A Solid-State Wire-Shaped Supercapacitor Based on Nylon/Ag/Polypyrrole and Nylon/Ag/MnO2 Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
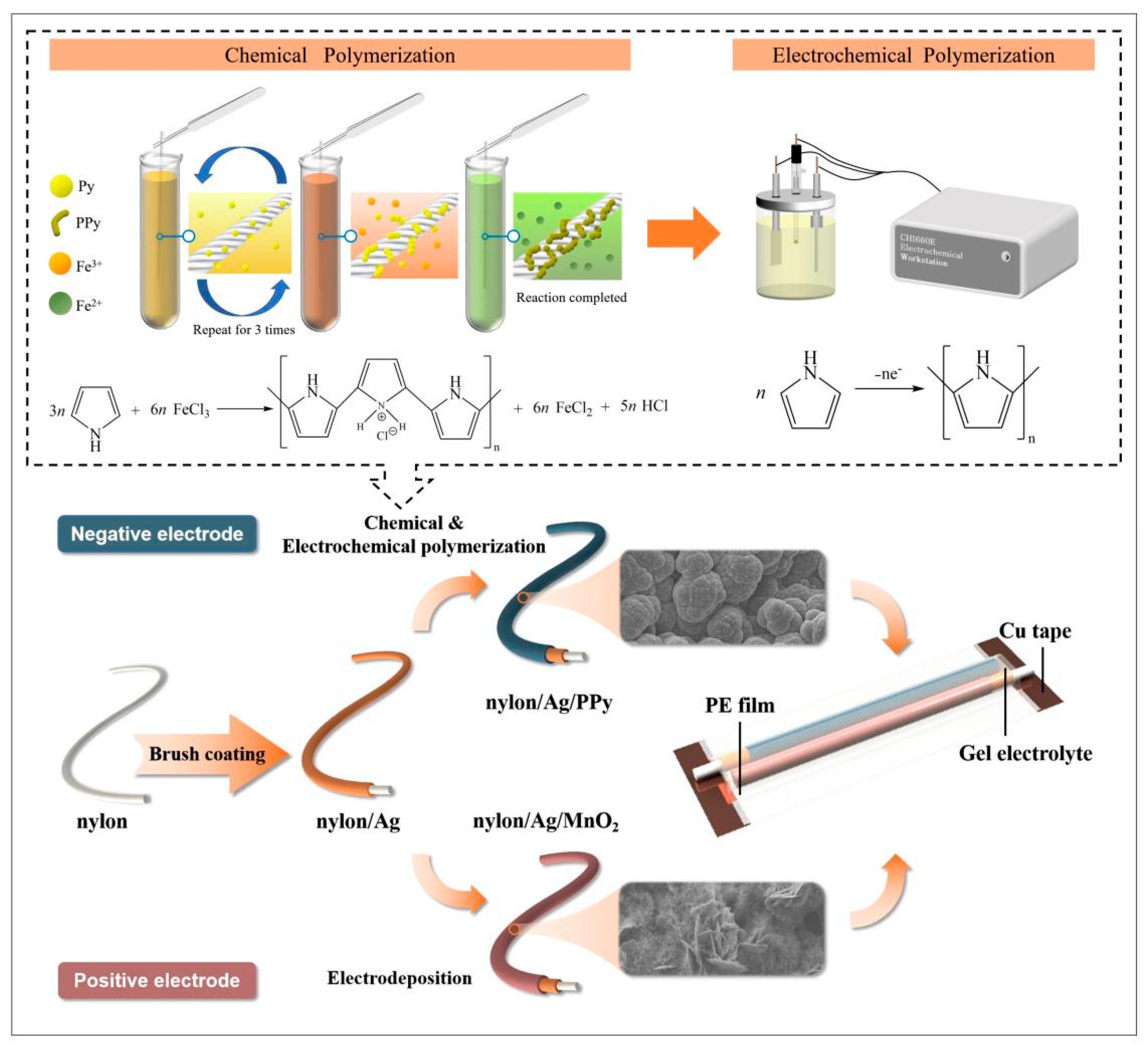
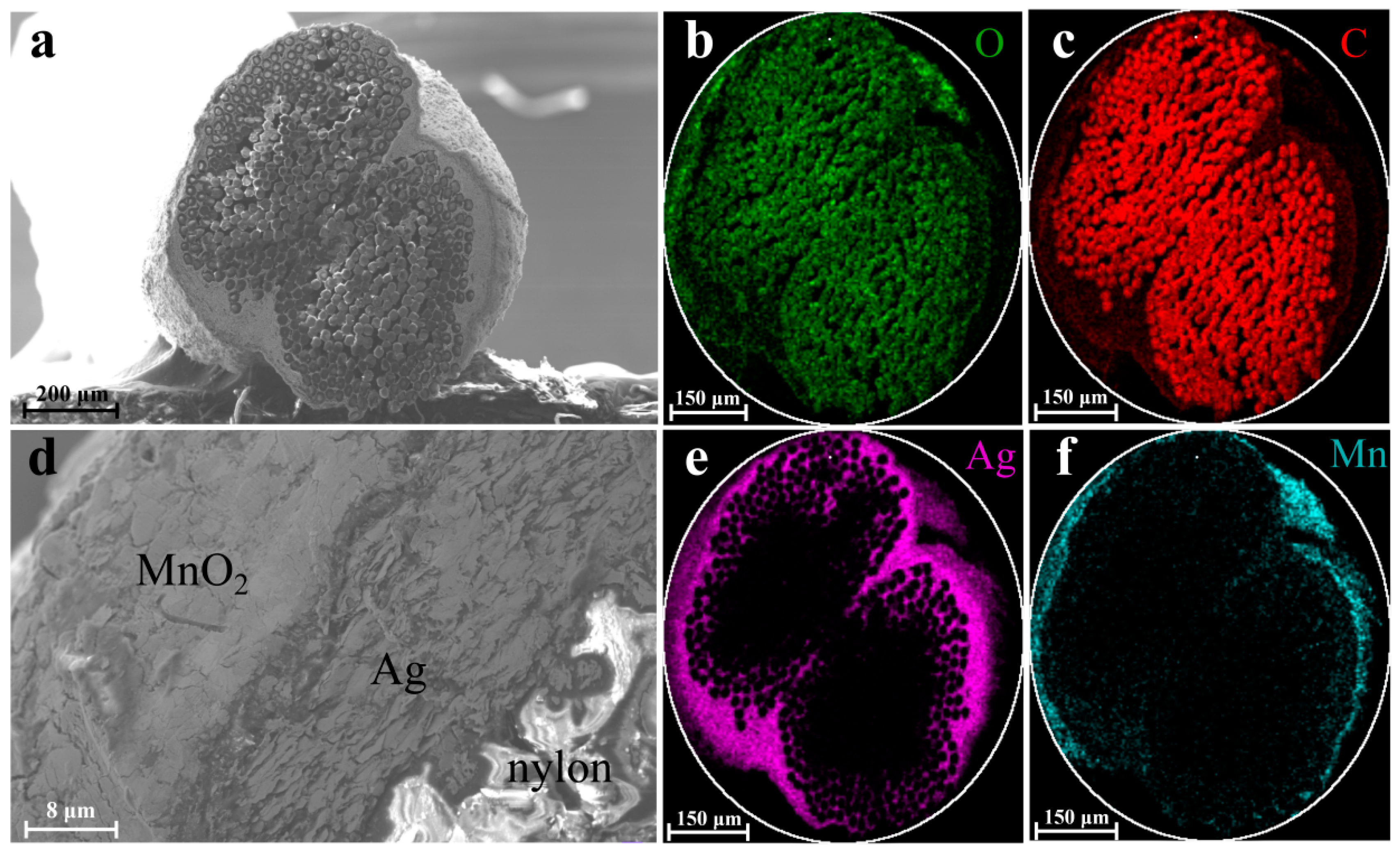
2.2. Preparation of Nylon/Ag/MnO2 Yarn Electrode
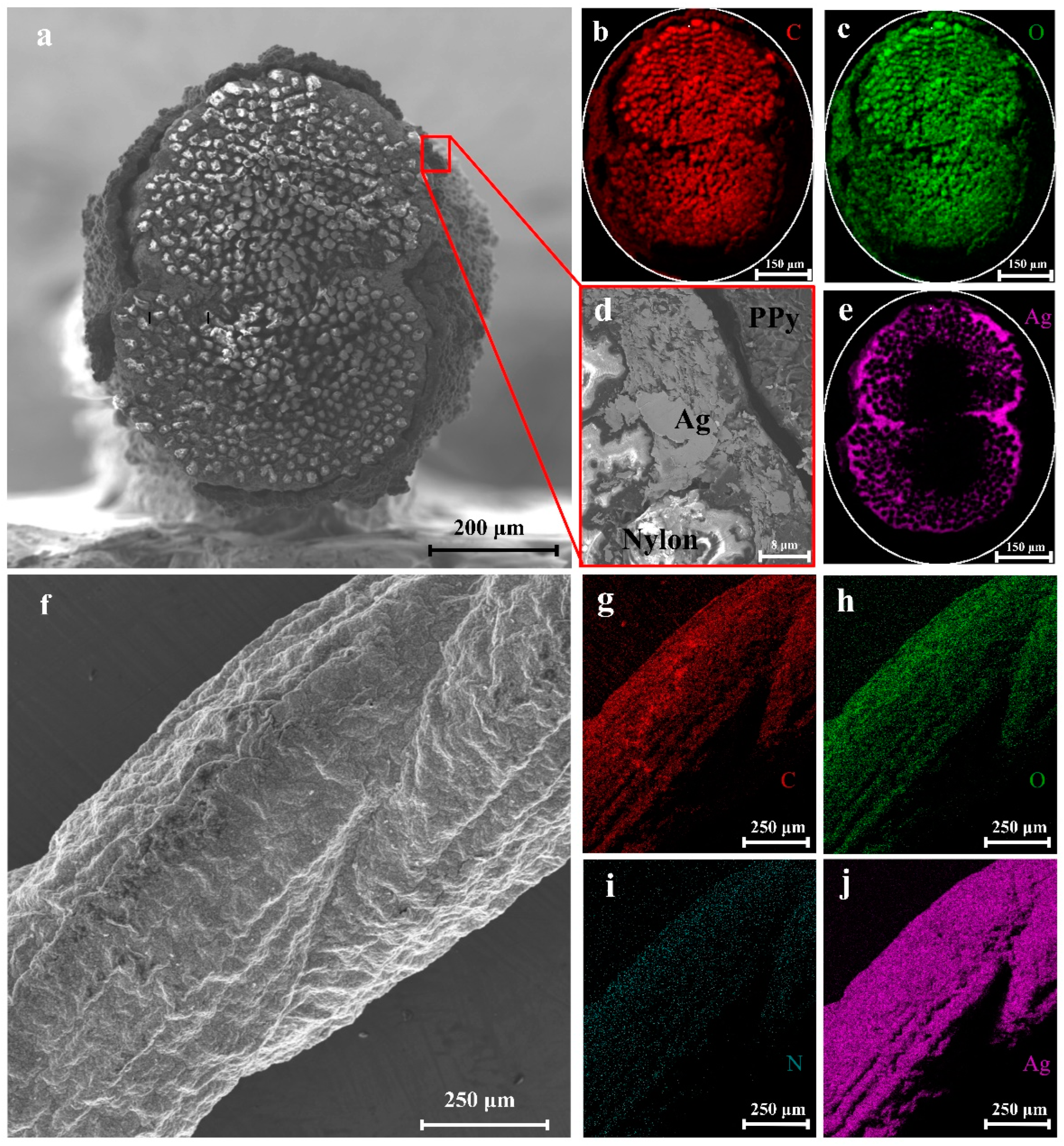
2.3. Preparation of Nylon/Ag/PPy Fiber Electrode
2.4. Fabrication of Solid-State Asymmetric Supercapacitor
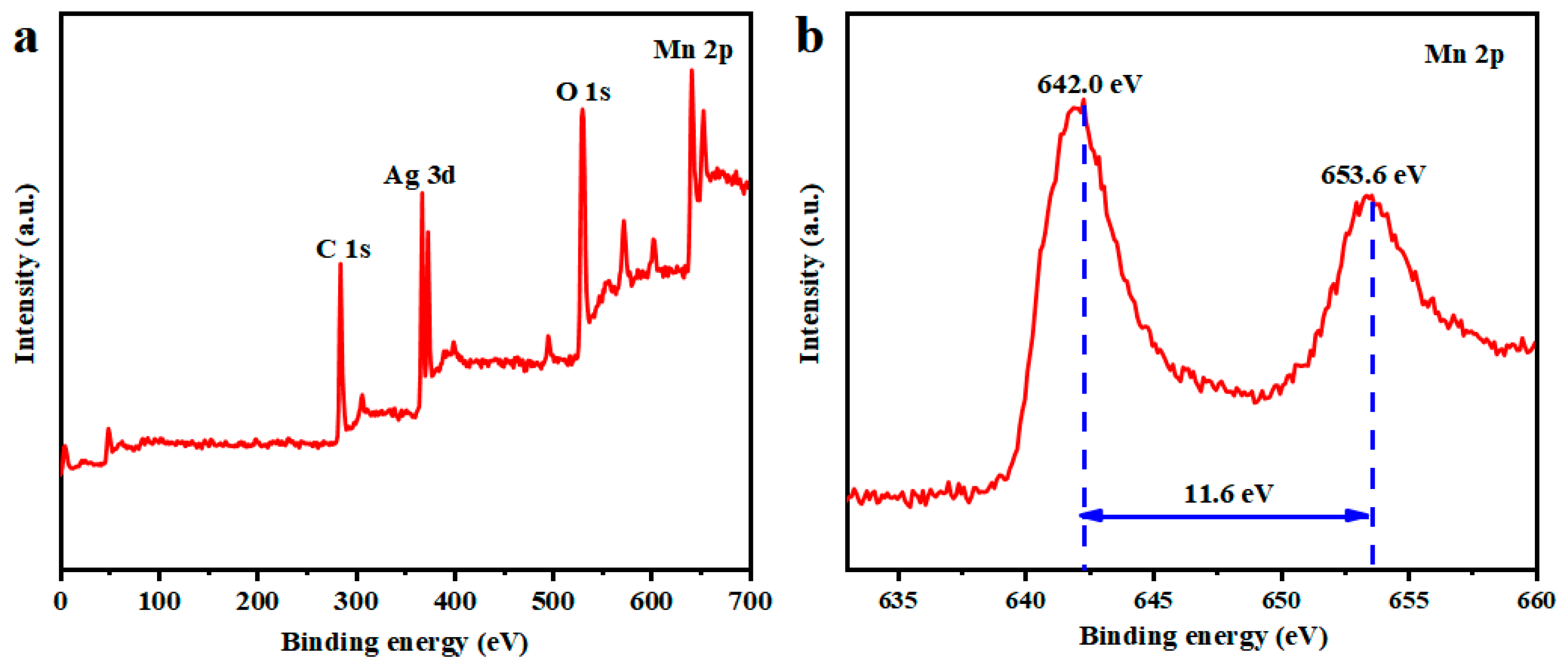
2.5. Materials Characterizations
2.6. Electrochemical Measurements
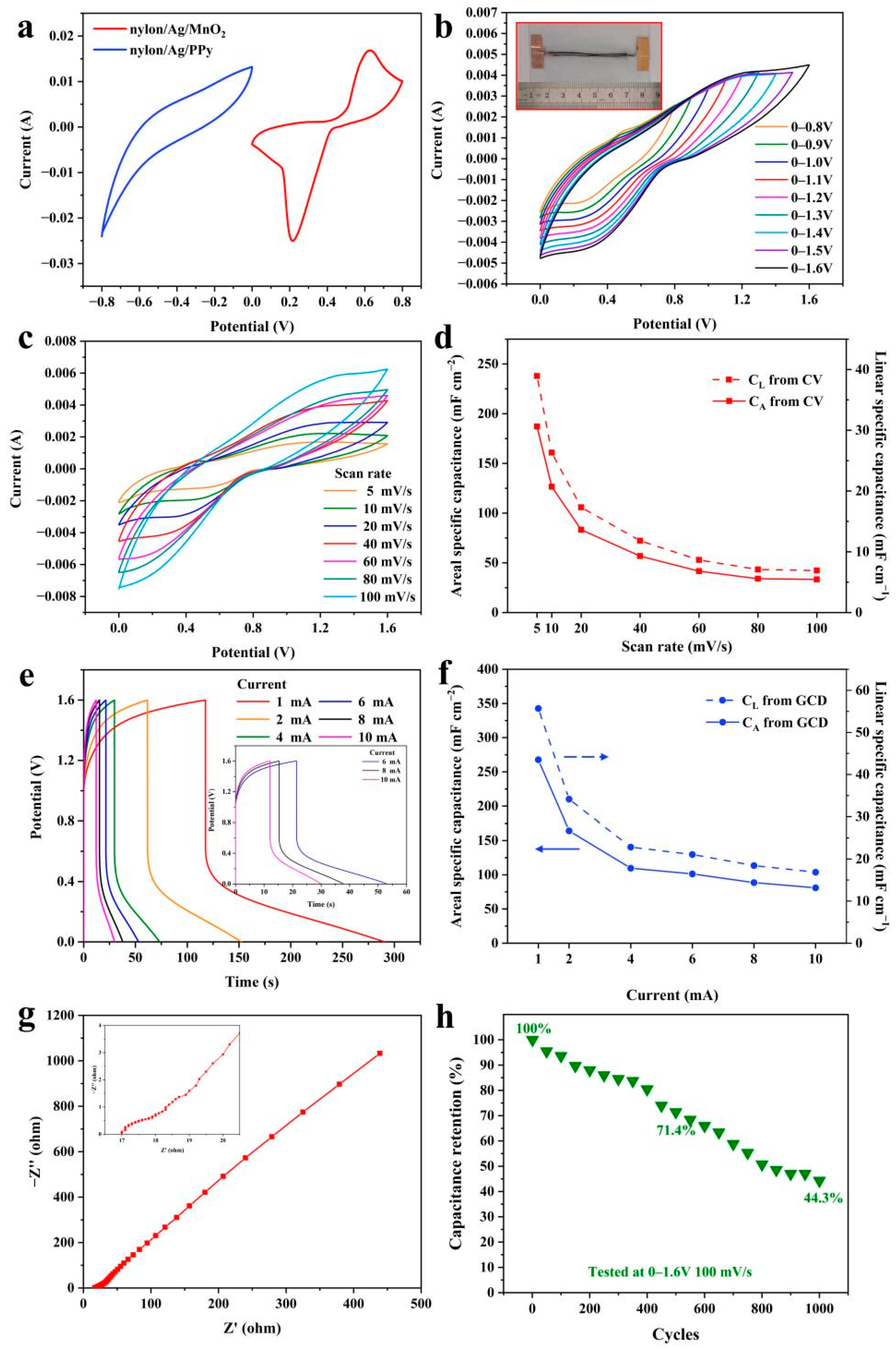
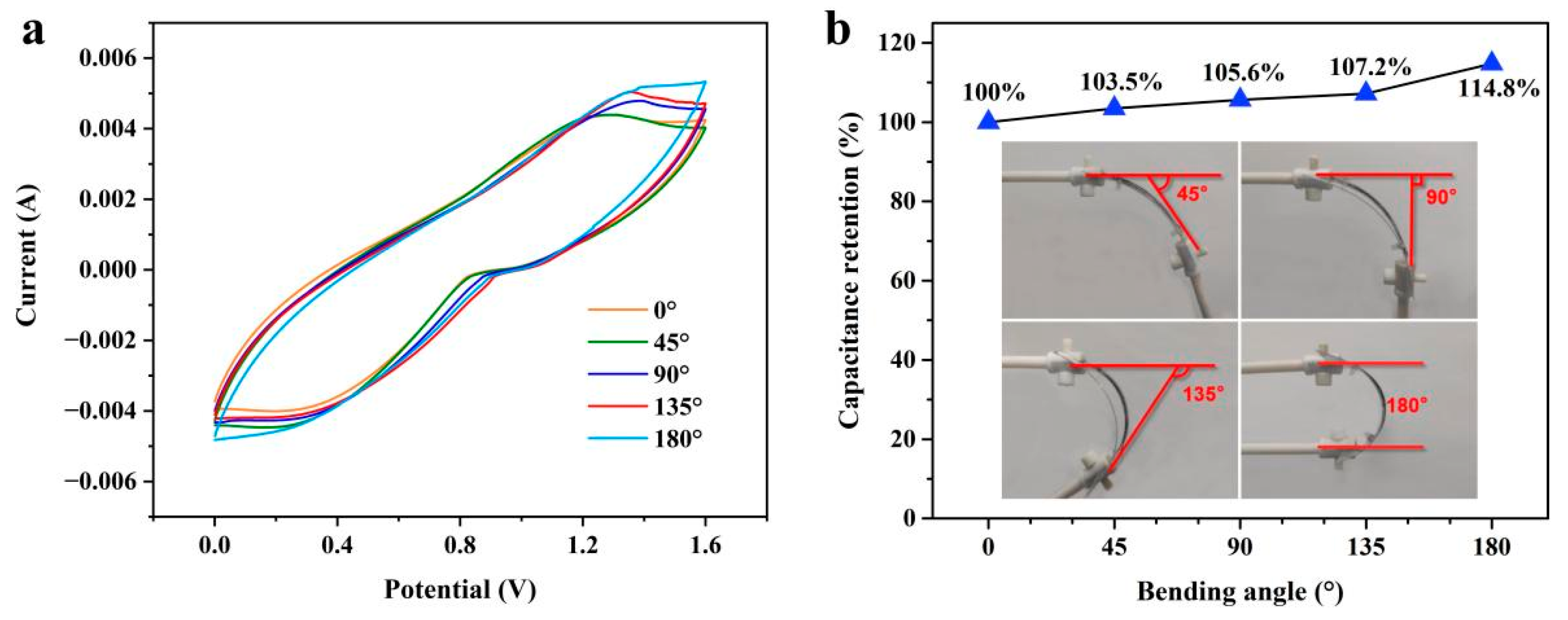
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, D.; Qian, Q.; Wei, L.; Jiang, W.; Goh, K.; Wei, J.; Zhang, J.; Chen, Y. Emergence of fiber supercapacitors. Chem. Soc. Rev. 2015, 44, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ren, P.; Guo, Z.; Hou, X.; He, W.; Ren, F.; Jin, Y. Silver nanoparticles as a conductive bridge for high-performance flexible all-solid-state asymmetric supercapacitor. RSC Adv. 2015, 5, 107716–107770. [Google Scholar] [CrossRef]
