Low Dielectric Constant Photocurable Fluorinated Poly (Phthalazinone Ether) Ink with Excellent Mechanical Properties and Heat Resistance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Eexperimental Methodology
2.2.1. Synthesis of FSt-FPPE
2.2.2. Purification of DPGDA
2.2.3. Preparation of Photosensitive Resin
2.2.4. UV Curing and Postprocessing
2.2.5. Printing Parameters and Post-Processing of 3D Sample
2.3. Characterizations
3. Results
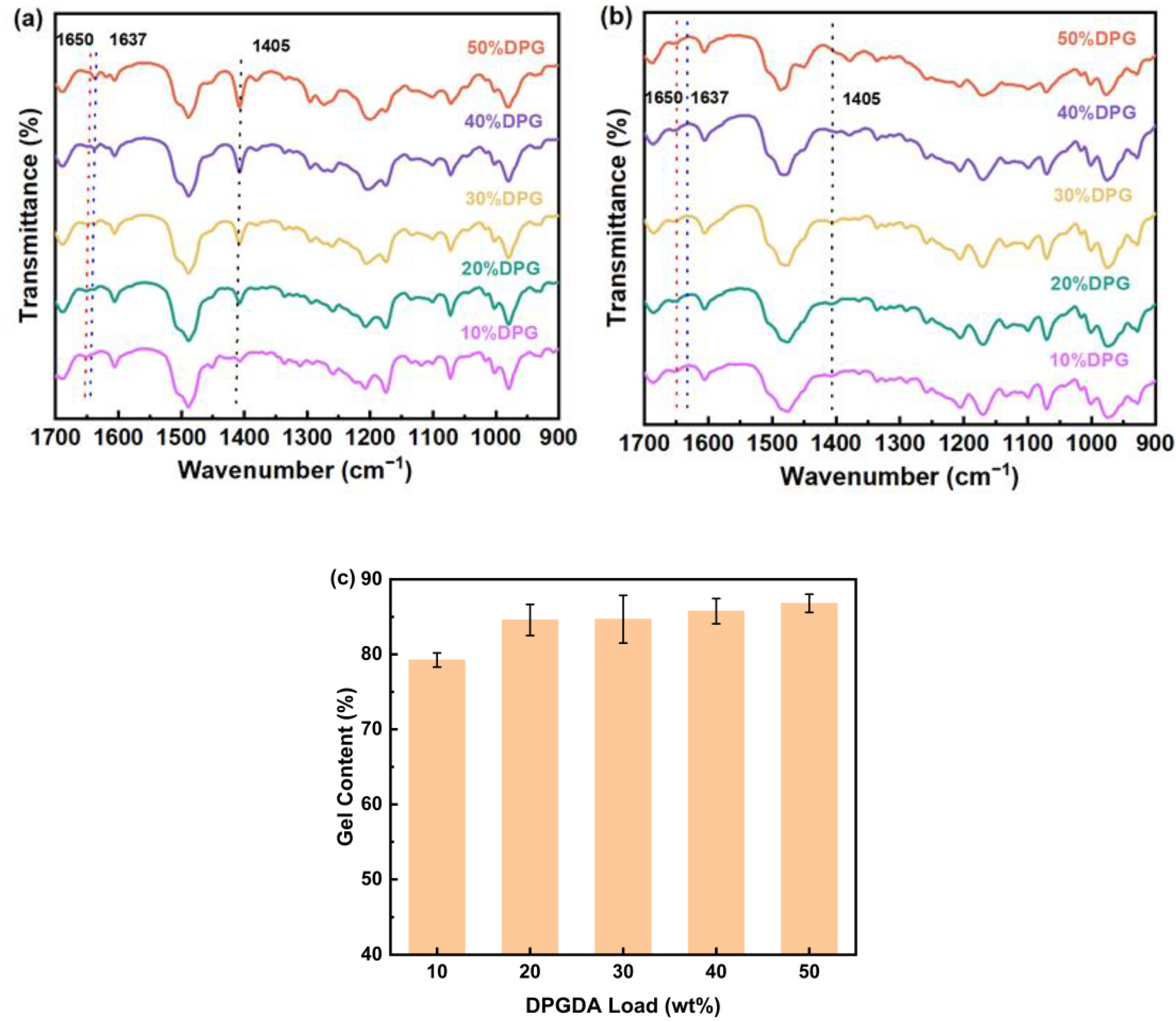
3.1. FTIRanalysis and Gel Content Analysis of FST/DPGs
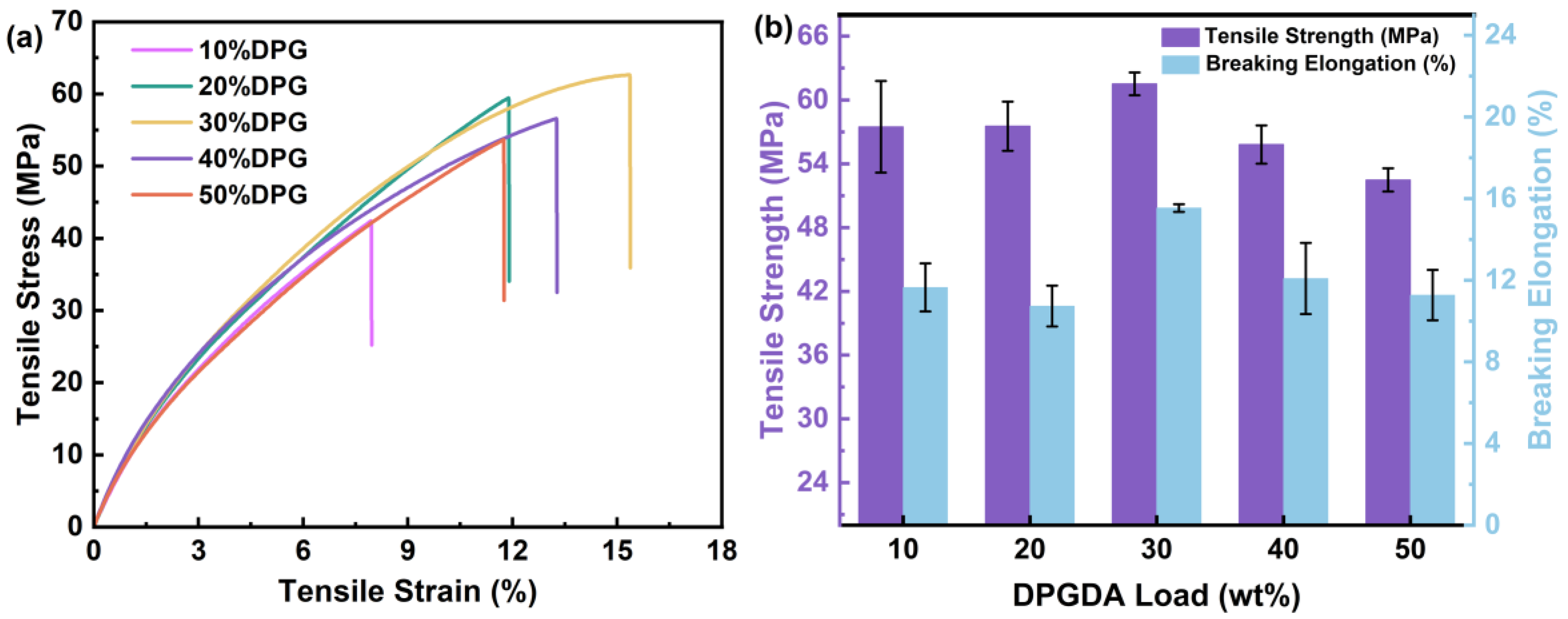
3.2. Mechanical Properties of FST/DPGs
3.3. SEM Analysis of FST/DPGs
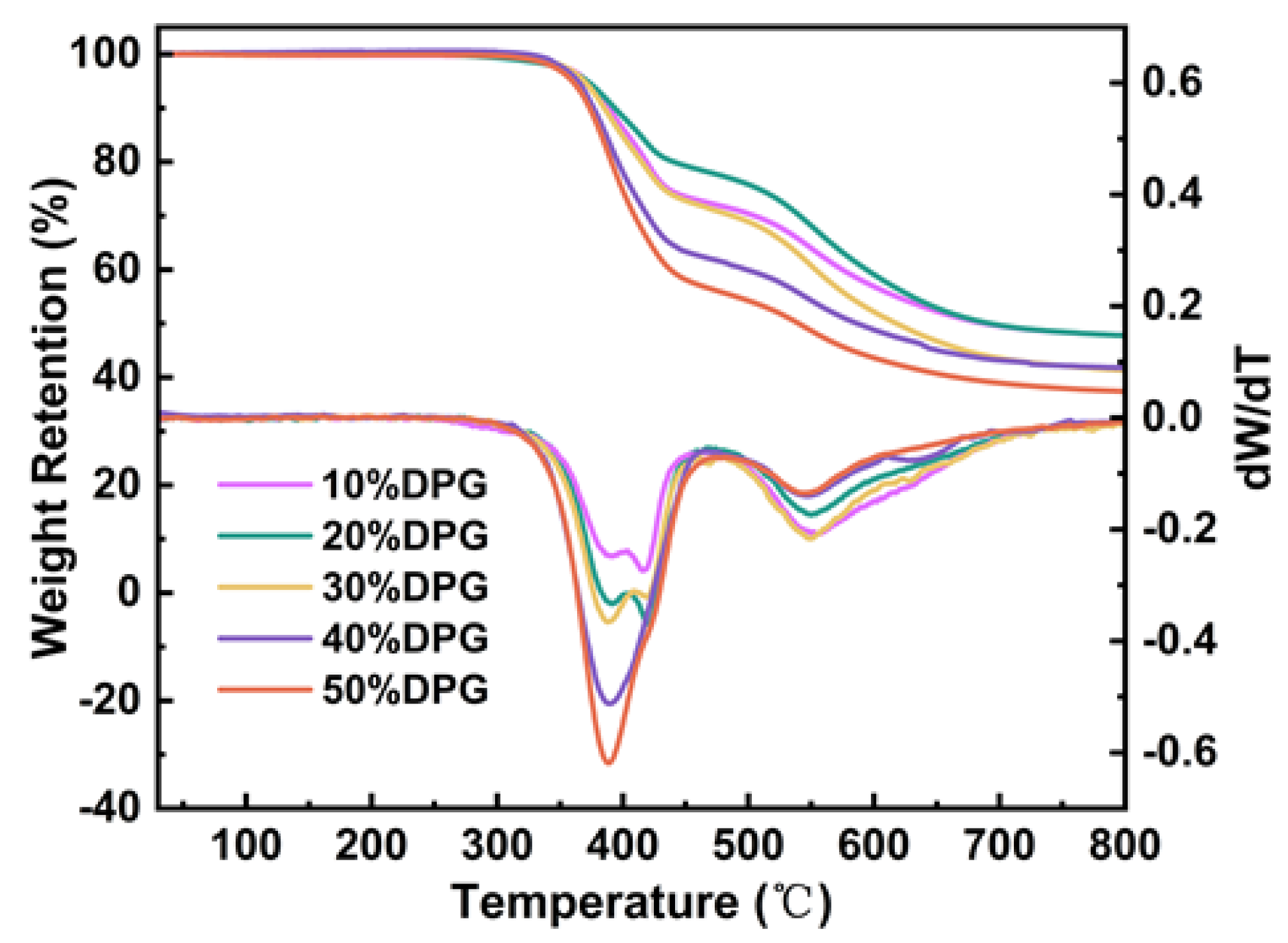
3.4. Thermal Characterization of FST/DPGs
3.5. Dielectric Properties of FST/DPGs
3.6. Water Contact Angle and Water Absorption of FST/DPGs
3.7. D Printing Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, A.M.; Giri, A.; Sangwan, V.K.; Xun, S.; Bartnof, M.; Torres-Castanedo, C.G.; Balch, H.B.; Rahn, M.S.; Bradshaw, N.P.; Vitaku, E.; et al. Thermally conductive ultra-low-k dielectric layers based on two-dimensional covalent organic frameworks. Nat. Mater. 2021, 20, 1142–1148. [Google Scholar] [CrossRef]
- Krishtab, M.; Stassen, I.; Stassin, T.; Cruz, A.J.; Okudur, O.O.; Armini, S.; Wilson, C.; De Gendt, S.; Ameloot, R. Vapor-deposited zeolitic imidazolate frameworks as gap-filling ultra-low-k dielectrics. Nat. Commun. 2019, 10, 3729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Y.; Zhou, Q.; Zhang, X.; Guo, S. Symmetrical “Sandwich” Polybutadiene Film with High-Frequency Low Dielectric Constants, Ultralow Dielectric Loss, and High Adhesive Strength. Ind. Eng. Chem. Res. 2019, 59, 1142–1150. [Google Scholar] [CrossRef]
- Zhou, D.L.; Li, J.H.; Guo, Q.Y.; Lin, X.; Zhang, Q.; Chen, F.; Han, D.; Fu, Q. Polyhedral Oligomeric Silsesquioxanes Based Ultralow-k Materials: The Effect of Cage Size. Adv. Funct. Mater. 2021, 31, 2102074. [Google Scholar] [CrossRef]
