Substantial Copper (Cu2+) Uptake by Metakaolin-Based Geopolymer and Its Resistance to Acid Leaching and Ion Exchange
Abstract
:1. Introduction
2. Materials, Experimental Procedures and Methods
2.1. Materials
2.2. Experimental Procedures
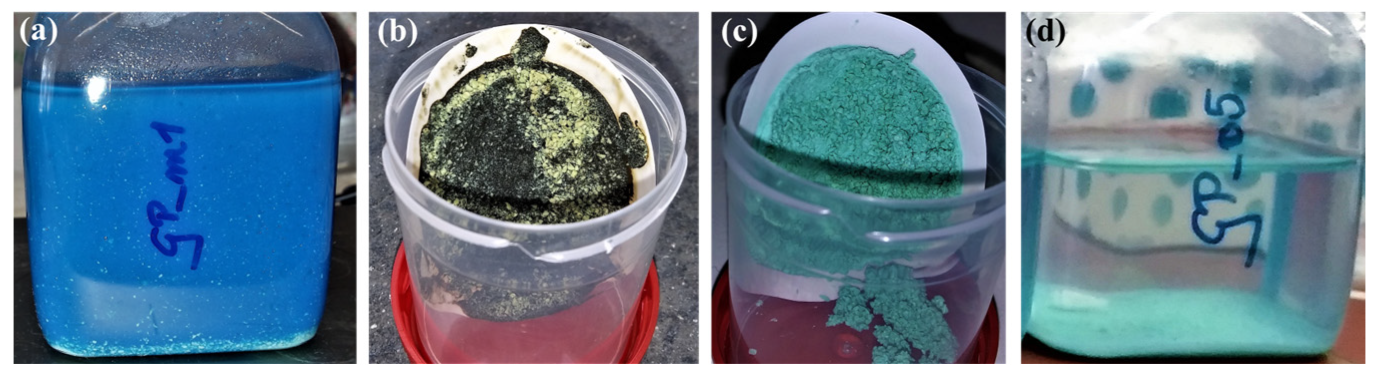
2.2.1. Cu Immobilization Experiments
2.2.2. Ion Exchange Experiments
2.2.3. Leaching Experiments
2.3. Analytical Methods
2.3.1. Fluid-Phase Characterization
2.3.2. Solid-Phase Characterization
3. Results and Discussion
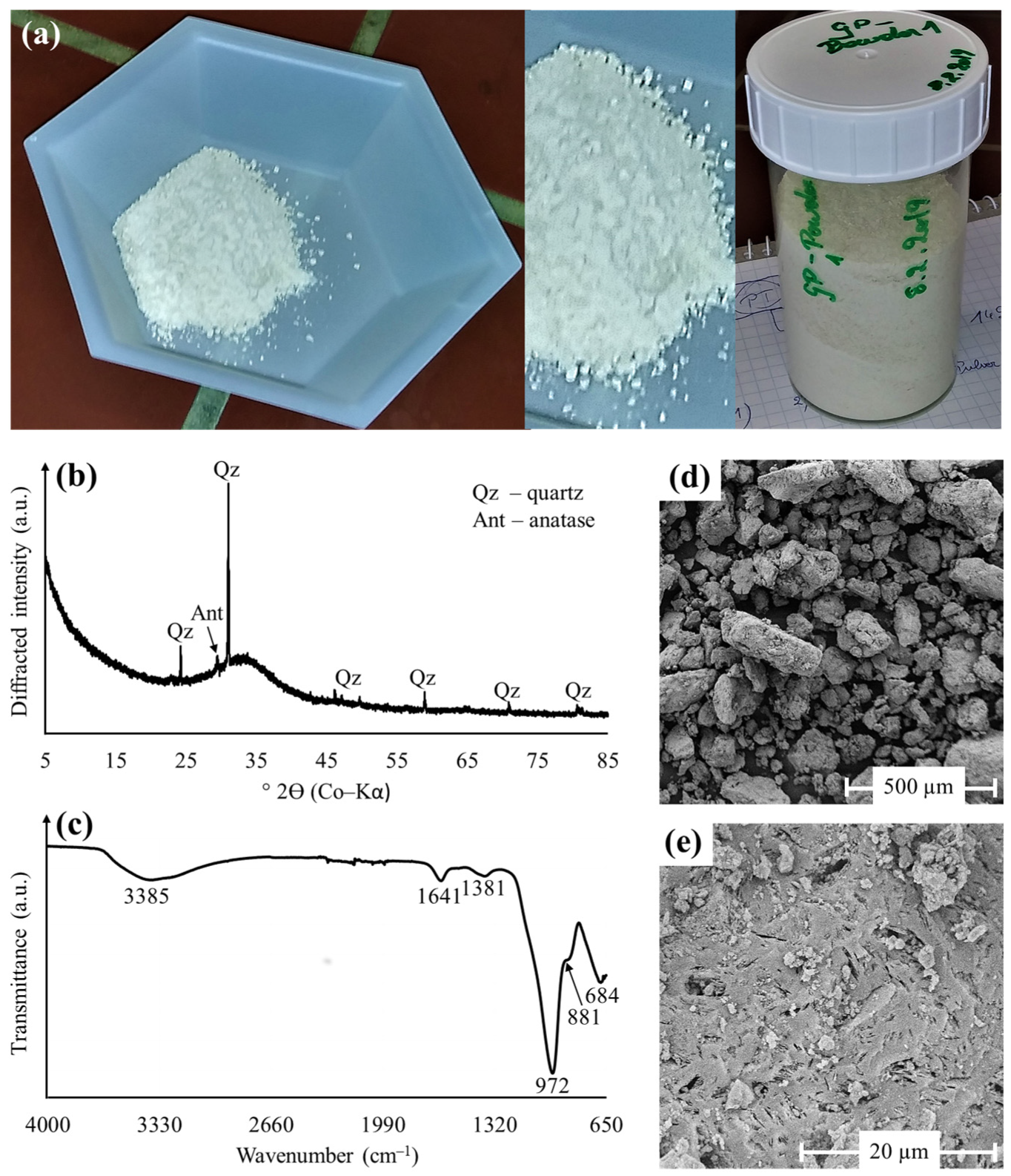
3.1. Geopolymer Characterization
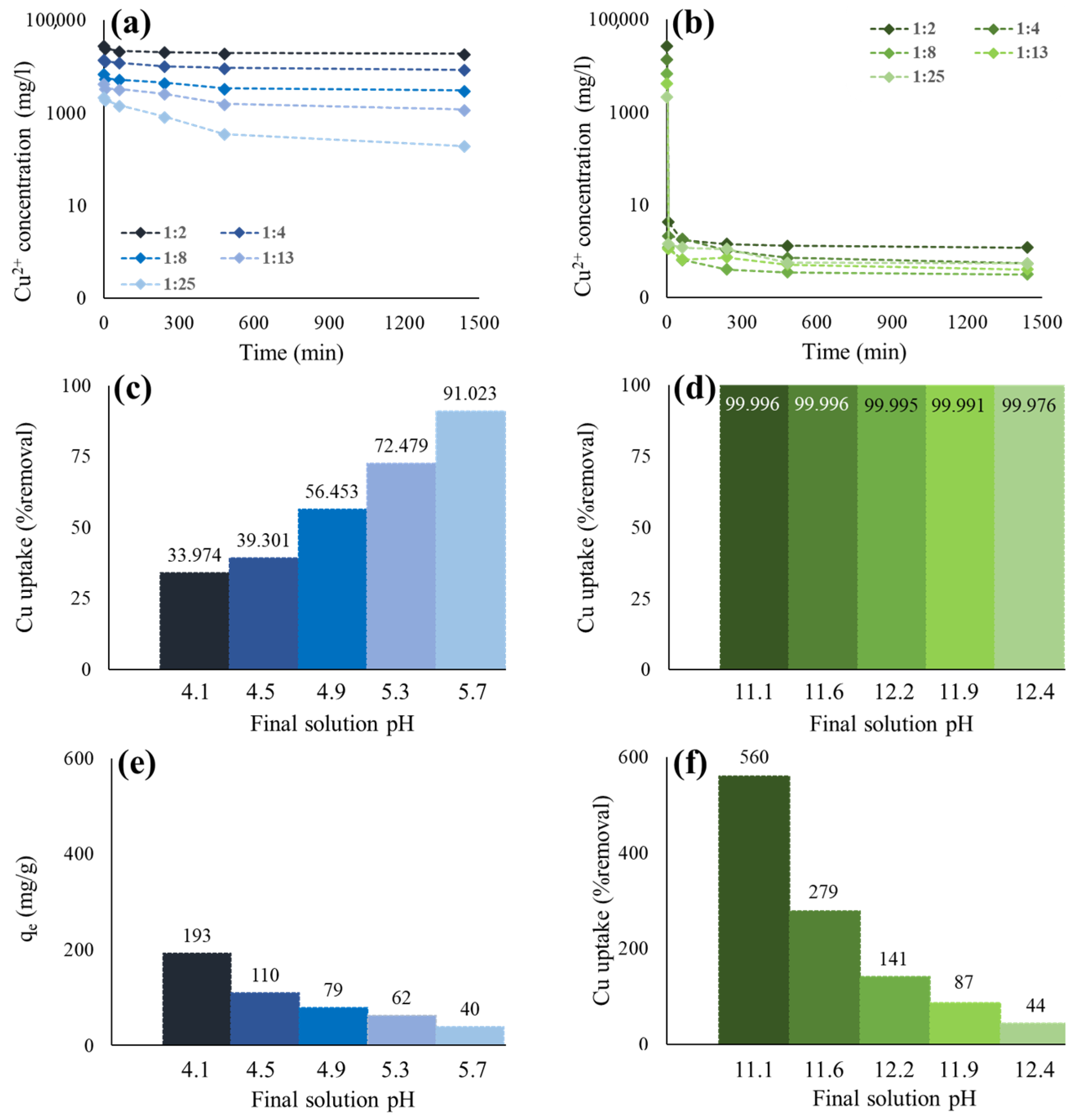
3.2. Cu2+ Immobilization by Geopolymers
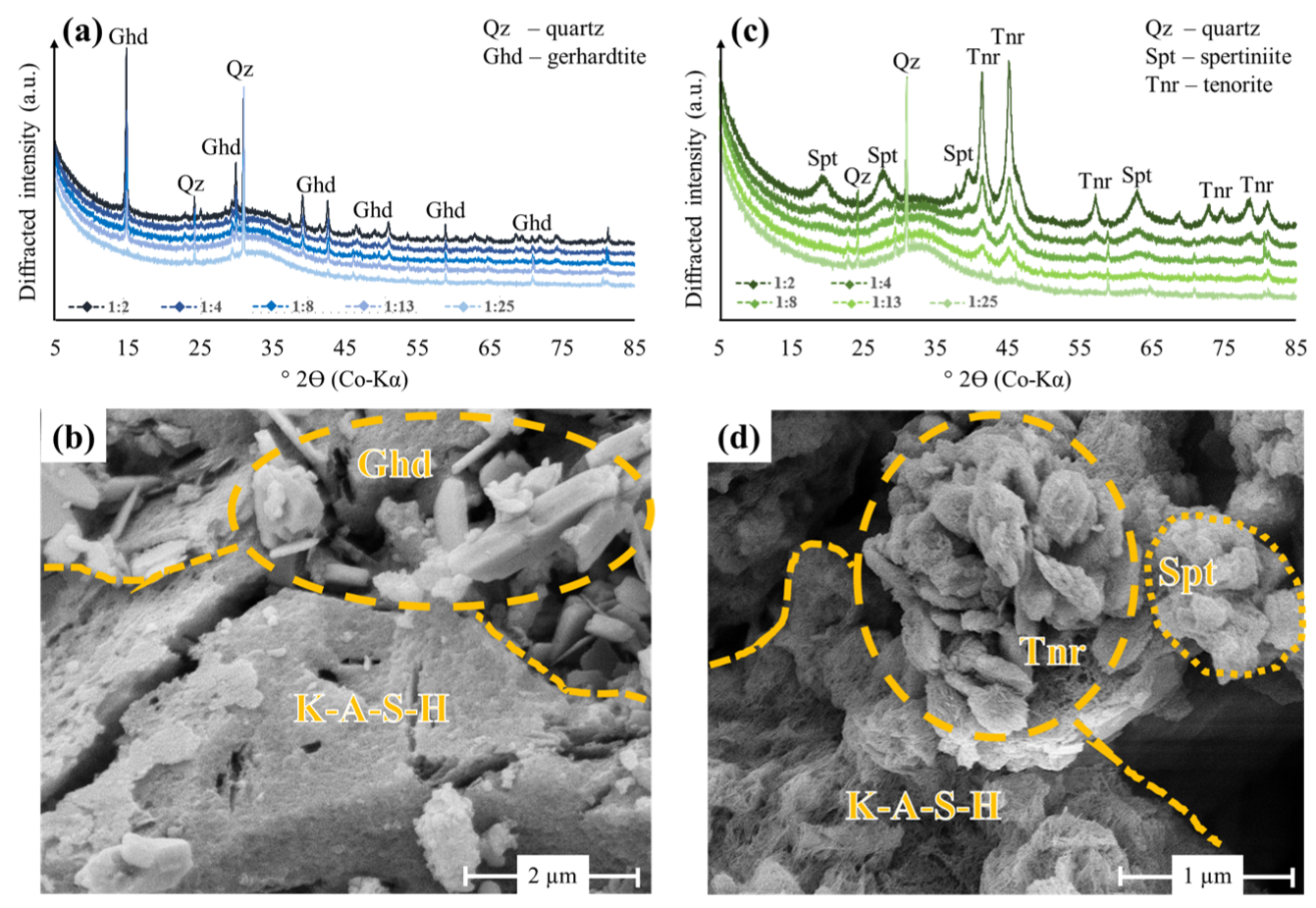
3.3. Mineralogy and Microstructure of Cu2+-Bearing Geopolymers
3.4. Chemical Resistance of Cu2+-Bearing Geopolymer
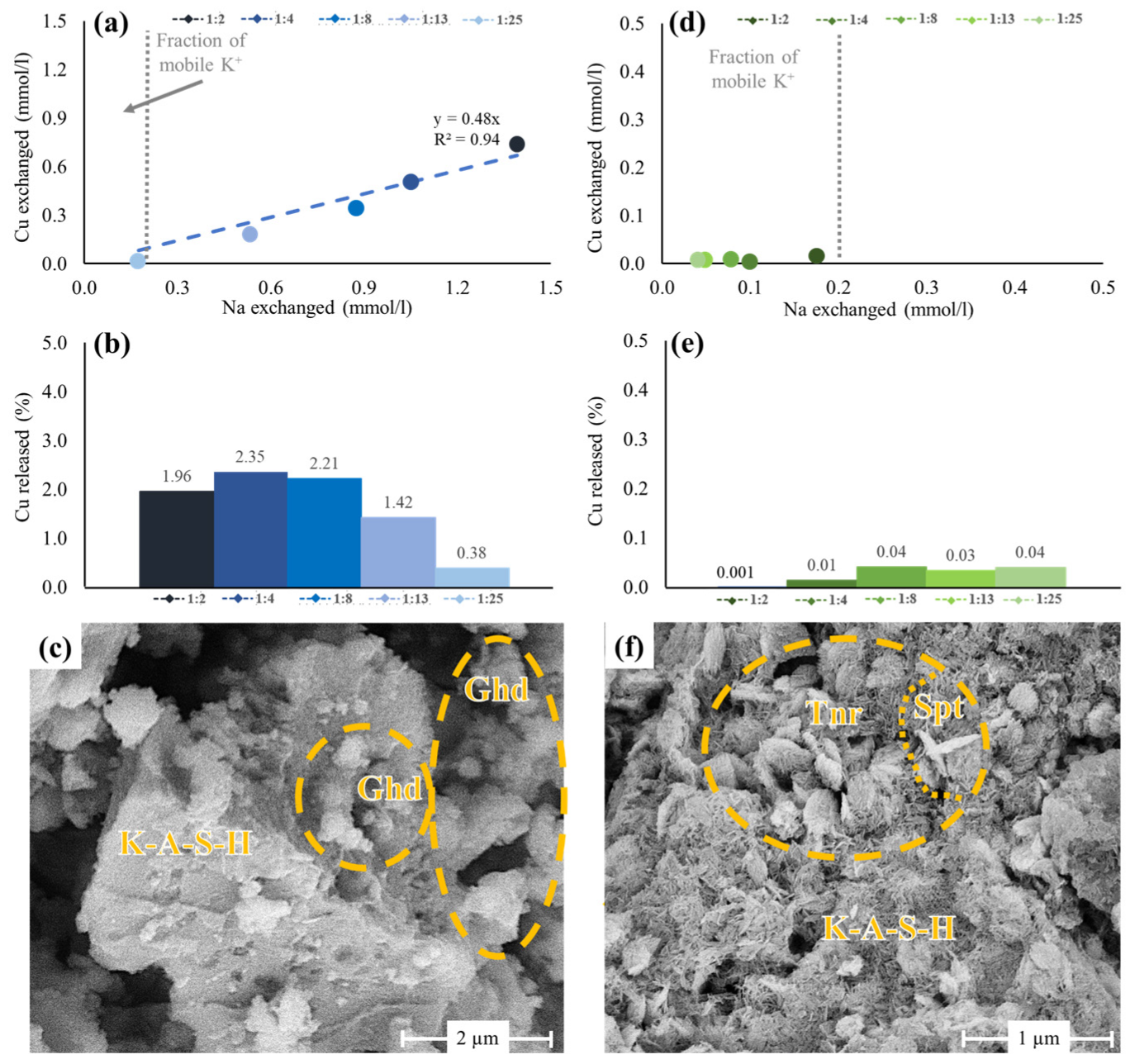
3.4.1. Ion Exchange
3.4.2. Acid Leaching
3.5. Implications for Water Treatment and Environmental Remediation
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malandrinos, G.; Hadjiliadis, N. Cu(II)–histones interaction related to toxicity-carcinogenesis. Coord. Chem. Rev. 2014, 262, 55–71. [Google Scholar] [CrossRef]
- Panhwar, A.H.; Kazi, T.G.; Afridi, H.I.; Arain, S.A.; Arain, M.S.; Brahaman, K.D.; Naeemullah; Arain, S.S. Correlation of cadmium and aluminum in blood samples of kidney disorder patients with drinking water and tobacco smoking: Related health risk. Environ. Geochem. Health 2016, 38, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Schlögl, S.; Dienhofer, P.; Baldermann, A.; Vollprecht, D. Use of industrial residues for heavy metals immobilization in contaminated site remediation: A brief review. Int. J. Environ. Sci. Technol. 2023, 20, 2313–2326. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Mao, Q.; Luo, L.; Deng, Y. Removal of Heavy Metals from Acid Mine Drainage by Red Mud–Based Geopolymer Pervious Concrete: Batch and Long–Term Column Studies. Polymers 2022, 14, 5355. [Google Scholar] [CrossRef]
- Bacquart, T.; Frisbie, S.; Mitchell, E.; Grigg, L.; Cole, C.; Small, C.; Sarkar, B. Multiple inorganic toxic substances contaminating the groundwater of Myingyan Township, Myanmar: Arsenic, manganese, fluoride, iron, and uranium. Sci. Total Environ. 2015, 517, 232–245. [Google Scholar] [CrossRef]