- Pan, S.; Lin, H.; Deng, J.; Chen, P.; Chen, X.; Yang, Z.; Peng, H. Novel wearable energy devices based on aligned carbon nanotube fiber textiles. Adv. Energy Mater. 2015, 5, 1401438. [Google Scholar] [CrossRef]
- Fu, Y.; Cai, X.; Wu, H.; Lv, Z.; Hou, S.; Peng, M.; Yu, X.; Zou, D. Fiber supercapacitors utilizing pen ink for flexible/wearable energy storage. Adv. Mater. 2012, 24, 5713–5718. [Google Scholar] [CrossRef]
- Zuo, W.; Zang, L.; Wang, X.; Liu, Q.; Qiu, J.; Liang, C.; Liu, X.; Yang, C. Flexible polypyrrole@Fe2O3@stainless steel yarn composite electrode for symmetric thread-like supercapacitor with extended operating voltage window in Li2SO4-based aqueous electrolyte. Adv. Sustain. Syst. 2020, 4, 2000173. [Google Scholar] [CrossRef]
- Wang, X.; Wei, H.; Du, W.; Sun, X.; Kang, L.; Zhang, Y.; Zhao, X.; Jiang, F. Recycled carbon fiber-supported polyaniline/manganese dioxide prepared via one-step electrodeposition for flexible supercapacitor integrated electrodes. Polymers 2018, 10, 1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Zhang, J.; Ren, J.; Liu, N.; Chen, P.; Zhang, Y.; Deng, J.; Wang, Y.; Peng, H. The