- Li, T.L.; Hsu, S.L.C. Preparation and Properties of Thermally Conductive Photosensitive Polyimide/Boron Nitride Nanocomposites. J. Appl. Polym. Sci. 2011, 121, 916–922. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Lyu, Z.Y.; Yang, X.T.; Lu, Y.J.; Ruan, K.P.; Wu, Y.L.; Kong, J.; Gu, J.W. Enhanced thermal conductivities and decreased thermal resistances of functionalized boron nitride/polyimide composites. Compos. Part B-Eng. 2019, 164, 732–739. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Barbosa, J.C.; Goncalves, S.; Pereira, N.; Costa, C.M.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. High dielectric constant UV curable polyurethane acrylate/indium tin oxide composites for capacitive sensing. Compos. Sci. Technol. 2020, 199, 108363. [Google Scholar] [CrossRef]
- Ding, C.; Li, R.; Yu, J.; Wang, X.; Huang, P. Synthesis of porous polyimide films with low dielectric constant and excellent mechanical properties by ambient pressure drying. J. Mater. Sci. 2022, 57, 9480–9492. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Yang, R. Ultra-low dielectric constant and high thermal stability of low-crosslinked polyimide with zinc tetraamino phthalocyanine. J. Mater. Sci. 2022, 57, 16064–16079. [Google Scholar] [CrossRef]
- Mosadegh, B.; Xiong, G.L.; Dunham, S.; Min, J.K. Current progress in 3D printing for cardiovascular tissue engineering. Biomed. Mater. 2015, 10, 034002. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Lu, B.; Li, D.; Tian, X. Development Trends in Additive Manufacturing and 3D Printing. Engineering 2015, 1, 085–089. [Google Scholar] [CrossRef] [Green Version]
- Kozior, T.; Kundera, C. Viscoelastic Properties of Cell Structures Manufactured Using a Photo-Curable Additive Technology—PJM. Poymers 2021, 13, 1895. [Google Scholar]
- Bagheri, A.; Jin, J.Y. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Xu, D.; Tang, L.; Sheng, B. Flexible humidity sensors composed with electrodes of laser induced graphene and sputtered sensitive films derived from poly(ether-ether-ketone). Sens. Actuators B Chem. 2023, 375, 132846. [Google Scholar] [CrossRef]
