Preparation and Properties of Hydrophobic Polyurethane Based on Silane Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of Waterborne Polyurethane Emulsion (WPU)
2.2.2. Synthesis of Silane-Modified Waterborne Polyurethane Acrylate Emulsion (SWPUA)
2.2.3. Preparation of Coating Film
2.2.4. Preparation of Paint Film
2.3. Characterization
3. Results and Discussion
3.1. FT-IR Analysis
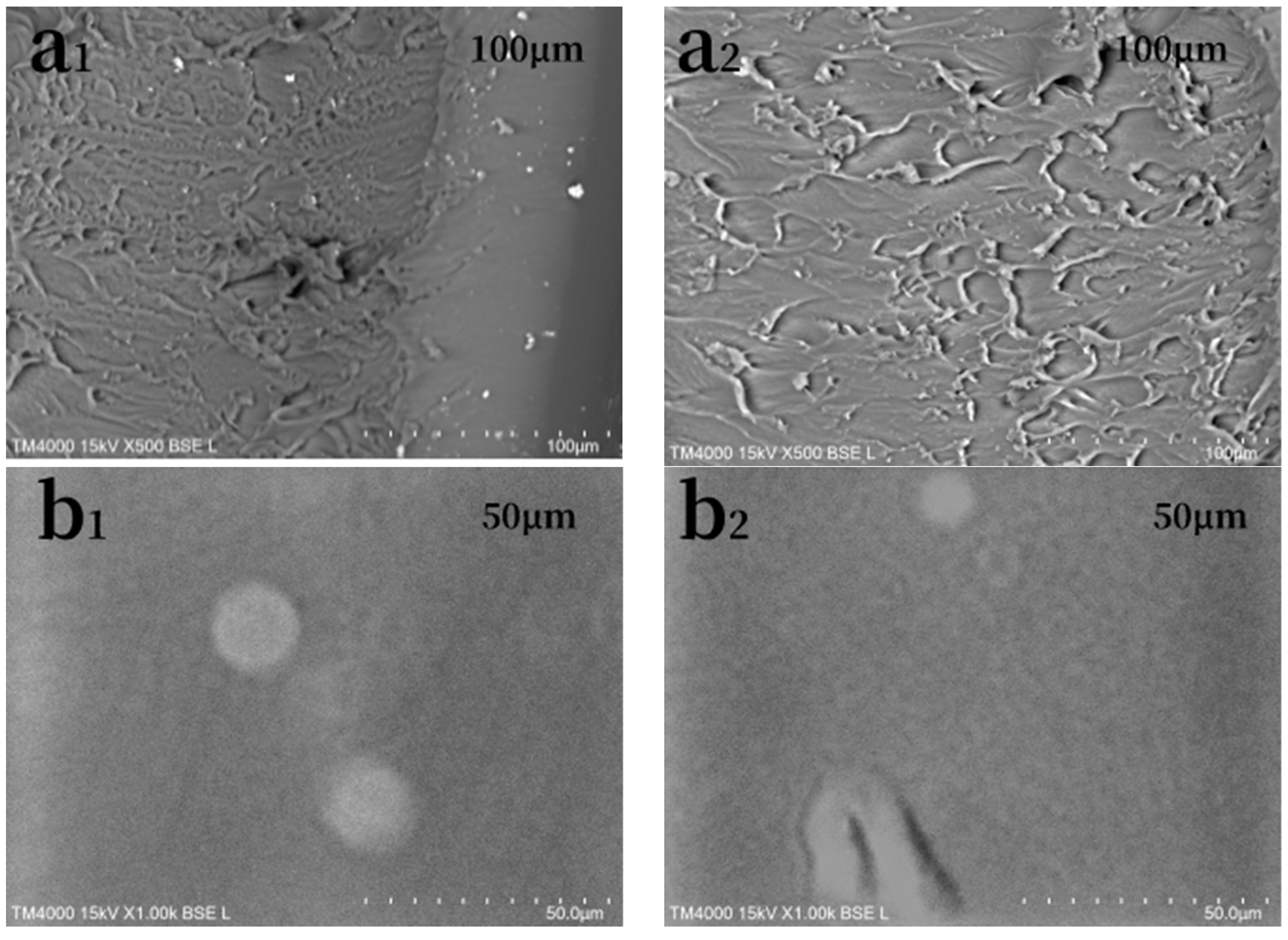
3.2. Surface Morphology Analysis
3.3. Particle Size and Stability Analysis
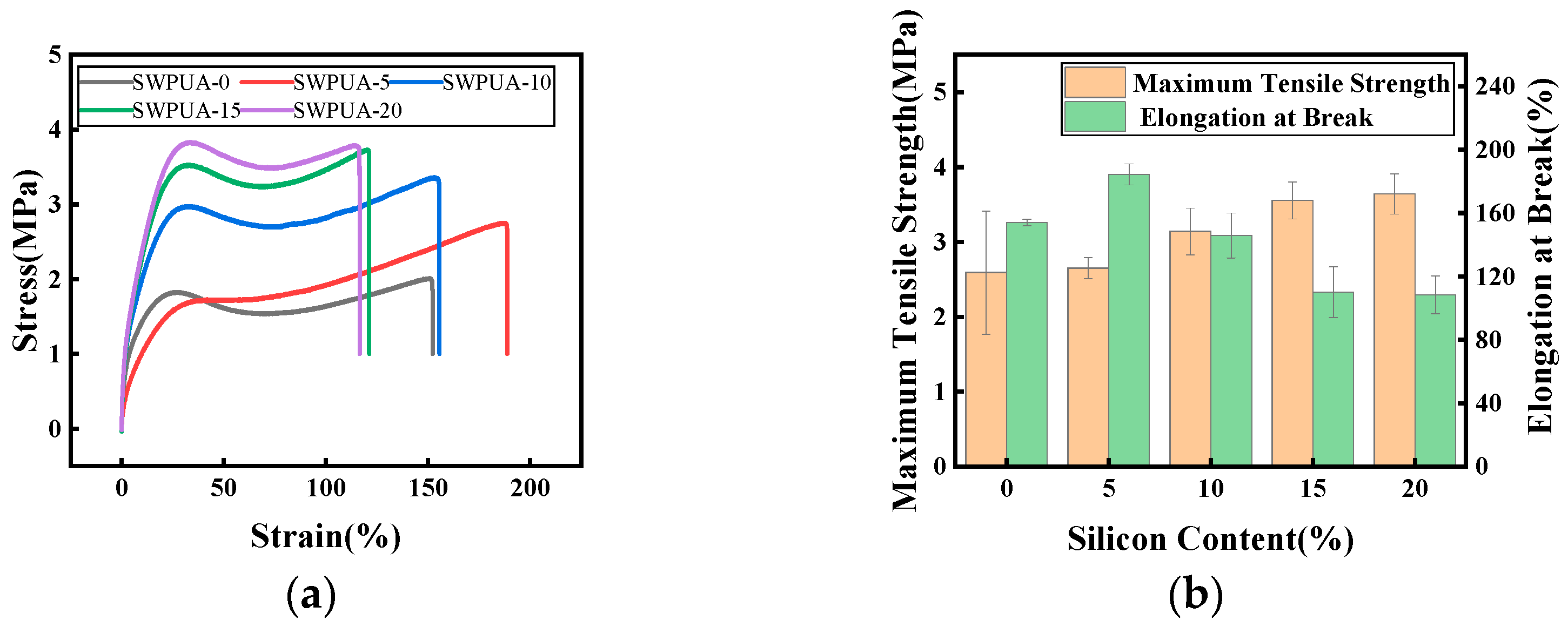
3.4. Mechanical Performance Analysis
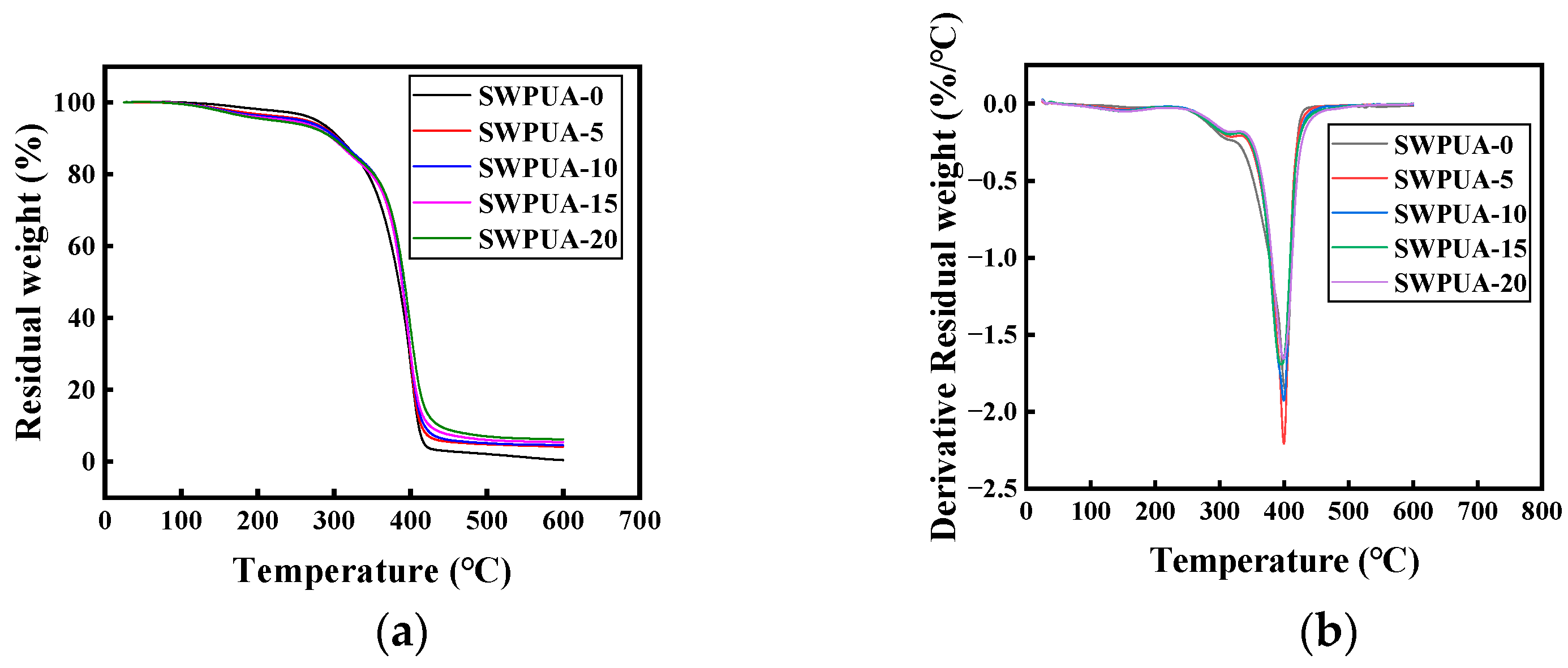
3.5. TG Analysis
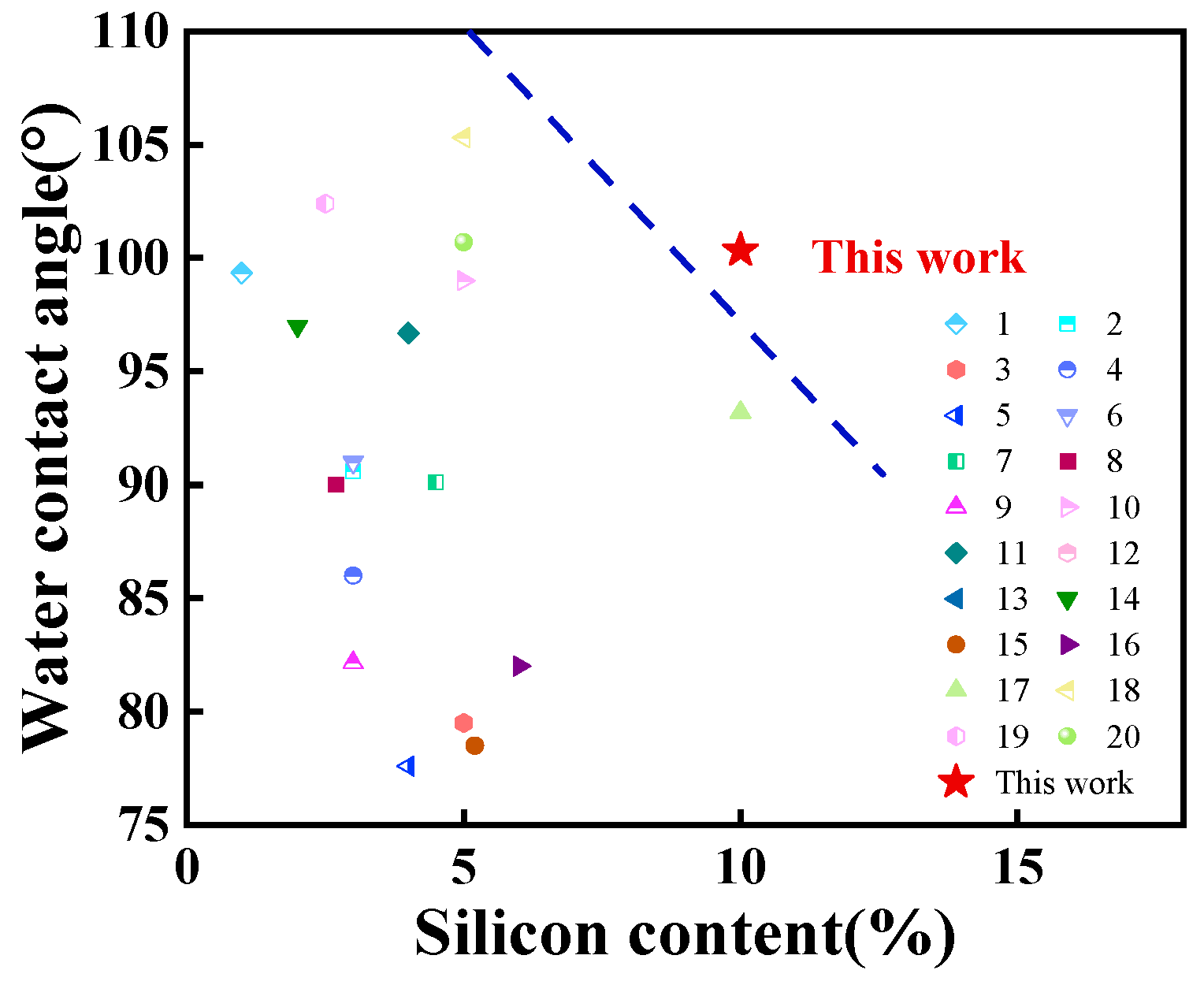
3.6. Water Absorption and Water Contact Angle Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhan, X.; Chen, J.; Yang, Z.; Wu, G.; Kong, Z. Superhydrophobic film from silicone-modified nanocellulose and waterborne polyurethane through simple sanding process. Int. J. Biol. Macromol. 2023, 232, 123431. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Li, Y.; Wu, Z.; Yi, Z. Synthesis of waterborne polyurethane by inserting polydimethylsiloxane and constructing dual crosslinking for obtaining the superior performance of waterborne coatings. Compos. Part B Eng. 2022, 238, 109889. [Google Scholar] [CrossRef]
