Effect of Tannins Addition on Thermal Stability of Furfurylated Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Impregnating Solutions and Resulting Polymers
2.2. Wood Modification
2.3. Thermogravimetric Analysis (TGA)
- T = 25 to 103 °C, t = 8 min, air condition (v = 0 mL/min);
- T = constant at 103 °C, t = 7.8 min, air condition (v = 0 mL/min);
- T = 103 to 700 °C (for wood samples) or 900 °C (for polymer), t = 59.7 or 79.7 min, N2 condition (v = 50 mL/min);
- T = 700 °C or 900 °C to 25 °C, t = 67.5/87.5 min, N2 condition (v = 50 mL/min).
3. Results and Discussion
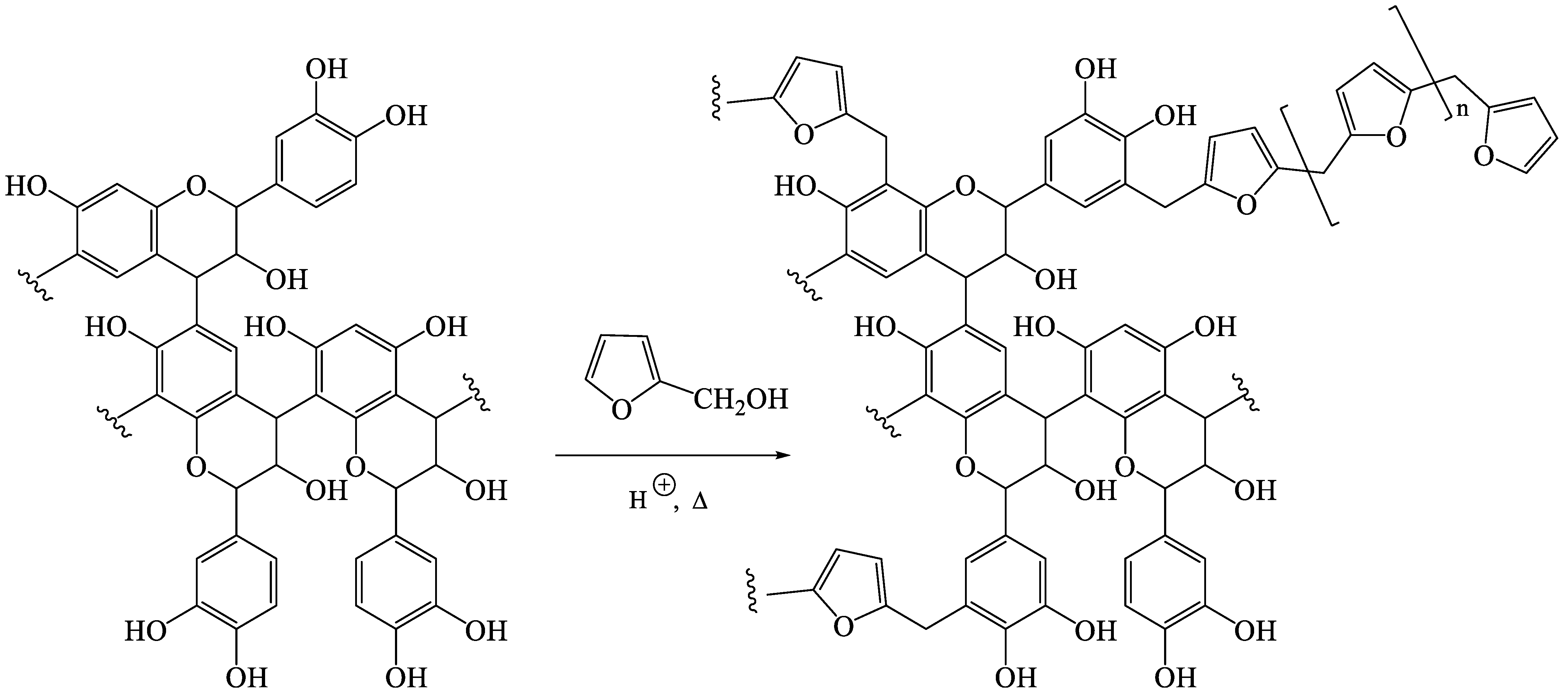
3.1. Polymerizationof Resin Alone
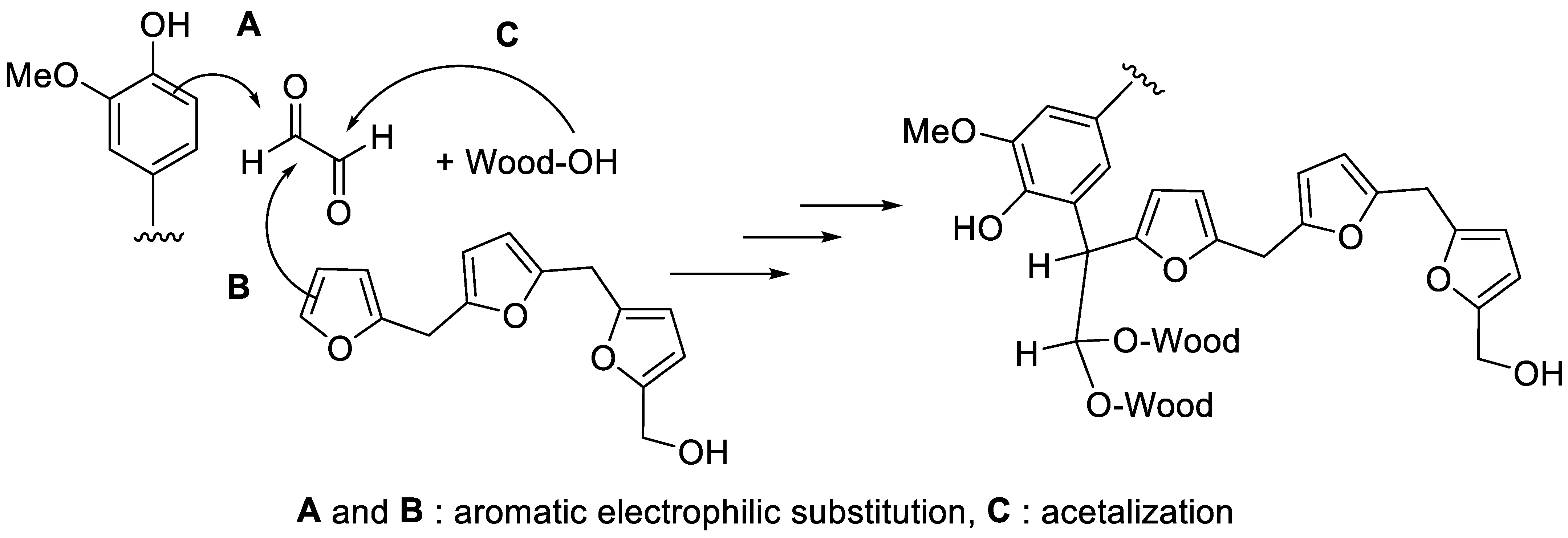
3.2. Polymerizationof Resin within the Wood Structure
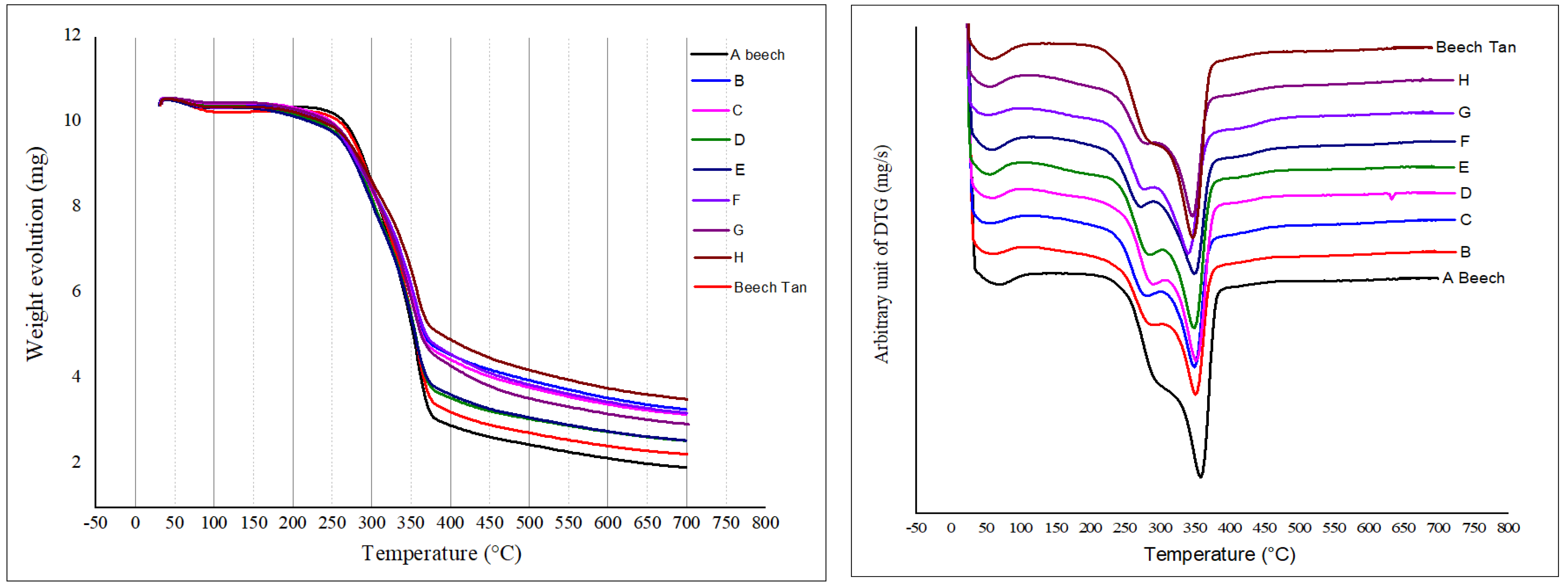
3.3. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grosse, C.; Grigsby, W.J.; Noël, M.; Treu, A.; Thévenon, M.F.; Gérardin, P. Optimizing Chemical Wood Modification with Oligomeric Lactic Acid by Screening of Processing Conditions. J. Wood Chem. Technol. 2019, 39, 385–398. [Google Scholar] [CrossRef]