- Liu, J.; Miao, P.; Zhang, W.; Song, G.; Feng, J.; Leng, X.; Li, Y. Synthesis and characterization of interpenetrating polymer networks (IPNs) based on UV curable resin and blocked isocyanate/polyols. Polymer 2022, 256, 125254. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, Z.; Zhang, Y.; Wang, X.; Zhou, F. Solvent-free and photocurable polyimide inks for 3D printing. J. Mater. Chem. A 2017, 5, 16307–16314. [Google Scholar]
- Wei, D.; Liao, B.; Huang, J.; Zhang, M.; Pang, H. Fabrication of castor oil-based hyperbranched urethane acrylate UV-curable coatings via thiol-ene click reactions. Prog. Org. Coat. 2019, 135, 114–122. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Li, G.; Sun, Q.; Jian, X.; Teng, J.; Zhang, H. Synthesis, characterization and optical properties of fluorinated poly(aryl ether)s containing phthalazinone moieties. Polymer 2008, 49, 4995–5001. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. 4D Printing-Encapsulated Polycaprolactone–Thermoplastic Polyurethane with High Shape Memory Performances. Adv. Eng. Mater. 2023, 25, 2201309. [Google Scholar] [CrossRef]
- Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. A comprehensive experimental investigation on 4D printing of PET-G under bending. J. Mater. Res. Technol. 2022, 18, 2552–2569. [Google Scholar] [CrossRef]
- Ge, X.; Yu, L.; Liu, Z.; Liu, H.; Chen, Y.; Chen, L. Developing acrylated epoxidized soybean oil coating for improving moisture sensitivity and permeability of starch-based film. Int. J. Biol. Macromol. 2019, 125, 370–375. [Google Scholar] [CrossRef]
