 1294–1303. [Google Scholar] [CrossRef]
- Hao, Z.; Dimov, N.; Chang, J.K.; Okada, S. Synthesis of bimetallic sulfide FeCoS4@carbon nanotube graphene hybrid as a high-performance anode material for sodium-ion batteries. Chem. Eng. J. 2021, 423, 130070. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Fan, X.; Shan, B.; Meyer, T.J. Promoting electrochemical reduction of CO2 to ethanol by B/N-doped sp3/sp2 nanocarbon electrode. Chin. Chem. Lett. 2022, 33, 4691–4694. [Google Scholar] [CrossRef]
- Lu, Z.; Zhai, Y.; Wang, N.; Zhang, Y.; Xue, P.; Guo, M.; Tang, B.; Huang, D.; Wang, W.; Bai, Z.; et al. FeS2 nanoparticles embedded in N/S co-doped porous carbon fibers as anode for sodium-ion batteries. Chem. Eng. J. 2020, 380, 122455. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Y.; Oh, J.A.S.; Gu, Q.; Zheng, W.; Goh, M.; Zeng, K.; Cheng, Y.; Lu, L. Insight into the structure-capacity relationship in biomass derived carbon for high-performance sodium-ion batteries. J. Energy Chem. 2021, 62, 497–504. [Google Scholar] [CrossRef]
- Lu, J.; Cai, L.; Zhang, N.; Qiu, B.; Chai, Y. Robust photoelectrochemical oxygen evolution with N, Fe-CoS2 nanorod arrays. ACS Appl. Mater. Interfaces 2019, 11, 44214–44222. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, D.; Wang, F.; Zhang, G.; Duan, J.; Han, F.; Duan, H.; Liu, J. Highly active Fe7S8 encapsulated in N-doped hollow carbon nanofibers for high-rate sodium-ion batteries. J. Energy Chem. 2021, 53, 26–35. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, C.; Li, C.M. Lychee-like FeS2@FeSe2 core–shell microspheres anode in sodium ion batteries for large capacity and ultralong cycle life. J. Mater. Chem. A 2017, 5, 19195–19202. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, X.; Wang, D.; Wang, H.; Yu, D.Y.W.; Rogach, A.L. Metal-organic framework derived CoS2 wrapped with nitrogen-doped carbon for enhanced lithium/sodium storage performance. ACS Appl. Mater. Interfaces 2020, 12, 12809–12820. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Han, L.; Liao, C.; Cai, W.; Kan, Y.; Hu, Y. Fabrication of an anode composed of a N, S co-doped carbon nanotube hollow architecture with CoS2 confined within: Toward Li and Na storage. Nanoscale 2019, 11, 20996–21007. [Google Scholar] [CrossRef]
- Chen, L.; Luo, N.; Huang, S.; Li, Y.; Wei, M. Metal-organic framework-derived hollow structure CoS2/nitrogen-doped carbon spheres for high-performance lithium/sodium ion batteries. Chem. Commun. 2020, 56, 3951–3954. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, S.; Li, C.; Liu, Z.; Li, D. Hollow CoS2@C nanocubes for high-performance sodium storage. Appl. Surf. Sci. 2020, 519, 146268. [Google Scholar] [CrossRef]
- Pinilla, S.; Park, S.H.; Fontanez, K.; Marquez, F.; Nicolosi, V.; Morant, C. 0D-1D hybrid silicon nanocomposite as lithium-ion batteries anodes. Nanomaterials 2020, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wu, J.; Fan, S.; Li, G. CoS2 /N-doped hollow spheres as an anode material for high-performance sodium-ion batteries. Chem. Eur. J. 2021, 27, 9820–9829. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, K.; Guo, Y.; Guo, J.; Xu, Q.; Zhang, J. CoS2 nanodots trapped within graphitic structured N-doped carbon spheres with efficient performances for lithium storage. J. Mater. Chem. A 2018, 6, 7148–7154. [Google Scholar] [CrossRef]
- Ghosh, S.; Qi, Z.; Wang, H.; Martha, S.K.; Pol, V.G. WS2 anode in Na and K-ion battery: Effect of upper cut-off potential on electrochemical performance. Electrochim. Acta 2021, 383, 138339. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, P.; Cheng, Z.; Song, J.; Zhao, Y.; Chen, L.; Cai, X.; Zhang, J.; Yuan, A. CoS2 nanoparticles embedded in two-dimensional sheet-shaped N-doped carbon for sodium storage. J. Energy Chem. 2021, 2021, 1536–1541. [Google Scholar] [CrossRef]
- Huang, P.; Ying, H.; Zhang, S.; Zhang, Z.; Han, W.Q. Multidimensional synergistic architecture of Ti3C2 MXene/CoS2@N-doped carbon for sodium-ion batteries with ultralong cycle lifespan. Chem. Eng. J. 2022, 429, 132396. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, Y.; Li, Q.; Zheng, Q.; Jiang, N.; Huo, Y.; Lin, D. SnS2-CoS2@C nanocubes as high initial coulombic efficiency and long-life anodes for sodium-ion batteries. Electrochim. Acta 2021, 387, 138525. [Google Scholar] [CrossRef]