- Sommerauer, L.; Thevenon, M.F.; Petutschnigg, A.; Tondi, G. Effect of Hardening Parameters of Wood Preservatives Based on Tannin Copolymers. Holzforschung 2019, 73, 457–467. [Google Scholar] [CrossRef]
- Tondi, G. Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers 2017, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Tondi, G.; Hu, J.; Rizzo, F.; Buh, J.; Medved, S.; Petutschnigg, A.; Thevenon, M.F. Tannin-Caprolactam and Tannin-PEG Formulations as Outdoor Wood Preservatives: Weathering Properties. Ann. For. Sci. 2017, 74, 9. [Google Scholar] [CrossRef]
- Li, X.; Nicollin, A.; Pizzi, A.; Zhou, X.; Sauget, A.; Delmotte, L. Natural tannin/furanic thermosetting moulding plastics. RSC Adv. 2017, 3, 17732–17740. [Google Scholar] [CrossRef]
- Lagel, M.C.; Pizzi, A.; Basso, M.C.; Abdalla, S. Development and characterisation of abrasive grinding wheels with a tannin-furfuryl alcohol resin matrix. Ind. Crops Prod. 2014, 65, 333–348. [Google Scholar]
- Lagel, M.C.; Zhang, J.; Pizzi, A. Cutting and grinding wheels for angle grinders with a bioresin matrix. Ind. Crops Prod. 2015, 67, 264–269. [Google Scholar] [CrossRef]
- Foo, L.Y.; Hemingway, R.W. Condensed Tannins: Reactions of Model Compounds with Furfuryl Alcohol and Furfuraldehyde. J. Wood Chem. Technol. 1985, 5, 135–158. [Google Scholar] [CrossRef]