- Malik, Q.A.; Khan, M.S. Effect on Human Health due to Drinking Water Contaminated with Heavy Metals. J. Pollut. Eff. Cont. 2016, 5, 1000179. [Google Scholar]
- Kayastha, V.; Patel, J.; Kathrani, N.; Varjani, S.; Bilal, M.; Show, P.L.; Kim, S.-H.; Bontempi, E.; Bhatia, S.K.; Bui, X.-T. New Insights in factors affecting ground water quality with focus on health risk assessment and remediation techniques. Environ. Res. 2022, 212, 113171. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, K.; Wang, Y.; Zhang, B.; Mao, K.; Zhang, H. Health Risk Assessments and Microbial Community Analyses of Groundwater from a Heavy Metal-Contaminated Site in Hezhou City, Southwest China. Int. J. Environ. Res. Public Health 2023, 20, 604. [Google Scholar] [CrossRef]
- European Food Safety Authority. Tolerable upper intake levels for vitamins and minerals. In Scientific Committee on Food Scientific Panel on Dietetic Products, Nutrition and Allergies; European Food Safety Authority: Parma, Italy, 2006; pp. 1–482. [Google Scholar]
- United States Environmental Protection Agency. Part 141—National Primary Drinking Water Regulations; United States Environmental Protection Agency: Washington, DC, USA, 2023; pp. 1–70. [Google Scholar]
- Fanni, D.; Fanos, V.; Gerosa, C.; Piras, M.; Dessi, A.; Atzei, A.; Van, E.P.; Gibo, Y.; Faa, G. Effects of Iron and Copper Overload on the Human Liver: An Ultrastructural Study. Curr. Med. Chem. 2014, 21, 3768–3774. [Google Scholar] [CrossRef]
- Grba, N.; Krčmar, D.; Maletić, S.; Bečelić-Tomin, M.; Grgić, M.; Pucar, G.; Dalmacija, B. Organic and inorganic priority substances in sediments of Ludaš Lake, a cross-border natural resource on the Ramsar list. Environ. Sci. Pollut. Res. Int. 2017, 24, 1938–1952. [Google Scholar] [CrossRef]
- Krčmar, D.; Tenodi, S.; Grba, N.; Kerkez, Đ.; Watson, M.; Rončević, S.; Dalmacija, B. Preremedial assessment of the municipal landfill pollution impact on soil and shallow groundwater in Subotica, Serbia, Sci. Total Environ. 2018, 615, 1341–1354. [Google Scholar] [CrossRef]
- Grba, N.; Baldermann, A.; Dietzel, M. Novel green technology for wastewater treatment: Geo-material/geopolymer applications for heavy metal removal from aquatic media. Int. J. Sed. Res. 2023, 38, 33–48. [Google Scholar] [CrossRef]
