Durability, Strength, and Erosion Resistance Assessment of Lignin Biopolymer Treated Soil
Abstract
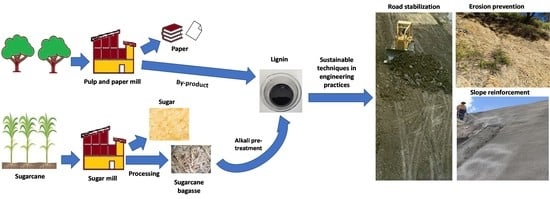
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Experimental Measurments
3. Results and Discussion
3.1. Atterberg Limits
3.2. Unconfined Compressive Strength (UCS)
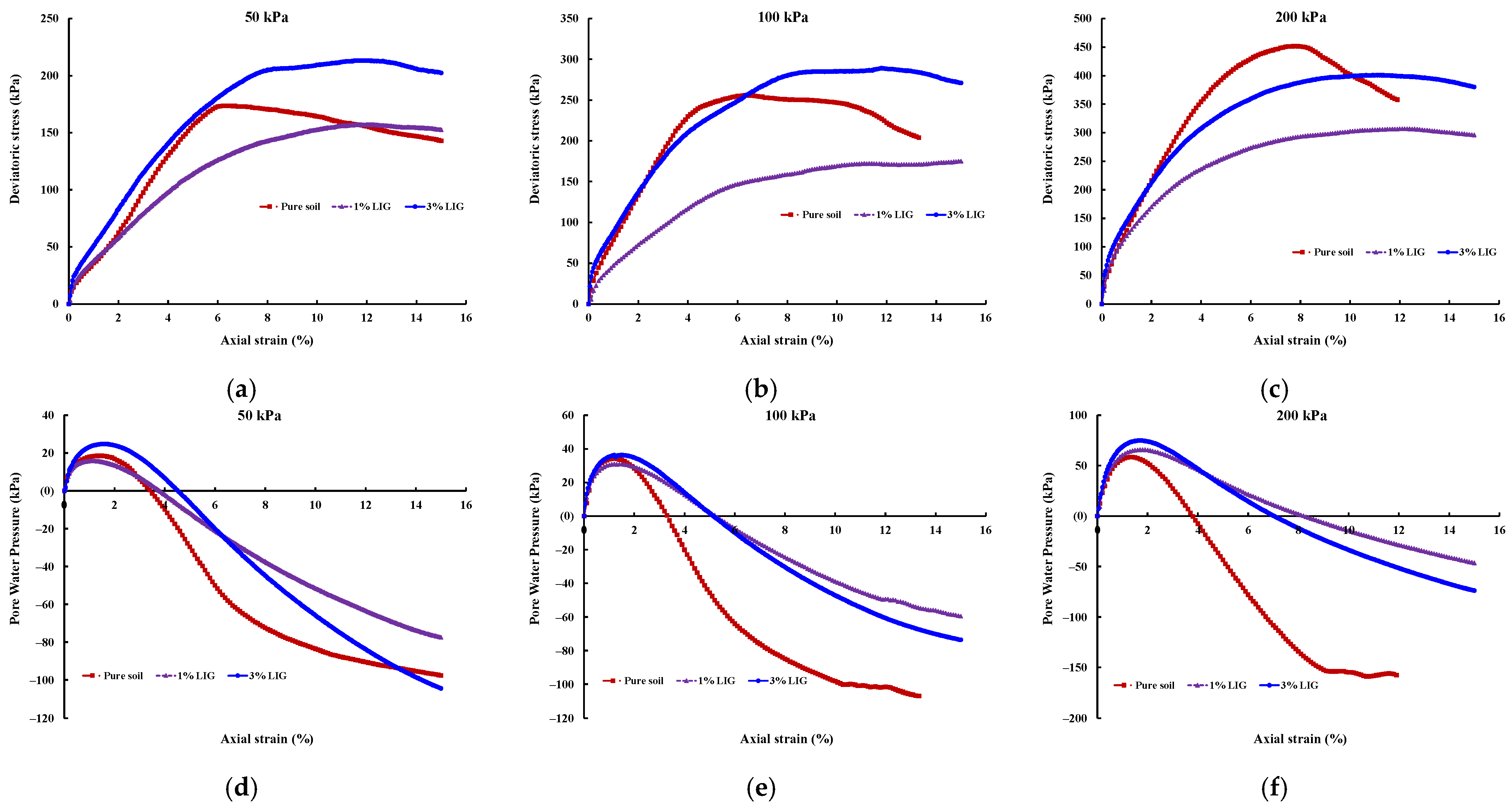
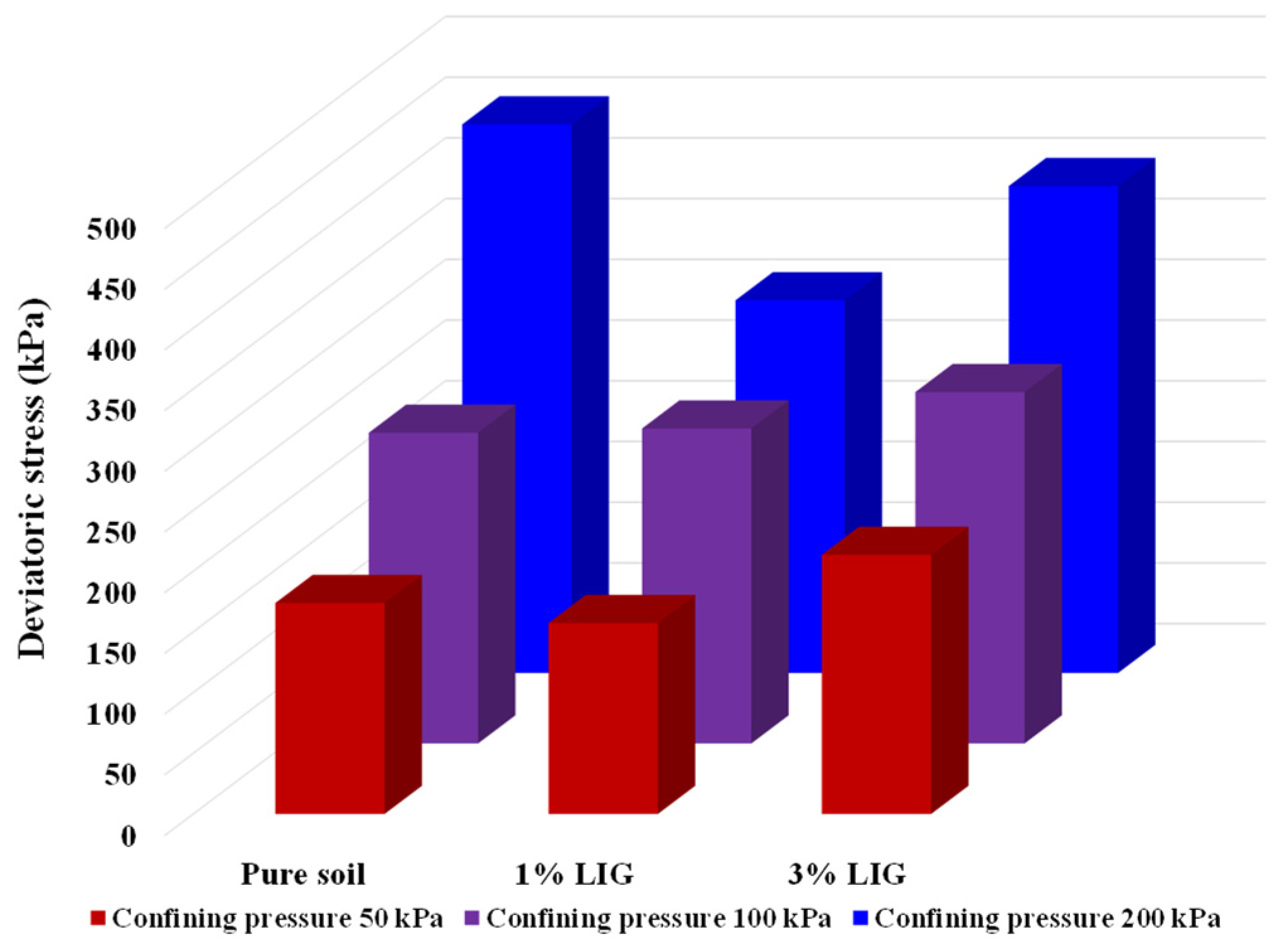