- Lin, Z.; Ke, Y.; Peng, X.; Wu, X.; Zhang, C.; Zhao, H.; Feng, P. Thermally Stable, Solvent Resistant, and Multifunctional Thermosetting Polymer Networks with High Mechanical Properties Prepared from Renewable Plant Phenols via Thiol–Ene Photo Click Chemistry. ACS Appl. Polym. Mater. 2022, 4, 5330–5340. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, T.; Ye, X.; Man, L.; Zhou, C.; Hu, Y.; Zhang, C.; Yang, Z. Study on the UV curing behavior of tung oil: Mechanism, curing activity and film-forming property. Ind. Crop. Prod. 2018, 112, 61–69. [Google Scholar] [CrossRef]
- Zhang, F.; Zong, L.; Bao, F.; Weng, Z.; Wang, C.; Wang, J.; Jian, X. Novel phthalazinone-bearing tetrafunctional epoxy: Synthesis, characterization, and their toughening application for TGDDM system. Polym. Adv. Technol. 2020, 31, 635–644. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Li, Y.-W.; Zheng, Y.-Q.; Luo, Z.; Gong, C.-R.; Xu, Y.; Wu, L.-X. Synthesis and characterization of a dual-curing resin for three-dimensional printing. J. Mater. Sci. 2018, 54, 5865–5876. [Google Scholar] [CrossRef]
- Yu, Z.H.; Cui, A.Y.; Zhao, P.Z.; Wei, H.K.; Hu, F.Y. Preparation and properties studies of UV-curable silicone modified epoxy resin composite system. J. Appl. Biomater. Funct. Mater. 2018, 16, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Shan, J.; Huang, Y.; Dong, X.; Zheng, W.; Jin, Y.; Zhou, W. Preparation and mechanism of free-radical/cationic hybrid photosensitive resin with high tensile strength for three-dimensional printing applications. J. Appl. Polym. Sci. 2020, 138, 49881. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Li, L.; Yan, B.; Bao, J.; Zhang, A. Acrylate-based photosensitive resin for stereolithographic three-dimensional printing. J. Appl. Polym. Sci. 2019, 136, 47487. [Google Scholar] [CrossRef]
- Roig, A.; Ramis, X.; De la Flor, S.; Serra, À. Sequential photo-thermal curing of (meth)acrylate-epoxy thiol formulations. Polymer 2021, 230, 124073. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Yan, S.; Yuan, Y.; Liu, S. Dielectric properties of photocrosslinkable polyimide/functional graphene oxide composites. Mater. Lett. 2015, 157, 201–204. [Google Scholar] [CrossRef]