- Tsupphayakorn-aek, P.; Suwan, A.; Tulyapitak, T.; Saetung, N.; Saetung, A. A novel UV-curable waterborne polyurethane-acrylate coating based on green polyol from hydroxyl telechelic natural rubber. Prog. Org. Coat. 2022, 163, 106585. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Sobhani, S.; Croutxé-Barghorn, C.; Allonas, X.; Bastani, S. Siloxane-modified waterborne UV-curable polyurethane acrylate coatings: Chemorheology and viscoelastic analyses. Prog. Org. Coat. 2021, 158, 106323. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Wang, Y.; Ban, T.; Zhang, Y.; Zhang, J.; Zhu, X. Preparation and characteristics of crosslinked fluorinated acrylate modified waterborne polyurethane for metal protection coating. Prog. Org. Coat. 2021, 158, 106371. [Google Scholar] [CrossRef]
- Hong, C.; Zhou, X.; Ye, Y.; Li, W. Synthesis and characterization of UV-curable waterborne Polyurethane–acrylate modified with hydroxyl-terminated polydimethylsiloxane: UV-cured film with excellent water resistance. Prog. Org. Coat. 2021, 156, 106251. [Google Scholar] [CrossRef]
- Hwang, H.-D.; Kim, H.-J. Enhanced thermal and surface properties of waterborne UV-curable polycarbonate-based polyurethane (meth) acrylate dispersion by incorporation of polydimethylsiloxane. React. Funct. Polym. 2011, 71, 655–665. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Lai, X.; Li, Z.; Liu, M. Synthesis and properties of crosslinked waterborne polyurethane. J. Polym. Res. 2011, 18, 469–476. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, X.; Cheng, F.; Fan, Y.; Wu, Y.; Yang, X. Fabrication and performance of UV cured transparent silicone modified polyurethane–acrylate coatings with high hardness, good thermal stability and adhesion. Prog. Org. Coat. 2020, 144, 105673. [Google Scholar] [CrossRef]
