Influence of Sulfododecenylsuccinylation on the Adhesion to Fibers and Film Properties of Corn Starch for Warp Sizing
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sulfododecenylsuccinylation of Starch
2.3. Fourier Transform Infrared Analysis
2.4. Determination of DS and Sulfonation Efficiency
2.5. Measurement of Surface Tension
2.6. Preparation and Measurement of Starch Films
2.7. Measurement of Bonding Force
2.8. Statistical Analysis
3. Results and Discussion
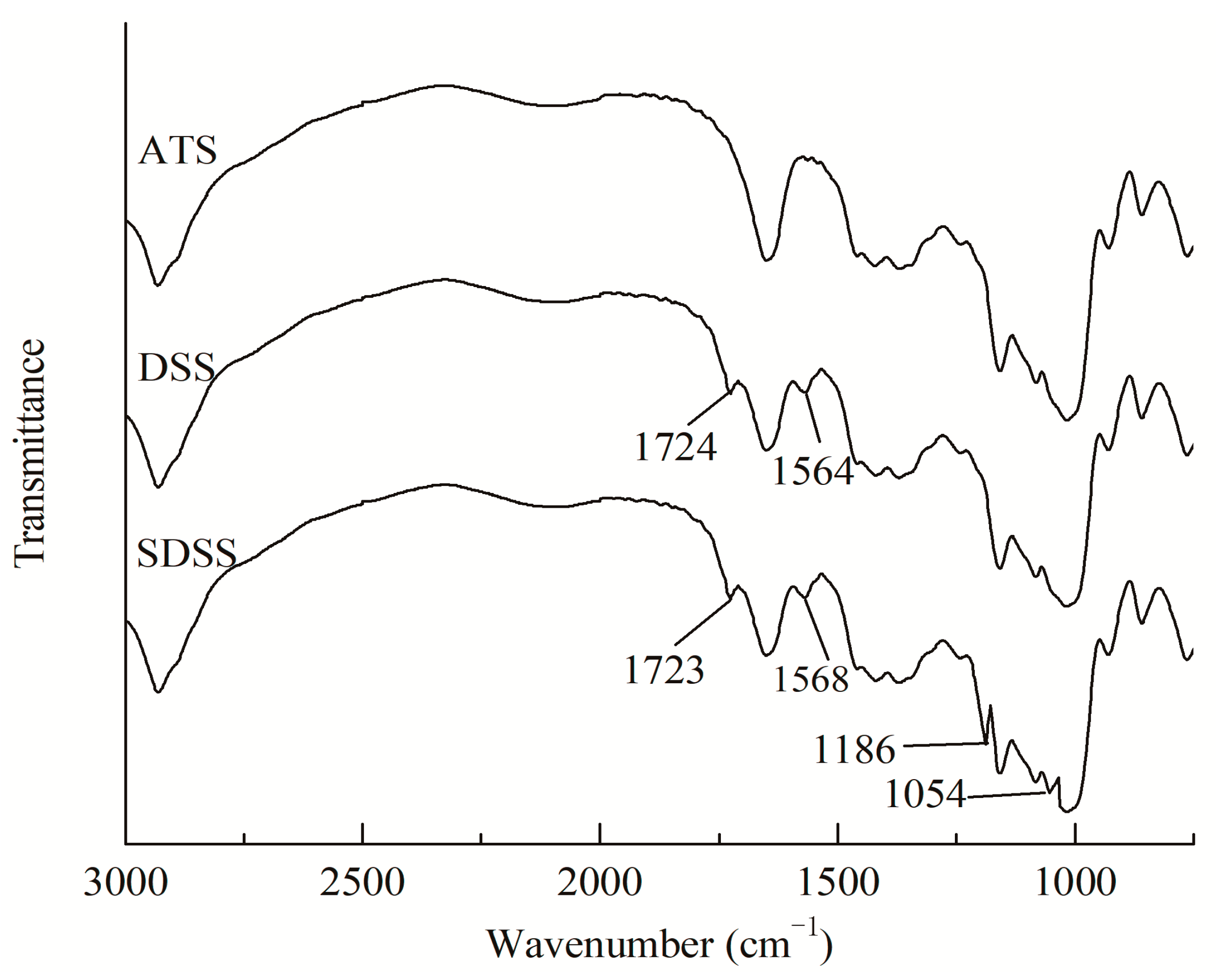
3.1. Characterization Analysis
3.2. Influence of Starch Sulfododecenylsuccination
3.2.1. Influence on Film Properties
3.2.2. Influence on Adhesion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Dodecenylsuccinated Starch | DSS |
| Sulfododecenylsuccinated Starch | SDSS |
| Degree of Substitution | DS |
| Acid-Thinned Starch | ATS |
| Dodecenylsuccinic Anhydride | DDSA |
| Fourier Transform Infrared | FTIR |
| X-ray Diffraction | XRD |
| Relative Humidity | RH |
References
- Zhu, Z.F.; Zhu, Z.Q.; Zhang, L.Y. Grafting poly(2-acryloyloxyethyl trimethyl ammonium chloride) branches onto the backbones of corn starch for toughening starch film. J. Polym. Eng. 2015, 35, 879–888. [Google Scholar] [CrossRef]
- Nurmi, L.; Holappa, S.; Mikkonen, H.; Seppälä, J. Controlled grafting of acetylated starch by atom transfer radical polymerization of MMA. Eur. Polym. J. 2007, 43, 1372–1382. [Google Scholar] [CrossRef]
- Abotbina, W.; Sapuan, S.M.; Sultan, M.T.H.; Alkbir, M.F.M.; Ilyas, R.A. Development and characterization of cornstarch-vased vioplastics packaging film using a combination of different plasticizers. Polymers 2021, 13, 3487. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.Q.; Zhu, Z.F.; Liu, F.D. Introduction of poly [(2-acryloyloxyethyl trimethyl ammonium chloride)-co-(acrylic acid)] branches onto starch for cotton warp sizing. Carbohydr. Polym. 2016, 138, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.F.; Cheng, Z.Q. Effect of inorganic phosphates on the adhesion of mono-phosphorylated cornstarch to fibers. Starch-Stärke 2008, 60, 315–320. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Cao, S.J. Modifications to improve the adhesion of crosslinked starch sizes to fiber substrates. Text. Res. J. 2004, 74, 253–258. [Google Scholar] [CrossRef]
- Behera, B.K.; Gupta, R.; Mishra, R. Comparative analysis of mechanical properties of size film. I. Performance of individual size materials. Fibers Polym. 2008, 9, 481–488. [Google Scholar] [CrossRef]
- Punia, B.S.; Nehra, M.; Siroha, A.K.; Petrů, M.; Ilyas, R.A.; Devi, U.; Devi, P. Development and characterization of physical modified pearl millet starch-based films. Foods 2021, 10, 1609. [Google Scholar] [CrossRef]
- Li, C.L.; Bao, L.; Zhu, Z.F. Effect of starch dodecenylsuccinylation on the adhesion and film properties of dodecenylsuccinylated starch for polyester warp sizing. J. Donghua Univ. (Eng. Ed.) 2014, 31, 747–752. [Google Scholar]
- Vries, H.J.D.; Semeijn, C.; Buwalda, P.L. Emulsifter Prepared Using a Glycosyl Transferase. U.S. Patent 817832382, 15 May 2012. [Google Scholar]