- Bashir, M.; Tyagi, S.; Annachhatre, A.P. Adsorption of copper from aqueous solution onto agricultural Adsorbents: Kinetics and isotherm studies. Mater. Today 2020, 28, 1833–1840. [Google Scholar] [CrossRef]
- Cao, Y.; Qian, X.; Zhang, Y.; Qu, G.; Xia, T.; Guo, X.; Jia, H.; Wang, T. Decomplexation of EDTA-chelated copper and removal of copper ions by non-thermal plasma oxidation/alkaline precipitation. Chem. Eng. J. 2019, 362, 487–496. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fan, J.; Ma, F. Asymmetric interaction and concurrent remediation of copper and atrazine by Acorus tatarinowii in an aquatic system. J. Hazard. Mater. 2022, 435, 128888. [Google Scholar] [CrossRef]
- Boulakradeche, O.M.; Merdoud, O.; Akretche, D.E. Enhancement of electrokinetic remediation of lead and copper contaminated soil by combination of multiple modified electrolyte conditioning techniques. Environ. Eng. Res. 2022, 27, 210167. [Google Scholar] [CrossRef]
- Emenike, E.C.; Adeniyi, A.G.; Omuku, P.E.; Okwu, K.C.; Iwuozor, K.O. Recent advances in nano-adsorbents for the sequestration of copper from water. J. Water Process. Eng. 2022, 47, 102715. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Herrera-Urbina, R.; McGlashan, D.W. Studies on the applicability of chelating agents as universal collectors for copper minerals. Int. J. Miner. Process. 2000, 58, 15–33. [Google Scholar] [CrossRef]
- Eriksson, N.; Destouni, G. Combined effects of dissolution kinetics, secondary mineral precipitation, and preferential flow on copper leaching from mining waste rock. Water Resour. Res. 1997, 33, 471–483. [Google Scholar] [CrossRef]
- Pollard, A.M.; Thomas, R.G.; Williams, P.A. Synthesis and stabilities of the basic copper(II) chlorides atacamite, paratacamite and botallackite. Mineral. Mag. 1989, 53, 557–563. [Google Scholar] [CrossRef]
- Fan, H.; Qin, J.; Liu, J.; Liu, G.; Yang, X. Investigation into the flotation of malachite, calcite and quartz with three phosphate surfactants. J. Mater. Res. Technol. 2019, 8, 5140–5148. [Google Scholar] [CrossRef]
- Kokes, H.; Morcali, M.H.; Acma, E. Dissolution of copper and iron from malachite ore and precipitation of copper sulfate pentahydrate by chemical process. Eng. Sci. Technol. Int. J. 2014, 17, 39–44. [Google Scholar] [CrossRef]