- Ren, L.; Yu, S.; Niu, Q.; Qiang, T. Construction of amphiphilic comb-like waterborne polyurethane to balance emulsion stability and film hydrophobicity. Prog. Org. Coat. 2022, 173, 107197. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Q.; Zhang, Y.; Wang, Y.; Zhou, Z.; Zhang, P.; Yuan, B. Waterborne polyurethane/3-amino-polyhedral oligomeric silsesquioxane (NH 2-POSS) nanocomposites with enhanced properties. Adv. Compos. Hybrid Mater. 2021, 4, 629–638. [Google Scholar] [CrossRef]
- Zheng, G.; Lu, M.; Rui, X. The effect of polyether functional polydimethylsiloxane on surface and thermal properties of waterborne polyurethane. Appl. Surf. Sci. 2017, 399, 272–281. [Google Scholar] [CrossRef]
- Wang, Y.; An, Q.; Yang, B. Synthesis of UV-curable polyurethane acrylate modified with polyhedral oligomeric silsesquioxane and fluorine for iron cultural relic protection coating. Prog. Org. Coat. 2019, 136, 105235. [Google Scholar] [CrossRef]
- Karna, N.; Joshi, G.M.; Mhaske, S. Structure-property relationship of silane-modified polyurethane: A review. Prog. Org. Coat. 2023, 176, 107377. [Google Scholar] [CrossRef]
- Deng, F.; Qin, S.; Liu, N.; Xu, W. The Synergistic Effect of Terminal and Pendant Fluoroalkyl Segments on Properties of Polyurethane Latex and Its Film. Coatings 2022, 12, 1271. [Google Scholar] [CrossRef]