3.3. Shear Strength
CU Triaxial
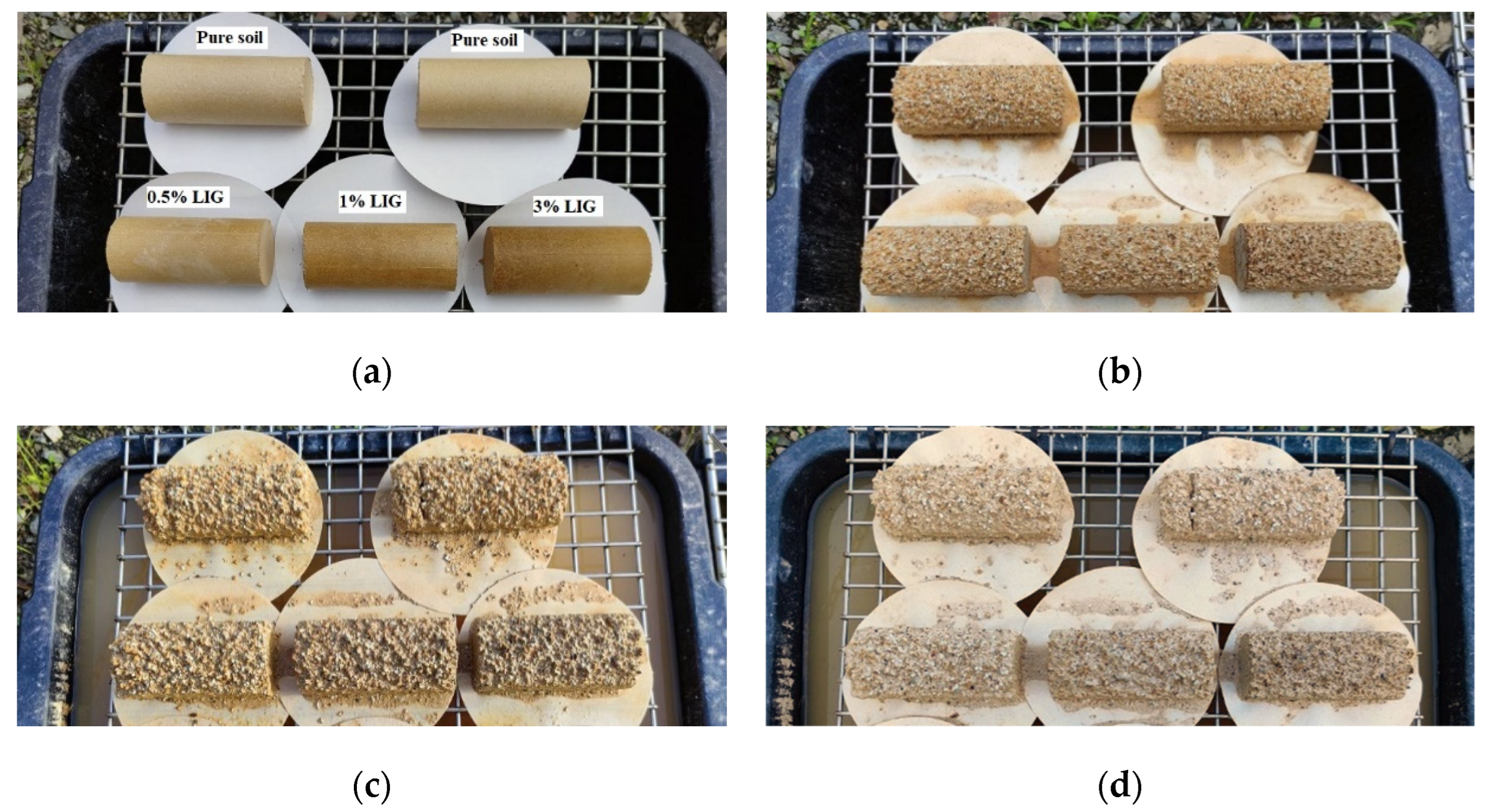
3.4. Wetting and Drying Cycles
3.5. Exposure to Atmosphere Conditions
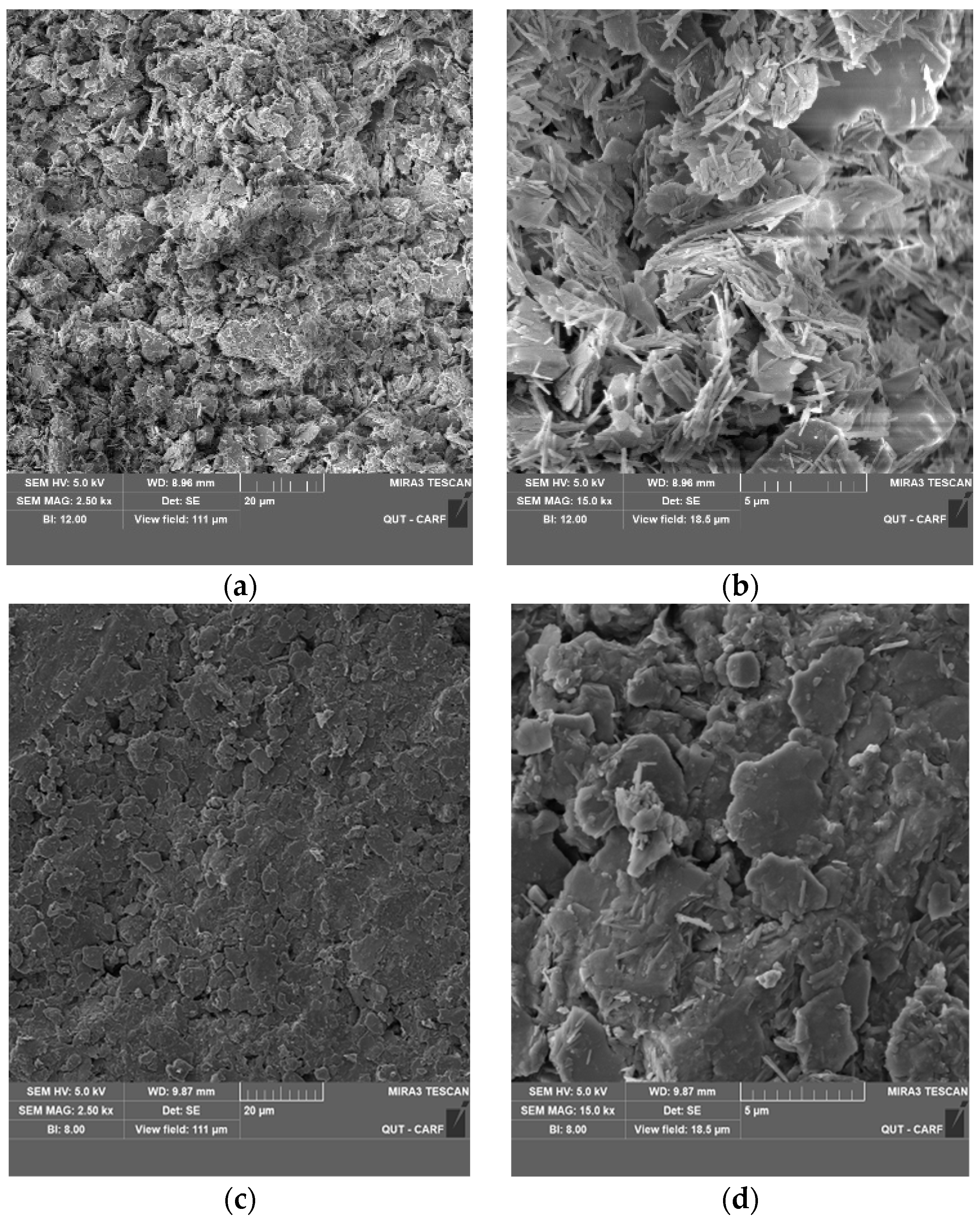
3.6. SEM Analysis
4. Conclusions
- -
- The effect of LIG additive appeared to be insignificant on the soil PL, and a slight reduction in LL and plasticity index was seen.