design of hierarchical ternary hybrid for fiber-shaped asymmetric supercapacitor with high volumetric energy density. J. Phys. Chem. C 2016, 120, 9685–9691. [Google Scholar] [CrossRef]
- Qu, G.; Cheng, J.; Li, X.; Yuan, D.; Chen, P.; Chen, X.; Wang, B.; Peng, H. A fiber supercapacitor with high energy density based on hollow graphene/conducting polymer fiber electrode. Adv. Mater. 2016, 28, 3646–3652. [Google Scholar] [CrossRef]
- Zhang, M.; Atkinson, K.R.; Baughman, R.H. Multifunctional carbon nanotube yarn by downsizing an ancient technology. Science 2004, 306, 1358–1361. [Google Scholar] [CrossRef]
- Kang, C.; Cao, D.; Liu, Y.; Liu, Z.; Liu, R.; Feng, X.; Wang, D.; Ma, Y. High loading carbon nanotubes deposited onto porous nickel yarns by solution imbibition as flexible wire-shaped supercapacitor electrodes. J. Energy Chem. 2018, 27, 836–842. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Kim, S.H.; Sim, H.J.; Lee, J.A.; Choi, A.Y.; Kim, Y.T.; Lepró, X.; Spinks, G.M.; Baughman, R.H.; Kim, S.J. Stretchable, weavable coiled carbon nanotube/MnO2/polymer