- Gurunathan, T.; Chung, J.S. Synthesis of aminosilane crosslinked cationomeric waterborne polyurethane nanocomposites and its physicochemical properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 124–132. [Google Scholar] [CrossRef]
- Gaddam, S.K.; Kutcherlapati, S.R.; Palanisamy, A. Self-cross-linkable anionic waterborne polyurethane–silanol dispersions from cottonseed-oil-based phosphorylated polyol as ionic soft segment. ACS Sustain. Chem. Eng. 2017, 5, 6447–6455. [Google Scholar] [CrossRef]
- Lyu, J.; Xu, K.; Zhang, N.; Lu, C.; Zhang, Q.; Yu, L.; Feng, F.; Li, X. In situ incorporation of diamino silane group into waterborne polyurethane for enhancing surface hydrophobicity of coating. Molecules 2019, 24, 1667. [Google Scholar] [CrossRef]
- Fu, C.; Yang, Z.; Zheng, Z.; Shen, L. Properties of alkoxysilane castor oil synthesized via thiol-ene and its polyurethane/siloxane hybrid coating films. Prog. Org. Coat. 2014, 77, 1241–1248. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y.; Ou, C.; Xia, Z.; Zhong, L. Synthesis and characterization of waterborne polyurethanes with alkoxy silane groups in the side chains for potential application in waterborne ink. Prog. Org. Coat. 2016, 92, 85–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, R.; Shi, Y.; Li, H.; Liu, Y.; Zhou, C. Synthesis and surface migration of polydimethylsiloxane and perfluorinated polyether in modified waterborne polyurethane. Polym. Bull. 2019, 76, 5517–5535. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, D.; Hao, T.H.; Hu, G.H.; Ye, G.B.; Jiang, T.; Zhang, Q.C. Synthesis and investigation of well-defined silane terminated and segmented waterborne hybrid polyurethanes. New J. Chem. 2017, 41, 9268–9275. [Google Scholar] [CrossRef]
- Wu, Y.; Du, Z.; Wang, H.; Cheng, X. Synthesis of aqueous highly branched silica sol as underlying crosslinker for corrosion protection. Prog. Org. Coat. 2017, 111, 381–388. [Google Scholar] [CrossRef]
- Zhang, P.; Fan, H.; Tian, S.; Chen, Y.; Yan, J. Synergistic effect of phosphorus–nitrogen and silicon-containing chain extenders on the mechanical properties, flame retardancy and thermal degradation behavior of waterborne polyurethane. RSC Adv. 2016, 6, 72409–72422. [Google Scholar] [CrossRef]
- Jing, R.; Wen, Y.; Yu, L.; Zhu, P. Synthesis and Properties of Vinyl Silane Coupling Agent Modified Waterborne Polyurethane. Leather Sci. Eng. 2015, 25, 45–49. [Google Scholar]
- Liu, Y.; Wei, M. Synthesis and Characterization of Pure Acrylic Emulsion Modified with Silane Coupling Agent. Paint. Coat. Ind. 2019, 42, 6–9. [Google Scholar]
- Jiao, C.; Shao, Q.; Wu, M.; Zheng, B.; Guo, Z.; Yi, J.; Zhang, J.; Lin, J.; Wu, S.; Dong, M.; et al. 2-(3, 4-Epoxy) ethyltriethoxysilane-modified waterborne acrylic resin: Preparation and property analysis. Polymer 2020, 190, 122196. [Google Scholar] [CrossRef]
- GB/T1727.92; General Preparation Method of Paint Film. Standardization Administration of the People’s Republic of China: Beijing, China, 1992.