- Baldermann, A.; Preissegger, V.; Šimić, S.; Letofsky-Papst, I.; Mittermayr, F.; Dietzel, M. Uptake of aqueous heavy metal ions (Co2+, Cu2+ and Zn2+) by calcium-aluminium-silicate-hydrate gels. Cem. Concr. Res. 2021, 147, 106521. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Zboon, K.A.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geopolymer for heavy metal removal: A case study on copper removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Sivasakthi, M.; Professor, R.J.; Rajamane, N.P. Fly ash geopolymer mortar: Impact of the substitution of river sand by copper slag as a fine aggregate on its thermal resistance properties. J. Clean. Prod. 2021, 279, 123766. [Google Scholar] [CrossRef]
- Kara, I.; Tunc, D.; Sayin, F.; Akar, S.T. Study on the performance of metakaolin based geopolymer for Mn(II) and Co(II) removal. Appl. Clay Sci. 2018, 161, 184–193. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Darmayanti, L.; Kadja, G.T.M.; Notodarmojo, S.; Damanhuri, E.; Mukti, R.R. Structural alteration within fly ash-based geopolymers governing the adsorption of Cu2+ from aqueous environment: Effect of alkali activation. J. Hazard. Mater. 2019, 377, 305–314. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Bingxue, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in concrete materials for sewer systems affected by microbial induced concrete corrosion: A review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W. Thermal Behavior of Portland Cement and Fly Ash–Metakaolin-Based Geopolymer Cement Pastes. Arab. J. Sci. Eng. 2015, 40, 2261–2269. [Google Scholar] [CrossRef]
- Le, V.S.; Louda, P.; Tran, H.N.; Nguyen, P.D.; Bakalova, T.; Ewa Buczkowska, K.; Dufkova, I. Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers 2020, 12, 2994. [Google Scholar] [CrossRef]
- Okoye, F.N.; Prakash, S.; Singh, N.B. Durability of fly ash based geopolymer concrete in the presence of silica fume. J. Clean. Prod. 2017, 149, 1062–1067. [Google Scholar] [CrossRef]
- Neupane, K.; Chalmers, D.; Kidd, P. High-Strength Geopolymer Concrete- Properties, Advantages and Challenges. Adv. Mater. 2018, 7, 15–25. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A. Novel porous fly ash-containing geopolymer monoliths for lead adsorption from wastewaters. J. Hazard. Mater. 2016, 318, 631–640. [Google Scholar] [CrossRef]