fiber solid-state supercapacitors. Sci. Rep. 2015, 5, 9387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Xu, X.; Yu, J.; Hong, J.; Liu, C.; Ouyang, X.; Lei, S.; Meng, X.; Tang, J.; Chen, D. Facile construction of 3D porous carbon nanotubes/polypyrrole and reduced graphene oxide on carbon nanotube fiber for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 314, 9–19. [Google Scholar] [CrossRef]
- Cen, T.; Chen, L.; Zhang, X.; Tian, Y.; Fan, X. A novel fiber-shaped asymmetric supercapacitor prepared by twisting carbon fiber/carbon nanotube/MnO2 and carbon fiber/carbon nanotube/polypyrrole electrodes. Electrochim. Acta 2021, 367, 137488. [Google Scholar] [CrossRef]
- Xu, P.; Wei, B.; Cao, Z.; Zheng, J.; Gong, K.; Li, F.; Yu, J.; Li, Q.; Lu, W.; Byun, J.H. Stretchable wire-shaped asymmetric supercapacitors based on pristine and MnO2 coated carbon nanotube fibers. ACS Nano 2015, 9, 6088–6096. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, C.; Lee, J.; Andrade, M.; Baughman, R.; Kim, S. Ag/MnO2 composite sheath-core structured yarn supercapacitors. Sci. Rep. 2018, 8, 13309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lin, R.; Fu, Y.; Wang, X.; Yu, X.; Li, J.; Zhu, Y.; Tan, S.; Wang, Z. Metal-organic framework derived Fe2O3 nanocubes on intertwined N-doped carbon nanowires for fiber-shaped supercapacitor. Mater. Lett. 2018, 228, 9–12. [Google Scholar] [CrossRef]
- Toledo, W.; Couto, A.; Almeida, D.; Ferreira, N. Facile synthesis of TiO2/rGO neatly electrodeposited on carbon fiber applied as ternary electrode for supercapacitor. Mater. Res. Express 2019, 6, 065040. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. Synthesis of highly crystalline polyaniline with the use of (Cyclohexylamino)-1-propanesulfonic acid for supercapacitor. J. Appl. Electrochem. 2015, 45, 51–56. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. Improving the electrochemical performance by sulfonation of polyaniline-graphene-silica composite for high performance supercapacitor. Int. J. Polym. Mater. Polym. Biomater. 2016, 16, 835–840. Available online: https://www.tandfonline.com/doi/full/10.1080/00914037.2016.1171221 (accessed on 16 March 2023).