- Zhang, F.; Liu, W.; Liang, L.; Liu, C.; Wang, S.; Shi, H.; Xie, Y.; Yang, M.; Pi, K. Applications of hydrophobic α, ω-bis (amino)-terminated polydimethylsiloxane-graphene oxide in enhancement of anti-corrosion ability of waterborne polyurethane. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124981. [Google Scholar] [CrossRef]
- Rao, Z.; Yan, H.; Tao, W.; Liu, C.; Jian, G.; Zhou, Y.; Chen, H.; Yang, M. Effects of aminopropyl-terminated polydimethylsiloxane on structure and properties of waterborne polyurethane-acrylate. Prog. Org. Coat. 2023, 174, 107314. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, R.; Li, H.; Shi, Y.; Liu, Y.; Zhou, C. Investigation of the migration ability of silicone and properties of waterborne polyurethanes. Int. J. Adhes. Adhes. 2020, 102, 102654. [Google Scholar] [CrossRef]
- Li, Q.; Guo, L.; Qiu, T.; Xiao, W.; Du, D.; Li, X. Synthesis of waterborne polyurethane containing alkoxysilane side groups and the properties of the hybrid coating films. Appl. Surf. Sci. 2016, 377, 66–74. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Li, Z.; Shen, Y.; Wang, P.; Wang, D.; Wang, X.; Hu, X. Preparation and properties of UV-curable waterborne silicon-containing polyurethane acrylate emulsion. Prog. Org. Coat. 2021, 160, 106503. [Google Scholar] [CrossRef]
- Yu, X.; Xiong, Y.; Zhou, C.; Li, G.; Ren, S.; Li, Z.; Tang, H. Polyurethane with tris (trimethylsiloxy) silyl propyl as the side chains: Synthesis and properties. Prog. Org. Coat. 2020, 142, 105605. [Google Scholar] [CrossRef]
- Chen, R.S.; Chang, C.J.; Chang, Y.H. Study on siloxane-modified polyurethane dispersions from various polydimethylsiloxanes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3482–3490. [Google Scholar] [CrossRef]
- Li, C.Y.; Chen, J.H.; Chien, P.C.; Chiu, W.Y.; Chen, R.S.; Don, T.M. Preparation of poly (IPDI-PTMO-siloxanes) and influence of siloxane structure on reactivity and mechanical properties. Polym. Eng. Sci. 2007, 47, 625–632. [Google Scholar] [CrossRef]
- Zong, J.; Zhang, Q.; Sun, H.; Yu, Y.; Wang, S.; Liu, Y. Characterization of polydimethylsiloxane–polyurethanes synthesized by graft or block copolymerizations. Polym. Bull. 2010, 65, 477–493. [Google Scholar] [CrossRef]
- Xu, C.A.; Qu, Z.; Tan, Z.; Nan, B.; Meng, H.; Wu, K.; Shi, J.; Lu, M.; Liang, L. High-temperature resistance and hydrophobic polysiloxane-based polyurethane films with cross-linked structure prepared by the sol-gel process. Polym. Test. 2020, 86, 106485. [Google Scholar] [CrossRef]
- Kim, M.-G.; Jo, K.-I.; Kim, E.; Park, J.-H.; Ko, J.-W.; Lee, J.H. Preparation of polydimethylsiloxane-modified waterborne polyurethane coatings for marine applications. Polymers 2021, 13, 4283. [Google Scholar] [CrossRef]