- Du, N.; Dal-Cin, M.M.; Robertson, G.P.; Guiver, M.D. Decarboxylation-Induced Cross-Linking of Polymers of Intrinsic Microporosity (PIMs) for Membrane Gas Separation. Macromolecules 2012, 45, 5134–5139. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Chen, C.-C.; Xu, L.; Cui, L.; Paul, D.R.; Koros, W.J. Sub-Tg Cross-Linking of a Polyimide Membrane for Enhanced CO2 Plasticization Resistance for Natural Gas Separation. Macromolecules 2011, 44, 6046–6056. [Google Scholar] [CrossRef]
- Kozior, T.; Mamun, A.; Trabelsi, M.; Sabantina, L. Comparative Analysis of Polymer Composites Produced by FFF and PJM 3D Printing and Electrospinning Technologies for Possible Filter Applications. Ploymers 2022, 12, 48. [Google Scholar] [CrossRef]







| Materials | Abbreviations | Purity | Company |
|---|---|---|---|
| 4-(4-hydroxyl-phenyl) (2H)-phthalazinone-1-one | DHPZ | >98% | Dalian Polymer New Material Co. Ltd. |
| 4,4′-(Hexafluoroisopropylidene)diphenol | HFBPA | >98% | Shanghai Aladdin. |
| Pentafluorostyrene | PFST | >98% | Shanghai Aladdin. |
| Decafluorobiphenyl | DFBP | 99% | Shanghai Macklin Inc. |
| Dipropylene glycol diacrylate | DPGDA | ≥80% | Shanghai Macklin Inc. |
| Triethanolamine | TEOA | ≥99% | Shanghai Aladdin. |
| 1-Hydroxycyclohexyl phenyl ketone | HCPK | >95% | Shanghai Bide Pharmatech Ltd. |
| Acetone | - | - | Tianjin third chemical agent Co. |
| Cyclohexanone | - | - | Tianjin third chemical agent Co. |
| Dimethylacetamide | DMAc | - | Tianjin third chemical agent Co. |
| Samples | FSt-FPPE (wt.%) | DPGDA (wt.%) |
|---|---|---|
| 10% DPG | 90 | 10 |
| 20% DPG | 80 | 20 |
| 30% DPG | 70 | 30 |
| 40% DPG | 60 | 40 |
| 50% DPG | 50 | 50 |
| Samples | E’ (MPa) | Tg (°C) | Td5% (°C) | Tdmax (°C) | Cy (%) |
|---|---|---|---|---|---|
| 10% DPG | 2163 | 233 | 371 | 416 | 48 |
| 20% DPG | 2412 | 225 | 371 | 420 | 48 |
| 30% DPG | 2106 | 220 | 369 | 389 | 42 |
| 40% DPG | 2262 | 206 | 364 | 389 | 42 |
| 50% DPG | 2168 | 174 | 360 | 389 | 37 |