- Gérardin, P. New Alternatives for Wood Preservation Based on Thermal and Chemical Modification of Wood—A Review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Lande, S.; Westin, M.; Schneider, M. Development of Modified Wood Products Based on Furan Chemistry. Mol. Cryst. Liq. Cryst. 2008, 484, 37–41. [Google Scholar] [CrossRef]
- Mantanis, G.I. Chemical Modification of Wood by Acetylation or Furfurylation: A Review of the Present Scaled-up Technologies. BioResources 2018, 12, 4478–4489. [Google Scholar] [CrossRef]
- Nordstierna, L.; Lande, S.; Westin, M.; Karlsson, O.; Furó, I. Towards Novel Wood-Based Materials: Chemical Bonds between Lignin-like Model Molecules and Poly(Furfuryl Alcohol) Studied by NMR. Holzforschung 2008, 62, 709–713. [Google Scholar] [CrossRef]
- Goldstein, I.; Dreher, W. Stable Furfuryl Alcohol Impregnating Solutions. Ind. Eng. Chem. 1960, 52, 57–58. [Google Scholar] [CrossRef]
- Lande, S.; Eikenes, M.; Westin, M. Chemistry and Ecotoxicology of Furfurylated Wood. Scand. J. For. Res. 2004, 19, 14–21. [Google Scholar] [CrossRef]