- Bolagam, R.; Boddula, R.; Srinivasan, P. Incorporation of graphene-Mn3O4 core into polyaniline shell: Supercapacitor electrode materia. Ionics 2018, 24, 1467–1474. [Google Scholar] [CrossRef]
- Bolagam, R.; Boddula, R.; Srinivasan, P. Simultaneous oxidation and doping of aniline to polyaniline by oxidative template: Electrochemical performance in supercapacitor. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 939–945. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, M.; Tong, L.; Cai, K. Polypyrrole/nylon membrane composite film for ultra-flexible all solid supercapacitor. J. Mater. 2020, 6, 339–347. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, S.; Liu, Y.; Yan, W.; Lin, S.; Cheng, G.; Yang, H.; Luo, J. Room-temperature fabrication of a nickel-functionalized copper metal–organic framework (Ni@ Cu-MOF) as a new pseudocapacitive material for asymmetric supercapacitors. Polymers 2019, 11, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Wang, G.; Zhao, Y.; Liu, Y.; Wu, Y.; Zhang, Y. A stretchable, asymmetric, coaxial fiber-shaped supercapacitor for wearable electronics. Nano Res. 2020, 13, 1686–1692. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, J.; Wang, X.; Lu, B.; Chen, M.; Liu, M. Construction and characterizations of hollow carbon microsphere@polypyrrole composite for the high performance supercapacitor. J. Energy Storage 2018, 18, 62–71. [Google Scholar] [CrossRef]
- Zheng, Y.; Pann, W.; Zhengn, D.; Sun, C. Fabrication of functionalized graphene-based MnO2 nanoflower through electrodeposition for high-performance supercapacitor electrodes. J. Electrochem. Soc. 2016, 163, D230. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Ren, J.; Wang, Y.; Wei, W. An effective interaction in polypyrrole/nickel phosphide (PPy/Ni2P) for high-performance supercapacitor. J. Solid State Electrochem. 2019, 23, 3409–3418. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.J.; Xia, W.X.; Akase, Z.; Shindo, D. Electron holography on charging effect in non-conductive materials. Mater. Sci. Forum 2007, 561, 2075–2078. [Google Scholar] [CrossRef]
- Chena, Y.; Chen, C.; Lv, R.; Shen, W.; Kang, F.; Tai, N.; Huang, Z. Flexible asymmetric supercapacitor based on MnO2 honeycomb structure. Chin. Chem. Lett. 2018, 29, 616–619. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene-MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Lösche, M.; Schmitt, J.; Decher, G.; Bouwman, W.G.; Kjaer, K. Detailed structure of molecularly thin polyelectrolyte multilayer films on solid substrates as revealed by neutron reflectometry. Macromolecules 1998, 31, 8893–8906. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.; Ma, Y. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- González-Tejera, M.; De Plaza, M.L.; Sánchez La Blanca, E.D.; Hernández-Fuentes, I. Electrochemical, FTIR and morphological study of polypyrrole-polystyrenesulphonate conducting films. Polym. Int. 1993, 31, 45–50. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.C. Graphene and nanostructured MnO2 composite electrodes for supercapacitors. Carbon 2011, 49, 2917–2925. [Google Scholar] [CrossRef]
- Ko, J.M.; Nam, J.H.; Won, J.H.; Kim, K.M. Supercapacitive properties of electrodeposited polyaniline electrode in acrylic gel polymer electrolytes. Synth. Met. 2014, 189, 152–156. [Google Scholar] [CrossRef]