- -
- The soil compressive strength significantly increased with increasing curing time and LIG dosage. After 14 days of curing, the compressive strength of 3% LIG-treated samples did not go under major changes, and further curing did not affect their strengths. A similar trend was seen for 0.5% and 1% LIG-treated specimens after 10 days of curing.
- -
- At lower confining pressures of CU triaxial tests, the 3% LIG-treated soil experienced slightly higher shear strength than the pure soil. The LIG additive caused significant improvement in soil cohesion and a slight reduction in soil friction angle.
- -
- Despite biopolymers susceptibility to water, during wetting/drying cycles, the LIG biopolymer showed its capability to improve the strength and durability of soil to water. While all samples experienced compressive strength reduction during wetting and drying cycles, the LIG treated samples showed significantly higher strength than pure soil.
- -
- The LIG-treated samples lost less soil mass compared to pure soil samples confirming the applicability of such treatment technique in improving soil erosion resistance.
- -
- This study showed that the LIG biopolymer, a byproduct of paper and sugarcane factories, can effectively improve soil strength and erosion resistance under various conditions. The durability of LIG additive to water during wetting/drying cycles proves its potential application for quick temporary constructions in an arid climate area. Additionally, lower soil mass loss of LIG-treated samples verifies its application in erosion-prone areas.
- -
- As the durability of biopolymers against soil erosion is an important factor and needs further investigation, large-scale field tests should be linked in future studies.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195. [Google Scholar]
- Bahmani, S.H.; Huat, B.B.K.; Asadi, A.; Farzadnia, N. Stabilization of residual soil using SiO2 nanoparticles and cement. Constr. Build. Mater. 2014, 64, 350–359. [Google Scholar]
- Amani, J.; Babu, K.; Lakshmipathi, R.; Rao, G.R.; Chandrasekhar, K. Effect of Cement Dust Deposition on Soil Microbial Properties. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1230–1234. [Google Scholar]
- Soldo, A.; Miletic, M.; Auad, M.L. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci. Rep. 2020, 10, 267. [Google Scholar] [PubMed] [Green Version]
- Bagheri, P.; Gratchev, I.; Rybachuk, M. Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties. Appl. Sci. 2023, 13, 887. [Google Scholar] [CrossRef]
- Chang, I.; Cho, G.-C. Shear strength behavior and parameters of microbial gellan gum-treated soils: From sand to clay. Acta Geotech. 2019, 14, 361–375. [Google Scholar] [CrossRef]
- Latifi, N.; Horpibulsuk, S.; Meehan, C.L.; Majid, M.Z.A.; Tahir, M.M.; Mohamad, E.T. Improvement of problematic soils with biopolymer—An environmentally friendly soil stabilizer. J. Mater. Civ. Eng. 2017, 29, 04016204. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.-C. Effects of Xanthan gum biopolymer on soil strengthening. Constr. Build. Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Chang, I.; Cho, G.-C. Strengthening of Korean residual soil with β-1, 3/1, 6-glucan biopolymer. Constr. Build. Mater. 2012, 30, 30–35. [Google Scholar] [CrossRef]
- Ta’negonbadi, B.; Noorzad, R. Physical and geotechnical long-term properties of lignosulfonate-stabilized clay: An experimental investigation. Transp. Geotech. 2018, 17, 41–50. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Budhu, M. Biopolymer stabilization of mine tailings. J. Geotech. Geoenviron. Eng. 2013, 139, 1802–1807. [Google Scholar] [CrossRef]
- Hataf, N.; Ghadir, P.; Ranjbar, N. Investigation of soil stabilization using chitosan biopolymer. J. Clean. Prod. 2018, 170, 1493–1500. [Google Scholar] [CrossRef]
- Ham, S.-M.; Noh, D.-H.; Kwon, T.-H.; Muhunthan, B. Improvement of surface erosion resistance of sand by microbial biopolymer formation. J. Geotech. Geoenviron. Eng. 2018, 144, 06018004. [Google Scholar] [CrossRef] [Green Version]
- Chang, I.; Im, J.; Cho, G.-C. Geotechnical engineering behaviors of gellan gum biopolymer treated sand. Can. Geotech. J. 2016, 53, 1658–1670. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cai, G.; Liu, S. Assessment of mechanical properties in recycled lignin-stabilized silty soil as base fill material. J. Clean. Prod. 2018, 172, 1788–1799. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S. Application of lignin-stabilized silty soil in highway subgrade: A macroscale laboratory study. J. Mater. Civ. Eng. 2018, 30, 04018034. [Google Scholar] [CrossRef]
- ASTM D2487-17; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D422-63; Standard Test Method for Particle-Size Analysis of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D4318-17; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D698-12; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D2166-06; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2010.