- Sejati, P.; Imbert, A.; Gérardin, C.; Dumarçay, S.; Fredon, E.; Masson, E.; Nandika, D.; Priadi, T.; Gérardin, P. Tartaric acid catalyzed furfurylation of beech wood. Wood Sci. Technol. 2017, 51, 379–394. [Google Scholar] [CrossRef]
- Mubarok, M.; Gérardin-Charbonnier, C.; Azadeh, E.; Akong, F.O.; Dumarçay, S.; Pizzi, A.; Gérardin, P. Modification of Wood by Tannin-Furfuryl Alcohol Resins-Effect on Dimensional Stability, Mechanical Properties and Decay Durability. J. Renew. Mater. 2013, 11, 505–521. [Google Scholar] [CrossRef]
- NF X 41-568; Wood Preservatives-Laboratory Method for Obtaining Samples for Analysis to Measure Losses by Leaching into Water or Synthetic Sea Water. European Committee for Standardization, AFNOR: Saint-Denis, France, 2014.
- Guigo, N.; Mija, A.; Zavaglia, R.; Vincent, L.; Sbirrazzuoli, N. New Insights on the Thermal Degradation Pathways of Neat Poly(Furfuryl Alcohol) and Poly(Furfuryl Alcohol)/SiO2 Hybrid Materials. Polym. Degrad. Stab. 2019, 94, 908–913. [Google Scholar] [CrossRef]
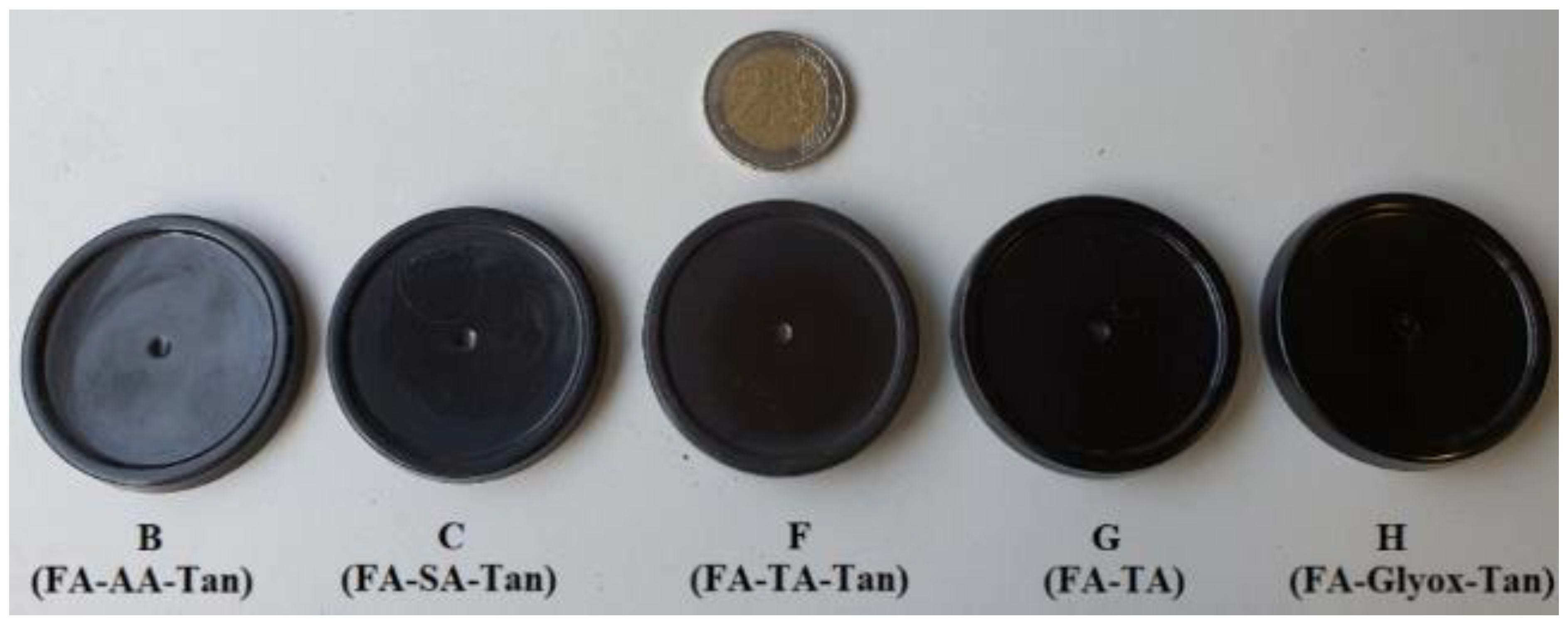

| ID | Impregnating Solution | pH |
|---|---|---|
| A | Untreated (without impregnation) | 6.8 (water) |
| B | AA 2.2% + FA 44.4% + Tan 8.9% + water 44.4% | 3.2 |