- Aoki, K.; Honda, K.; Tokuda, K.; Matsuda, H. Voltammetry at microcylinder electrodes: Part I. Linear sweep voltammetry. J. Electroanal. Chem. 1985, 182, 267–279. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, C.; Fan, L.; Rao, W.; Xu, W.; Liang, H.; Xu, J. Polypyrrole/titania-coated cotton fabrics for flexible supercapacitor electrodes. Appl. Surf. Sci. 2018, 460, 84–91. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, R.; Li, G.; Lu, H.; Liu, Z.; Xiao, F.; Zhang, M.; Tong, Y. Polyaniline nanotube arrays as high-performance flexible electrodes for electrochemical energy storage devices. J. Mater. Chem. 2012, 22, 2401–2404. [Google Scholar] [CrossRef]
- Tong, H.; Li, T.; Liu, J.; Gong, D.; Xiao, J.; Shen, L.; Ding, B.; Zhang, X. Fabrication of the oxygen vacancy amorphous MnO2/Carbon nanotube as cathode for advanced aqueous zinc-ion batteries. Energy Technol. 2021, 9, 2000769. [Google Scholar] [CrossRef]
- Moazzen, E.; Timofeeva, E.V.; Segre, C.U. Controlled synthesis of MnO2 nanoparticles for aqueous battery cathodes: Polymorphism-capacity correlation. J. Mater. Sci. 2017, 52, 8107–8118. [Google Scholar] [CrossRef]
- Ameri, B.; Davarani, S.S.H.; Roshani, R.; Moazami, H.R.; Tadjarodi, A. A flexible mechanochemical route for the synthesis of copper oxide nanorods/nanoparticles/nanowires for supercapacitor applications: The effect of morphology on the charge storage ability. J. Alloys Compd. 2017, 695, 114–123. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Noh, J.; Yoon, C.M.; Kim, Y.K.; Jang, J. High performance asymmetric supercapacitor twisted from carbon fiber/MnO2 and carbon fiber/MoO3. Carbon 2017, 116, 470–478. [Google Scholar] [CrossRef]
- Gnanakan, S.; Karthikeyan, K.; Amaresh, S.; Cho, S.; Park, G.; Lee, Y. New application and electrochemical characterization of a nickel-doped mesoporous carbon for supercapacitors. J. Alloys Compd. 2011, 509, 9858–9864. [Google Scholar] [CrossRef]
- Banerjee, J.; Dutta, K.; Kader, M.A.; Nayak, S.K. An overview on the recent developments in polyaniline-based supercapacitors. Polym. Adv. Technol. 2019, 30, 1902–1921. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y. Addressing the Achilles’ heel of pseudocapacitive materials: Long-term stability. InfoMat 2020, 2, 807–842. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, X.; Wang, J.; Seveno, D.; Fransaer, J.; Locquet, J.-P.; Seo, J.W. Carbon nanotube fibers decorated with MnO2 for wire-shaped supercapacitor. Molecules 2021, 26, 3479. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, C.; Yu, H.; Cai, S.; Xu, Y.; Yang, Y.; Chang, H. All-solid-state wire-shaped asymmetric supercapacitor based on binder-free CuO nanowires on copper wire and PPy on carbon fiber electrodes. J. Electroanal. Chem. 2021, 893, 115323. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, Y.; Lu, W.; Yarlagadda, S.; Xu, G. High-performance flexible asymmetric fiber-shaped supercapacitor based on CF/PPy and CNT/MnO2 composite electrodes. ACS Appl. Energ. Mater. 2021, 4, 10639–10645. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Fu, C.; Wang, Z.; Huang, Y.; Zhu, M.; Zhi, C.; Hu, H. High-performance stretchable yarn supercapacitor based on PPy@CNTs@urethane elastic fiber core spun yarn. Nano Energy 2016, 27, 230–237. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Sun, Y.; Yang, H.; Liu, X.; Huang, Y. Flexible fiber-shaped supercapacitors based on hierarchically nanostructured composite electrodes. Nano Res. 2015, 8, 1148–1158. [Google Scholar] [CrossRef]