- Onutai, S.; Kobayashi, T.; Thavorniti, P.; Jiemsirilers, S. Porous fly ash-based geopolymer composite fiber as an adsorbent for removal of heavy metal ions from wastewater. Mater. Lett. 2019, 236, 30–33. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. SW-846 Test Method 1311: Toxicity Characteristic Leaching Procedure; United States Environmental Protection Agency: Washington, DC, USA, 1992; pp. 1–35. [Google Scholar]
- Eichinger, S.; Boch, R.; Leis, A.; Baldermann, A.; Domberger, G.; Schwab, C.; Dietzel, M. Green inhibitors reduce unwanted calcium carbonate precipitation: Implications for technical settings. Water Res. 2022, 208, 117850. [Google Scholar] [CrossRef]
- Baldermann, A.; Mittermayr, F.; Bernasconi, S.M.; Dietzel, M.; Grengg, C.; Hippler, D.; Kluge, T.; Leis, A.; Lin, K.; Wang, X.; et al. Fracture dolomite as an archive of continental palaeo-environmental conditions. Commun. Earth Environ. 2020, 1, 35. [Google Scholar] [CrossRef]
- Baldermann, A.; Dietzel, M.; Reinprecht, V. Chemical weathering and progressing alteration as possible controlling factors for creeping landslides. Sci. Total Environ. 2021, 778, 146300. [Google Scholar] [CrossRef]
- Baldermann, A.; Dohrmann, R.; Kaufhold, S.; Nickel, C.; Letofsky-Papst, I.; Dietzel, M. The Fe-Mg-saponite solid solution series—A hydrothermal synthesis study. Clay Miner. 2014, 49, 391–415. [Google Scholar] [CrossRef]
- Baldermann, A.; Wasser, O.; Abdullayev, E.; Bernasconi, S.; Löhr, S.; Wemmer, K.; Piller, W.E.; Rudmin, M.; Richoz, S. Palaeo-environmental evolution of Central Asia during the Cenozoic: New insights from the continental sedimentary archive of the Valley of Lakes (Mongolia). Clim. Past. 2021, 17, 1955–1972. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers Based on Natural and Synthetic Metakaolin a Critical Review. In Proceedings of the 41st International Conference on Advanced Ceramics and Composites: Ceramic Engineering and Science Proceedings, Daytona Beach, FL, USA, 22–27 January 2017; Kriven, W.M., Bansal, N.P., Kusnezoff, M., Ohji, T., Zhou, Y., Moon, K., Matyas, J., Shimamura, K., Kirihara, S., Eds.; John Wiley & Sons: New York, NY, USA, 2018; Volume 38, pp. 201–214. [Google Scholar]
- Kumar, S.; Kristály, F.; Mucsi, G. Geopolymerisation behaviour of size fractioned fly ash. Adv. Powder Technol. 2015, 26, 24–30. [Google Scholar] [CrossRef]