- Stefanović, I.S.; Džunuzović, J.V.; Džunuzović, E.S.; Brzić, S.J.; Jasiukaitytė-Grojzdek, E.; Basagni, A.; Marega, C. Tailoring the properties of waterborne polyurethanes by incorporating different content of poly (dimethylsiloxane). Prog. Org. Coat. 2021, 161, 106474. [Google Scholar] [CrossRef]
- Sui, Z.; Li, Y.; Guo, Z.; Zhang, Q.; Xu, Y.; Zhao, X. Preparation and properties of polysiloxane modified fluorine-containing waterborne polyurethane emulsion. Prog. Org. Coat. 2022, 166, 106783. [Google Scholar] [CrossRef]
- He, Z.; Xue, J.; Ke, Y.; Luo, Y.; Lu, Q.; Xu, Y.; Zhang, C. Enhanced Water Resistance Performance of Castor Oil—Based Waterborne Polyurethane Modified by Methoxysilane Coupling Agents via Thiol-Ene Photo Click Reaction. J. Renew. Mater. 2022, 10, 591. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Guo, M.; Bai, H.; Liu, X. Synthesis and characterization of waterborne UV-curable polyurethane modified with side-chain triethoxysilane and colloidal silica. Colloids Surf. A Physicochem. Eng. Asp. 2015, 468, 1–9. [Google Scholar] [CrossRef]
- Sun, D.; Miao, X.; Zhang, K.; Kim, H.; Yuan, Y. Triazole-forming waterborne polyurethane composites fabricated with silane coupling agent functionalized nano-silica. J. Colloid Interface Sci. 2011, 361, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Hu, X.; Yang, Z.; Shen, L.; Zheng, Z. Preparation and properties of waterborne bio-based polyurethane/siloxane cross-linked films by an in situ sol–gel process. Prog. Org. Coat. 2015, 84, 18–27. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, T.; Qu, X.; Ding, H.; Li, F. Synthesis of waterborne polyurethane modified by nano-SiO2 silicone and properties of the WPU coated RDX. China Pet. Process. Petrochem. Technol. 2015, 17, 39–45. [Google Scholar]
- Du, Y.; Yang, Z.; Zhou, C. Study on waterborne polyurethanes based on poly (dimethyl siloxane) and perfluorinated polyether. Macromol. Res. 2015, 23, 867–875. [Google Scholar] [CrossRef]
- Jiang, W.; Dai, A.; Zhou, T.; Xie, H. Hybrid polysiloxane/polyacrylate/nano-SiO2 emulsion for waterborne polyurethane coatings. Polym. Test. 2019, 80, 106110. [Google Scholar] [CrossRef]
- Christopher, G.; Kulandainathan, M.A.; Harichandran, G. Biopolymers nanocomposite for material protection: Enhancement of corrosion protection using waterborne polyurethane nanocomposite coatings. Prog. Org. Coat. 2016, 99, 91–102. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Z.; Zhou, T.; Cai, X.; Ren, X. Synthesis of silane coupling agent modified high solid waterborne polyurethane. China Synth. Resin Plast. 2012, 29, 16–20. [Google Scholar]