- Ta’negonbadi, B.; Noorzad, R. Stabilization of clayey soil using lignosulfonate. Transp. Geotech. 2017, 12, 45–55. [Google Scholar] [CrossRef]
- Xin, H.; Wei, M.; Li, G.-Y.; Mu, Y.-H.; Zhou, Z.-W.; Fei, W. Influence of lignosulfonate on mechanical properties of Lanzhou loess. Rock Soil Mech. 2017, 38, 18–26. [Google Scholar]







| Soil Reference | Liquid Limit, LL (%) | Plastic Limit, PL (%) | Plasticity Index, PI (%) |
|---|---|---|---|
| Pure soil | 38.0 | 26.9 | 11.1 |
| 0.5% LIG | 36.9 | 26.1 | 10.8 |
| 1% LIG | 36.1 | 25.6 | 10.5 |
| 3% LIG | 34.6 | 25.6 | 9.0 |
| Soil Reference | Effective Cohesion, c′ (kPa) | Effective Internal Friction Angle, ϕ′ (°) |
|---|---|---|
| Pure soil | 24 | 28.9 |
| 1% LIG | 38 | 24.0 |
| 3% LIG | 53 | 22.5 |
| Cycle | Rf (100%) | Cycles | ∆Rf | ||||
|---|---|---|---|---|---|---|---|
| Pure Soil | 1% LIG | 3% LIG | Pure Soil | 1% LIG | 3% LIG | ||
| 0 | 0.0 | 0.0 | 0.0 | 0–1 | 19.0 | 50.1 | 30.3 |
| 1 | 19.0 | 50.1 | 30.3 | 1–2 | 3.2 | 5.3 | 13.1 |
| 2 | 22.2 | 55.4 | 43.4 | 2–3 | 16.3 | 4.8 | 13.0 |
| 3 | 38.5 | 60.2 | 56.4 | 3–4 | 0.3 | 1.6 | −1.0 |
| 4 | 38.9 | 61.8 | 55.4 | 4–5 | 7.8 | 3.6 | 8.6 |
| 5 | 46.7 | 65.4 | 63.9 | 5–6 | 6.4 | −0.7 | 1.1 |
| 6 | 53.1 | 64.7 | 65.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri, P.; Gratchev, I.; Son, S.; Rybachuk, M. Durability, Strength, and Erosion Resistance Assessment of Lignin Biopolymer Treated Soil. Polymers 2023, 15, 1556. https://doi.org/10.3390/polym15061556
Bagheri P, Gratchev I, Son S, Rybachuk M. Durability, Strength, and Erosion Resistance Assessment of Lignin Biopolymer Treated Soil. Polymers. 2023; 15(6):1556. https://doi.org/10.3390/polym15061556
Chicago/Turabian StyleBagheri, Pouyan, Ivan Gratchev, Suwon Son, and Maksym Rybachuk. 2023. "Durability, Strength, and Erosion Resistance Assessment of Lignin Biopolymer Treated Soil" Polymers 15, no. 6: 1556. https://doi.org/10.3390/polym15061556
APA StyleBagheri, P., Gratchev, I., Son, S., & Rybachuk, M. (2023). Durability, Strength, and Erosion Resistance Assessment of Lignin Biopolymer Treated Soil. Polymers, 15(6), 1556. https://doi.org/10.3390/polym15061556