- Guo, K.; Ma, Y.; Li, H.; Zhai, T. Flexible wire-shaped supercapacitors in parallel double helix configuration with stable electrochemical properties under static/dynamic bending. Small 2016, 12, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Song, M.K.; Park, Y.J.; Kim, J.M.; Liu, M.; Wang, Z.L. Fiber supercapacitors made of nanowire-fiber hybrid structures for wearable/flexible energy storage. Angew. Chem. Int. Ed. 2011, 50, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Jiang, K.; Zhai, G.; Ji, H.; Li, N.; Zhu, J.; Yang, C.; Qiu, F.; Lu, C.; Wang, T.; et al. A novel 2D conjugated coordination framework with a narrow bandgap for micro-supercapacitors. Energy Technol. 2022, 10, 2200133. [Google Scholar] [CrossRef]
- Wu, C.; Unnikrishnan, B.; Chen, I.; Harroun, S.G.; Chang, H.T.; Huang, C.C. Excellent oxidation resistive MXene aqueous ink for micro-supercapacitor application. Energy Storage Mater. 2020, 25, 563–571. [Google Scholar] [CrossRef]



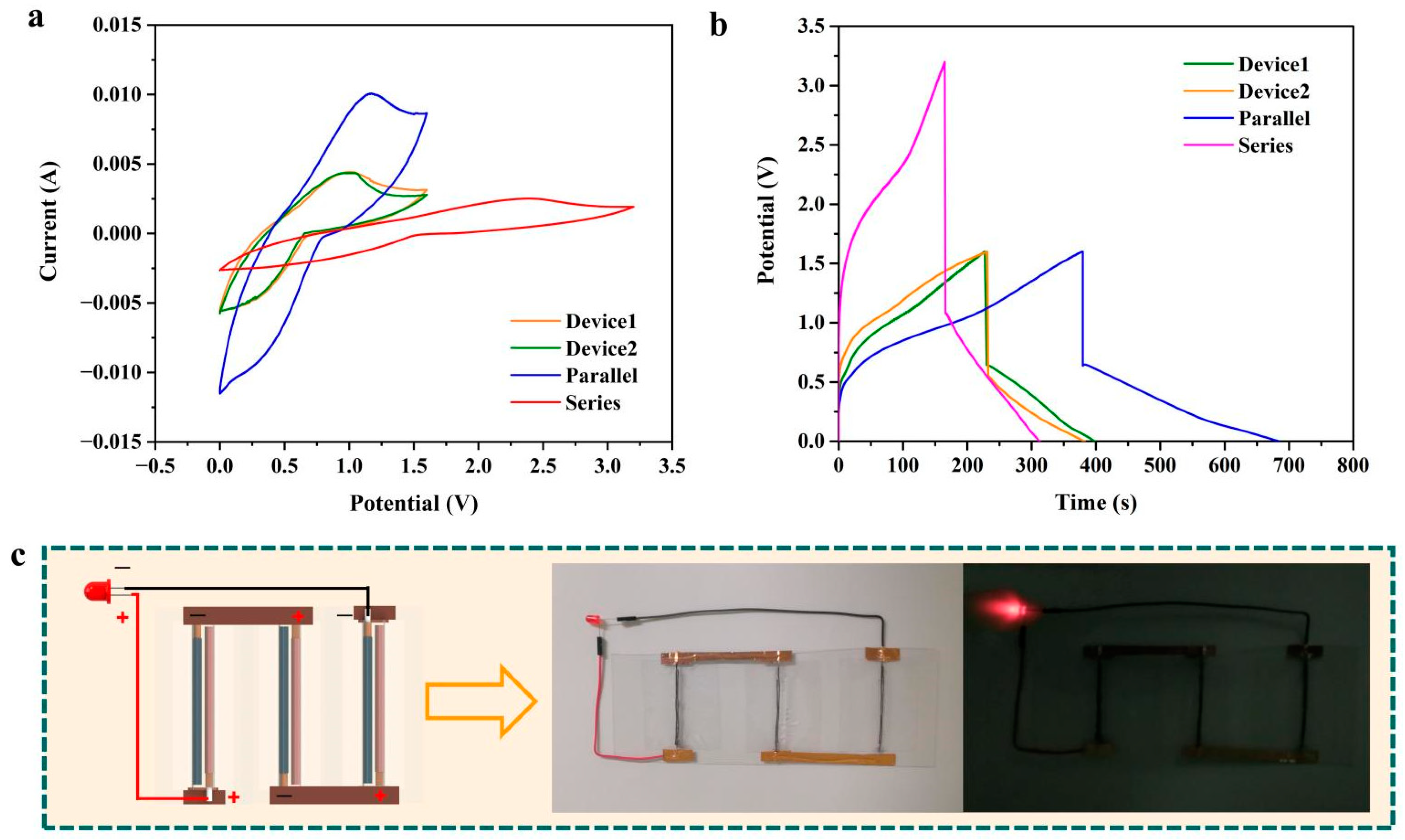

| CV Test (40 mV s−1) | GCD Test (1 mA) | |||||
|---|---|---|---|---|---|---|
| Theoretical Value/mF | Experimental Value/mF | Error | Theoretical Value/mF | Experimental Value/mF | Error | |
| Device1 | - | 61.4 | - | - | 260.9 | - |
| Device2 | - | 60.8 | - | - | 272.6 | - |
| Parallel | 122.2 | 137.7 | 12.7% | 533.5 | 499.5 | −6.8% |
| Series | 30.5 | 32.4 | 6.2% | 133.3 | 136.5 | 2.4% |
| Electrodes | Configuration | Gel Electrolyte | Potential Window (V) | CL (mF cm−1) | CA (mF cm−2) | Ref. |
|---|---|---|---|---|---|---|
| nylon/Ag/MnO2 ||nylon/Ag/PPy | Parallel | 0.5 M PAANa/Na2SO4 | 0–1.6 | 38.9 | 181.7 | This work |
| CNT/MnO2 ||CNT/MnO2 | Parallel | 2 M PVA/H3PO4 | 0–1.0 | 0.75 | - | [47] |
| CF/MnO2||CF/MoO3 | Twisted | 1 M PVA/KOH | 0–2.0 | - | 4.86 | [43] |
| Cu wire/CuO NWs ||CF/PPy | Twisted | 2 M PVA/KOH | 0–1.3 | 24.91 | 39.67 | [48] |
| CF/PPy ||CNT/MnO2 | Coaxial | 5 M PVA/LiCl | 0–1.6 | 13.5 | 66.3 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, X.; Cai, S.; Tao, K.; Xu, Y. A Solid-State Wire-Shaped Supercapacitor Based on Nylon/Ag/Polypyrrole and Nylon/Ag/MnO2 Electrodes. Polymers 2023, 15, 1627. https://doi.org/10.3390/polym15071627
Zhang R, Wang X, Cai S, Tao K, Xu Y. A Solid-State Wire-Shaped Supercapacitor Based on Nylon/Ag/Polypyrrole and Nylon/Ag/MnO2 Electrodes. Polymers. 2023; 15(7):1627. https://doi.org/10.3390/polym15071627
Chicago/Turabian StyleZhang, Ruirong, Xiangao Wang, Sheng Cai, Kai Tao, and Yanmeng Xu. 2023. "A Solid-State Wire-Shaped Supercapacitor Based on Nylon/Ag/Polypyrrole and Nylon/Ag/MnO2 Electrodes" Polymers 15, no. 7: 1627. https://doi.org/10.3390/polym15071627
APA StyleZhang, R., Wang, X., Cai, S., Tao, K., & Xu, Y. (2023). A Solid-State Wire-Shaped Supercapacitor Based on Nylon/Ag/Polypyrrole and Nylon/Ag/MnO2 Electrodes. Polymers, 15(7), 1627. https://doi.org/10.3390/polym15071627