- Feng, B.; Liu, J.; Chen, Y.; Tan, X.; Zhang, M.; Sun, Z. Properties and microstructure of self-waterproof metakaolin geopolymer with silane coupling agents. Constr. Build. Mater. 2022, 342, 128045. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Abo-El-Enein, S.A. A novel method to produce dry geopolymer cement powder. HBRC J. 2016, 12, 13–24. [Google Scholar] [CrossRef]
- Pereira, M.A.; Vasconcelos, D.C.L.; Vasconcelos, W.L. Synthetic Aluminosilicates for Geopolymer Production. Mater. Res. 2019, 22, e20180508. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S. Review on characteristics of metakaolin-based geopolymer and fast setting. J. Korean Ceram. Soc. 2020, 57, 368–377. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Fuchs, Y.; Grybos, M.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Characterization and pH-dependent leaching behaviour of historical and modern copper slags. J. Geochem. Explor. 2016, 160, 1–15. [Google Scholar] [CrossRef]
- Yu, Z.; Song, W.; Li, J.; Li, Q. Improved simultaneous adsorption of Cu(II) and Cr(VI) of organic modified metakaolin-based geopolymer. Arab. J. Chem. 2020, 13, 4811–4823. [Google Scholar] [CrossRef]
- Baykara, H.; Mendoza Solorzano, M.D.L.; Delgado Echeverria, J.J.; Cornejo, M.H.; Tapia-Bastidas, C.V. The use of zeolite-based geopolymers as adsorbent for copper removal from aqueous media. R. Soc. Open Sci. 2022, 9, 211644. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Yaseen, M.; Tong, Z.; Cui, X. Synthesis of highly efficient porous inorganic polymer microspheres for the adsorptive removal of Pb2+from wastewater. J. Clean. Prod. 2018, 193, 351–362. [Google Scholar] [CrossRef]
- Yoder, C.H.; Bushong, E.; Weidner, V.; McWilliams, P.; Martin, K.; Lorgunpai, J.; Haller, J.; Schaeffer, R.W. The synthesis and solubility of the copper hydroxyl nitrates: Gerhardtite, rouaite and likasite. Mineral. Mag. 2010, 74, 433–440. [Google Scholar] [CrossRef]
- Aguirre, J.M.; Gutiérrez, A.; Giraldo, O. Simple route for the synthesis of copper hydroxy salts. J. Braz. Chem. Soc. 2011, 22, 546–551. [Google Scholar] [CrossRef]
- Hidmi, L.; Edwards, M. Role of Temperature and pH in Cu(OH)2 Solubility. Environ. Sci. Technol. 1999, 33, 2607–2610. [Google Scholar] [CrossRef]