| Samples | Dk | Df |
|---|---|---|
| 10% DPG | 2.81 | 0.0212 |
| 20% DPG | 2.75 | 0.0193 |
| 30% DPG | 2.86 | 0.0234 |
| 40% DPG | 2.95 | 0.0383 |
| 50% DPG | 2.96 | 0.0418 |
| 10% DPG | 20% DPG | 30% DPG | 40% DPG | 50% DPG | |
|---|---|---|---|---|---|
| 2θ (°) | 15.48 | 14.69 | 16.58 | 17.43 | 18.61 |
| d-spacing (Å) | 5.72 | 6.02 | 5.34 | 5.08 | 4.76 |
| Samples | Weights 1 (g) | Weights 2 (g) | Water Absorption (%) |
|---|---|---|---|
| 10% DPG | 0.2731 | 0.2741 | 0.3662 |
| 20% DPG | 0.3655 | 0.3663 | 0.2189 |
| 30% DPG | 0.3769 | 0.3790 | 0.5572 |
| 40% DPG | 0.4291 | 0.4330 | 0.9089 |
| 50% DPG | 0.4089 | 0.4127 | 0.9293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, C.; Jiang, L.; Wang, Y.; Wang, B.; Wang, X.; Liu, H.; Zong, L.; Wang, J.; Jian, X. Low Dielectric Constant Photocurable Fluorinated Poly (Phthalazinone Ether) Ink with Excellent Mechanical Properties and Heat Resistance. Polymers 2023, 15, 1531. https://doi.org/10.3390/polym15061531
Zhang G, Wang C, Jiang L, Wang Y, Wang B, Wang X, Liu H, Zong L, Wang J, Jian X. Low Dielectric Constant Photocurable Fluorinated Poly (Phthalazinone Ether) Ink with Excellent Mechanical Properties and Heat Resistance. Polymers. 2023; 15(6):1531. https://doi.org/10.3390/polym15061531
Chicago/Turabian StyleZhang, Guangsheng, Chenghao Wang, Lingmei Jiang, Yibo Wang, Bing Wang, Xiaoxu Wang, Haoran Liu, Lishuai Zong, Jinyan Wang, and Xigao Jian. 2023. "Low Dielectric Constant Photocurable Fluorinated Poly (Phthalazinone Ether) Ink with Excellent Mechanical Properties and Heat Resistance" Polymers 15, no. 6: 1531. https://doi.org/10.3390/polym15061531
APA StyleZhang, G., Wang, C., Jiang, L., Wang, Y., Wang, B., Wang, X., Liu, H., Zong, L., Wang, J., & Jian, X. (2023). Low Dielectric Constant Photocurable Fluorinated Poly (Phthalazinone Ether) Ink with Excellent Mechanical Properties and Heat Resistance. Polymers, 15(6), 1531. https://doi.org/10.3390/polym15061531