- Wang, C.; Wang, Q.; Dai, Z.; Xu, G. Research of Waterborne Polyurethane Modified With Silane Coupling Agent KH-602. Appl. Technol. 2010, 31, 23–27. [Google Scholar]
- Allauddin, S.; Narayan, R.; Raju, K. Synthesis and properties of alkoxysilane castor oil and their polyurethane/urea–silica hybrid coating films. ACS Sustain. Chem. Eng. 2013, 1, 910–918. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Javaherian Naghash, H. Synthesis and characterization of a novel hydroxyl-terminated polydimethylsiloxane for application in the silicone-modified acrylic-grafted polyester resins. J. Adhes. Sci. Technol. 2015, 29, 1341–1359. [Google Scholar] [CrossRef]
- Yu, Q.; Pan, P.; Du, Z.; Du, X.; Wang, H.; Cheng, X. The study of cationic waterborne polyurethanes modified by two different forms of polydimethylsiloxane. RSC Adv. 2019, 9, 7795–7802. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, X.; Zhang, H.; Liu, T.; Hong, C.; Ren, Q.; Zhou, C. Preparation of waterborne polyurethane-silica nanocomposites by a click chemistry method. Mater. Today Commun. 2020, 23, 100911. [Google Scholar] [CrossRef]
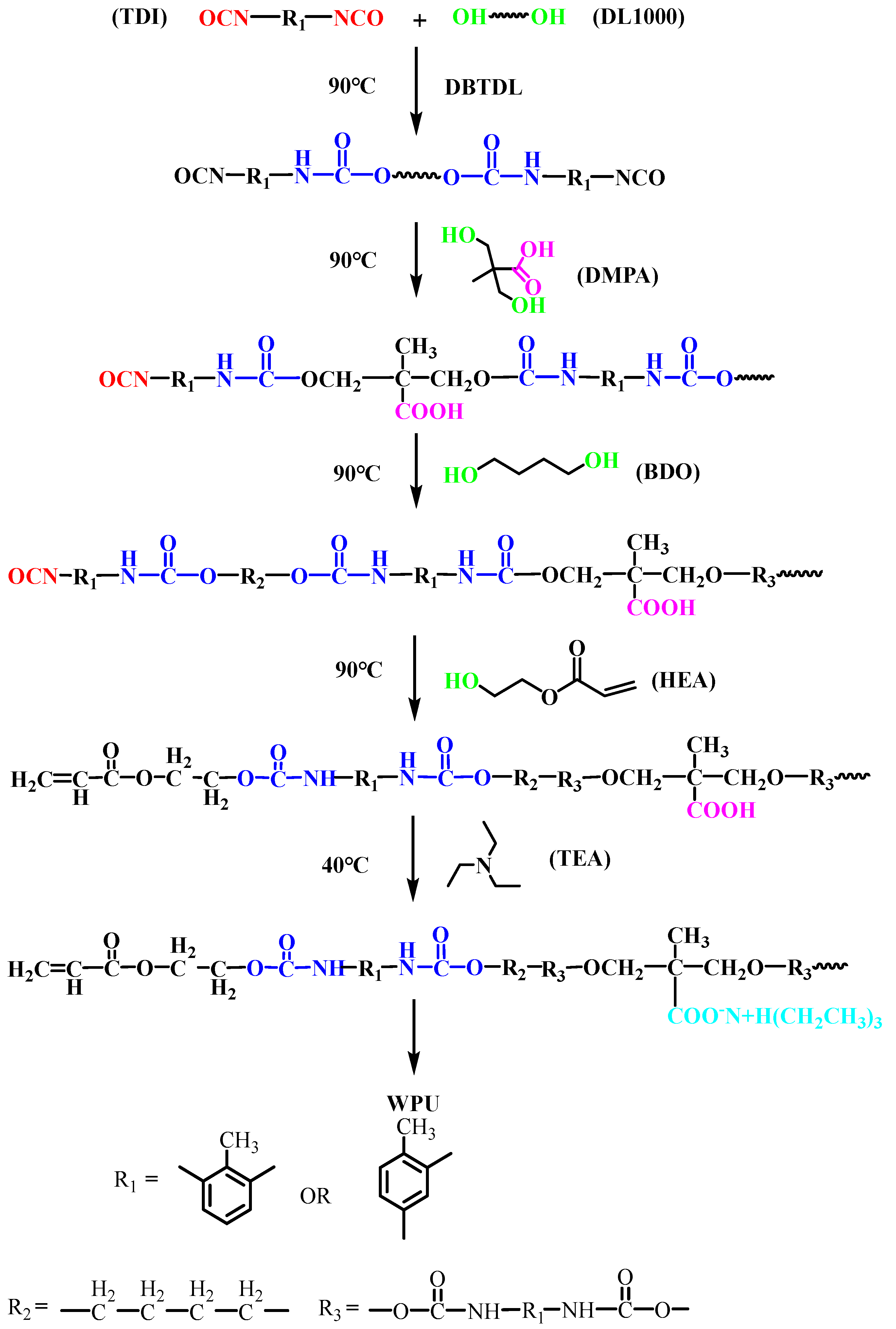




| WPU | MMA | BA | HEA | TMPTA | A172 | |
|---|---|---|---|---|---|---|
| g | g | g | g | g | ωA172/% | g |
| 100 | 39.9 | 18.6 | 1.5 | 1.2 | 0 | 0 |
| 100 | 39.9 | 18.6 | 1.5 | 1.2 | 5 | 3 |
| 100 | 39.9 | 18.6 | 1.5 | 1.2 | 10 | 6 |
| 100 | 39.9 | 18.6 | 1.5 | 1.2 | 15 | 9 |
| 100 | 39.9 | 18.6 | 1.5 | 1.2 | 20 | 12 |
| Sample | SWPUA-0 | SWPUA-5 | SWPUA-10 | SWPUA-15 | SWPUA-20 |
|---|---|---|---|---|---|
| Silicon content/% | / | 5 | 10 | 15 | 20 |
| Maximum tensile strength/MPa | 2.59 ± 0.82 | 2.65 ± 0.14 | 3.14 ± 0.31 | 3.56 ± 0.25 | 3.64 ± 0.27 |
| Elongation at break/% | 154 ± 2 | 184 ± 7 | 146 ± 14 | 110 ± 16 | 108 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, M.; Du, W.; Sun, S.; Zhao, B.; Cheng, Y. Preparation and Properties of Hydrophobic Polyurethane Based on Silane Modification. Polymers 2023, 15, 1759. https://doi.org/10.3390/polym15071759
Ma Y, Zhang M, Du W, Sun S, Zhao B, Cheng Y. Preparation and Properties of Hydrophobic Polyurethane Based on Silane Modification. Polymers. 2023; 15(7):1759. https://doi.org/10.3390/polym15071759
Chicago/Turabian StyleMa, Yuxian, Minghui Zhang, Wenhao Du, Shixiong Sun, Benbo Zhao, and Yuan Cheng. 2023. "Preparation and Properties of Hydrophobic Polyurethane Based on Silane Modification" Polymers 15, no. 7: 1759. https://doi.org/10.3390/polym15071759
APA StyleMa, Y., Zhang, M., Du, W., Sun, S., Zhao, B., & Cheng, Y. (2023). Preparation and Properties of Hydrophobic Polyurethane Based on Silane Modification. Polymers, 15(7), 1759. https://doi.org/10.3390/polym15071759