- Li, W.; Xu, Z.Z.; Wang, Z.Q.; Liu, X.H.; Li, C.L.; Ruan, F.T. Double etherification of corn starch to improve its adhesion to cotton and polyester fibers. Int. J. Adhes. Adhes. 2018, 84, 101–107. [Google Scholar] [CrossRef]
- Jansson, A.; Thuvander, F. Influence of thickness on the mechanical properties for starch films. Carbohydr. Polym. 2004, 56, 499–503. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Chen, P.H. Carbamoyl ethylation of starch for enhancing the adhesion capacity to fibers. J. Appl. Polym. Sci. 2007, 106, 2763–2768. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Zhang, L.Q.; Li, M.L.; Zhou, Y.S. Effects of starch alkenylsuccinylation on the grafting efficiency, paste viscosity, and film properties of alkenylsuccinylated starch-g-poly(acrylic acid). Starch-Stärke 2012, 64, 704–712. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Zhang, Z.Q.; Wu, L.J.; Lu, Y.H. Investigation on the synthesis process of bromoisobutyryl esterified starch and its sizing properties: Viscosity stability, adhesion and film properties. Polymers 2019, 11, 1936. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Xu, W.Z.; Wei, A.F.; Xu, Z.Z.; Zhang, C.H. Quaternization/maleation of cornstarch to improve its adhesion and film properties for warp sizing. Fibers Polym. 2016, 17, 1589–1597. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; Lashin, I.; Hassan, S.A.M. In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, L.L.; Tong, J.; Xie, L.; Liu, Z.Q. Surface esterification of corn starch films: Reaction with dodecenyl succinic anhydride. Carbohydr. Polym. 2009, 78, 888–893. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, R.G.; Zhang, L.; Liu, M.H. Growth of CaCO3 in the templated langmuir-blodgett film of a bolaamphiphilic diacid. New J. Chem. 2004, 28, 614–617. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.Z.; Wang, Z.Q.; Xing, J. One-step quaternization/hydroxypropylsulfonation to improve paste stability, adhesion, and film properties of oxidized starch. Polymers 2018, 10, 1110. [Google Scholar] [CrossRef] [Green Version]
- Thiré, R.M.S.; Simão, M.R.A.; Andrade, C.T. High resolution imaging of the microstructure of maize starch films. Carbohydr. Polym. 2003, 54, 149–158. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.Z.; Wang, Z.Q.; Li, C.L.; Feng, Q.; Zhu, Y.N. Tertiary amination/hydroxypropylsulfonation of cornstarch to improve the adhesion-to-fibers and film properties for warp sizing. Fibers Polym. 2018, 19, 1386–1394. [Google Scholar] [CrossRef]
- Hu, G.F.; Chen, J.Y.; Gao, J.P. Preparation and characteristics of oxidized potato starch films. Carbohydr. Polym. 2009, 76, 291–298. [Google Scholar] [CrossRef]
- Li, W.; Wu, L.J.; Zhang, Z.Q.; Ke, H.Z.; Zhu, Z.F.; Xu, Z.Z.; Wei, A.F.; Cheng, X.D. Introduction of poly(2-acrylamide-2-methylpro panesulfonic acid) branches into starch molecules for improving its paste stability, adhesion and desizability. Int. J. Adhes. Adhes. 2021, 110, 102939. [Google Scholar] [CrossRef]