- Cao, D.; Kang, W.; Wang, S.; Wang, Y.; Sun, K.; Yang, L.; Zhou, X.; Sun, D.; Cao, Y. In situ N-doped carbon modified (Co0.5Ni0.5)9S8 solid-solution hollow spheres as high-capacity anodes for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 8268–8276. [Google Scholar] [CrossRef]
- He, Y.; Dong, C.; He, S.; Li, H.; Sun, X.; Cheng, Y.; Zhou, G.; Xu, L. Bimetallic nickel cobalt sulfides with hierarchical coralliform architecture for ultrafast and stable Na-ion storage. Nano Res. 2021, 14, 4014–4024. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, Z.; Li, C.; Zhang, J.; Shen, S.; Yao, Z.; Liu, T.; Li, W.; Xia, X.; Yang, Y. Spatially confinedsynthesis of SnSe spheres encapsulated in N, Se dual-doped carbon networks toward fast and durable sodium storage. ACS Appl. Mater. Interfaces 2022, 14, 4230–4241. [Google Scholar] [CrossRef]
- Li, C.; Hou, J.; Zhang, J.; Li, X.; Jiang, S.; Zhang, G.; Yao, Z.; Liu, T.; Shen, S.; Liu, Z.; et al. Heterostructured NiS2@SnS2 hollow spheres as superior high-rate and durable anodes for sodium-ion batteries. Sci. China Chem. 2022, 65, 1420–1432. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Fan, H.; Hou, B.H.; Rui, X.H.; Ning, Q.L.; Cui, Z.; Guo, J.Z.; Yang, Y.; Wu, X.L. Ni1.5CoSe5 nanocubes embedded in 3D dual N-doped carbon network as advanced anode material in sodium-ion full cells with superior low-temperature and high-power properties. J. Mater. Chem. A 2018, 6, 22966–22975. [Google Scholar] [CrossRef]
- Cheng, D.; Ye, L.; Wei, A.; Xu, G.; Cao, Z.; Zhu, P.; Chen, Y. Constructing SnS/Fe2O3 heterostructure anchored on few-layered graphene as an ion-adsorption/diffusion enhancer for ultrafast and cycle-stable sodium storage. Chem. Eng. J. 2023, 457, 141243. [Google Scholar] [CrossRef]
- Yang, D.; Yadav, D.; Jeon, I.; Seo, J.; Jeong, S.Y.; Cho, C.R. Enhanced high-rate capability and long cycle stability of FeS@NCG nanofibers for sodium-ion battery anodes. ACS Appl. Mater. Inter. 2022, 14, 44303–44316. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Tang, X.; Qiu, R.; Wang, S.; Cao, G.; Zhang, M. Graphene-encapsulated FeS2 in carbon fibers as high reversible anodes for Na+/K+ batteries in a wide temperature range. Small 2019, 15, 1804740. [Google Scholar] [CrossRef]
- Yao, G.; Niu, P.; Li, Z.; Xu, Y.; Wei, L.; Niu, H.; Yang, Y.; Zheng, F.; Chen, Q. Construction of flexible V3S4@CNF films as long-term stable anodes for sodium-ion batteries. Chem. Eng. J. 2021, 423, 130229. [Google Scholar] [CrossRef]
- Huang, P.; Ying, H.; Zhang, S.; Zhang, Z.; Han, W.Q. Molten salts etching route driven universal construction of MXene/transition metal sulfides heterostructures with interfacial electronic coupling for superior sodium storage. Adv. Energy Mater. 2022, 12, 2202052. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Hou, J.; Zhang, J.; Wang, L.; Wang, P.; Yao, Z.; Yang, Y. Bimetallic copper tin sulfide nanosheet arrays encapsulated in nitrogen-doped carbon shells for boosted sodium storage performance. ACS Appl. Energ. Mater. 2021, 4, 8572–8582. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Liu, C.; Cai, C.; Sun, Q.; Lu, M.; Yao, Z.; Yang, Y. Prussian Blue Analogue-Derived Fe-Doped CoS2 Nanoparticles Confined in Bayberry-like N-Doped Carbon Spheres as Anodes for Sodium-Ion Batteries. Polymers 2023, 15, 1496. https://doi.org/10.3390/polym15061496
Hu J, Liu C, Cai C, Sun Q, Lu M, Yao Z, Yang Y. Prussian Blue Analogue-Derived Fe-Doped CoS2 Nanoparticles Confined in Bayberry-like N-Doped Carbon Spheres as Anodes for Sodium-Ion Batteries. Polymers. 2023; 15(6):1496. https://doi.org/10.3390/polym15061496
Chicago/Turabian StyleHu, Jiajia, Cheng Liu, Chen Cai, Qianqian Sun, Mixue Lu, Zhujun Yao, and Yefeng Yang. 2023. "Prussian Blue Analogue-Derived Fe-Doped CoS2 Nanoparticles Confined in Bayberry-like N-Doped Carbon Spheres as Anodes for Sodium-Ion Batteries" Polymers 15, no. 6: 1496. https://doi.org/10.3390/polym15061496