- Grengg, C.; Gluth, G.J.G.; Mittermayr, F.; Ukrainczyk, N.; Bertmer, M.; Buzanich, A.G.; Radtke, M.; Leis, A.; Dietzel, M. Deterioration mechanism of alkali-activated materials in sulfuric acid and the influence of Cu: A micro-to-nano structural, elemental and stable isotopic multi-proxy study. Cem. Concr. Res. 2021, 142, 106373. [Google Scholar] [CrossRef]
- Pandey, B.; Kinrade, S.D.; Catalan, L.J.J. Effects of carbonation on the leachability and compressive strength of cement-solidified and geopolymer-solidified synthetic metal wastes. J. Environ. Manag. 2012, 101, 59–67. [Google Scholar] [CrossRef]
- Ilcheva, L.; Bjerrum, J. Metal Ammine Formation in Solution. XVII. Stability Constants of Copper(II) Methylamine and Diethylamine Complexes Obtained from Solubility Measurements with Gerhardtite, Cu(OH)1.5(NO3)0.5. Acta Chem. Scand. A 1976, 30, 343–350. [Google Scholar] [CrossRef]
- Aquino, A.; Lezzerini, M.; Giaccherini, A.; Montegrossi, G.; Di Benedetto, F. Thermochemical stability of delafossite and other relevant ternary phases in the Cu–Fe–S–O–H system. Appl. Geochem. 2020, 123, 104795. [Google Scholar] [CrossRef]
- Schmutzler, B.; Eggert, G.; Kuhn-Wawrzinek, C.F. Copper(II) hydroxide on artefacts: Corrosion, conservation, colourants. Stud. Conserv. 2016, 62, 61–67. [Google Scholar] [CrossRef]
- Clavier, B.; Baptiste, T.; Zhadan, A.; Guiet, A.; Boucher, F.; Brezová, V.; Roques, C.; Corbel, G. Understanding the bactericidal mechanism of Cu(OH)2 nanorods in water through Mg-substitution: High production of toxic hydroxyl radicals by non-soluble particles. J. Mater. Chem. B 2022, 10, 77. [Google Scholar] [CrossRef]
- Baldermann, A.; Preissegger, V.; Dietzel, M. Solubility of C-A-S-H phases with high degree of heavy metal ion substitution. Constr. Build. Mater. 2022, 327, 126926. [Google Scholar] [CrossRef]
- Chen, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Painer, F.; Baldermann, A.; Gallien, F.; Eichinger, S.; Steindl, F.; Dohrmann, R.; Dietzel, M. Synthesis of Zeolites from Fine-Grained Perlite and Their Application as Sorbents. Materials 2022, 15, 4474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, X.; Wang, F.; Wang, J. Mechanical Properties and Durability of Geopolymer Recycled Aggregate Concrete: A Review. Polymers 2023, 15, 615. [Google Scholar] [CrossRef] [PubMed]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. LLDPE Composites with Nanosized Copper and Copper Oxides for Water Disinfection. Polymers 2020, 12, 1713. [Google Scholar] [CrossRef]