- Hasanin, M.S. Simple, economic, ecofriendly method to extract starch nanoparticles from potato peel waste for biological applications. Starch–Stärke 2021, 73, 2100055. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abboud, M.; Alawlaqi, M.M.; Abdelghany, T.M.; Hasanin, M. Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity. Starch–Stärke 2022, 74, 2100165. [Google Scholar] [CrossRef]
- Li, W.; Wu, L.J.; Zhu, Z.F.; Zhang, Z.Q.; Liu, Q.; Lu, Y.H.; Ke, H.Z. Incorporation of poly(sodium allyl sulfonate) branches on corn starch chains for enhancing its sizing properties: Viscosity stability, adhesion, film properties and desizability. Int. J. Biol. Macromol. 2021, 166, 1460–1470. [Google Scholar] [CrossRef]
- Li, W.; Cheng, X.D.; Wang, Y.F.; Xu, Z.Z.; Ke, H.Z. Quaternization-butyrylation to improve the viscosity stability, adhesion to fibers, film properties and desizability of starch for warp sizing. Int. J. Biol. Macromol. 2022, 204, 500–509. [Google Scholar] [CrossRef]




| Dodecenylsuccination | Sulfonation | |||
|---|---|---|---|---|
| Dry Mass of DDSA (g) | DS of DSS (DS1) | Dry Mass of NaHSO3 (g) | Sulfonation Efficiency (%) | DS of SDSS (DS2) |
| 11 | 0.008 | 9.28 | 97.5 | 0.0078 |
| 24 | 0.016 | 18.37 | 93.8 | 0.015 |
| 43 | 0.025 | 28.39 | 96.0 | 0.024 |
| 63 | 0.032 | 36.01 | 93.8 | 0.030 |
| 96 | 0.041 | 45.64 | 92.7 | 0.038 |
| Starches | DS | Breaking Elongation | Tensile Strength | ||
|---|---|---|---|---|---|
| Average (%) | CV (%) | Average (MPa) | CV (%) | ||
| ATS | / | 2.1 a | 8.14 | 28.6 a | 7.85 |
| DSS | 0.008 | 2.5 b | 9.26 | 27.6 ab | 7.36 |
| 0.032 | 3.3 c | 8.35 | 25.1 ab | 6.79 | |
| SDSS | 0.0078 | 2.7 b | 9.75 | 26.9 ab | 6.97 |
| 0.015 | 3.2b c | 9.42 | 26.1 ab | 7.51 | |
| 0.024 | 3.5 c | 9.64 | 25.2 ab | 7.76 | |
| 0.030 | 3.7 c | 9.38 | 24.4 b | 7.48 | |
| 0.038 | 3.3 c | 9.17 | 24.2 b | 6.70 | |
| Starches | DS | Bonding Force to Polyester Fibers | Bonding Force to Cotton Fibers | ||
|---|---|---|---|---|---|
| Average/N | CV/% | Average/N | CV/% | ||
| ATS | / | 103 a | 6.9 | 55 a | 6.0 |
| DSS | 0.008 | 110 ab | 6.7 | 58 ac | 6.0 |
| 0.032 | 118 bc | 6.5 | 61 ac | 6.7 | |
| SDSS | 0.0078 | 115 bc | 6.0 | 60 ac | 5.7 |
| 0.015 | 121 bc | 6.9 | 63 bc | 5.9 | |
| 0.024 | 126 c | 7.1 | 65 bc | 6.4 | |
| 0.030 | 123 c | 7.3 | 63 bc | 6.8 | |
| 0.038 | 118 bc | 7.6 | 61 ab | 6.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, W.; Xu, Z. Influence of Sulfododecenylsuccinylation on the Adhesion to Fibers and Film Properties of Corn Starch for Warp Sizing. Polymers 2023, 15, 1495. https://doi.org/10.3390/polym15061495
Zhang C, Li W, Xu Z. Influence of Sulfododecenylsuccinylation on the Adhesion to Fibers and Film Properties of Corn Starch for Warp Sizing. Polymers. 2023; 15(6):1495. https://doi.org/10.3390/polym15061495
Chicago/Turabian StyleZhang, Chaohui, Wei Li, and Zhenzhen Xu. 2023. "Influence of Sulfododecenylsuccinylation on the Adhesion to Fibers and Film Properties of Corn Starch for Warp Sizing" Polymers 15, no. 6: 1495. https://doi.org/10.3390/polym15061495