- Hashimoto, S.; Machino, T.; Takeda, H.; Daiko, Y.; Honda, S.; Iwamoto, Y. Antimicrobial activity of geopolymers ion-exchanged with copper ions. Ceram. Int. 2015, 41, 13788–13792. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Lv, K.; Chen, X. S-Scheme photocatalyst TaON/Bi2WO6 nanofibers with oxygen vacancies for efficient abatement of antibiotics and Cr(VI): Intermediate eco-toxicity analysis and mechanistic insights. Chin. J. Catal. 2022, 43, 2652–2664. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Zhang, Y.; Cai, J.; He, M.; Li, M.; Chen, Z.; Zhang, L. Growth of BiOBr/ZIF-67 Nanocomposites on Carbon Fiber Cloth as Filter-Membrane-Shaped Photocatalyst for Degrading Pollutants in Flowing Wastewater. Adv. Fiber Mater. 2022, 4, 1620–1631. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Wang, C.; Liu, K.; Wang, D.; Wang, G.; Chu, P.K.; Meng, Z.; Wang, X. Hierarchical CuO–ZnO/SiO2 Fibrous Membranes for Efficient Removal of Congo Red and 4-Nitrophenol from Water. Adv. Fiber Mater. 2022, 4, 1069–1080. [Google Scholar] [CrossRef]
- Jia, C.; Xu, Z.; Luo, D.; Xiang, H.; Zhu, M. Flexible Ceramic Fibers: Recent Development in Preparation and Application. Adv. Fiber Mater. 2022, 4, 573–603. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grba, N.; Grengg, C.; Petronijević, M.; Dietzel, M.; Baldermann, A. Substantial Copper (Cu2+) Uptake by Metakaolin-Based Geopolymer and Its Resistance to Acid Leaching and Ion Exchange. Polymers 2023, 15, 1971. https://doi.org/10.3390/polym15081971
Grba N, Grengg C, Petronijević M, Dietzel M, Baldermann A. Substantial Copper (Cu2+) Uptake by Metakaolin-Based Geopolymer and Its Resistance to Acid Leaching and Ion Exchange. Polymers. 2023; 15(8):1971. https://doi.org/10.3390/polym15081971
Chicago/Turabian StyleGrba, Nenad, Cyrill Grengg, Mirjana Petronijević, Martin Dietzel, and Andre Baldermann. 2023. "Substantial Copper (Cu2+) Uptake by Metakaolin-Based Geopolymer and Its Resistance to Acid Leaching and Ion Exchange" Polymers 15, no. 8: 1971. https://doi.org/10.3390/polym15081971