| C | SA 2.2% + FA 44.4% + Tan 8.9% + water 44.4% | 2.8 |
| D | AA 2.2% + FA 53.3% + water 44.4% | 4.0 |
| E | SA 2.2% + FA 53.3% + water 44.4% | 3.6 |
| F | TA 2.2% + FA 44.4% + Tan 8.9% + water 44.4% | 2.0 |
| G | TA 2.2% + FA 53.3% + water 44.4% | 2.0 |
| H | Glyoxal 20% + FA 40% + Tan 8% + water 32% | 2.9 |
| ID | Solution Uptake (%) * | WPG (%) * | Bulking (%) * | Leaching (%) ** | ASE Cycle III (%) *** |
|---|---|---|---|---|---|
| A | - | - | - | 1.5 ± 0.2 | 9.2 ± 5.9 |
| B | 120.1 ± 5.0 | 36.2 ± 2.4 | 13.2 ± 2.1 | 3.8 ± 0.6 | 46.1 ± 2.5 |
| C | 121.4 ± 7.0 | 39.4 ± 0.7 | 15.0 ± 1.6 | 2.2 ± 0.3 | 49.8 ± 2.2 |
| D | 120.1 ± 5.4 | 21.3 ± 2.5 | 8.1 ± 1.4 | 5.4 ± 1.3 | 39.5 ± 3.7 |
| E | 116.1 ± 6.4 | 27.1 ± 1.5 | 11.8 ± 1.0 | 5.2 ± 0.9 | 47.5 ± 3.0 |
| F | 118.5 ± 6.3 | 46.2 ± 2.4 | 13.8 ± 0.8 | 2.0 ± 0.1 | 44.6 ± 1.8 |
| G | 124.2 ± 2.8 | 38.4 ± 1.1 | 17.8 ± 0.9 | 2.8 ± 0.2 | 66.1 ± 7.9 |
| H | 121.4 ± 7.2 | 50.8 ± 4.4 | 14.9 ± 1.0 | 2.1 ± 0.3 | 51.1 ± 3.0 |
| Treatment | Sample | TGA | The Main Tinf in DTG (°C) | |||
|---|---|---|---|---|---|---|
| Total ML (%) | Stage I | Stage II | Stage III | Stage IV | ||
| B | FA-AA-Tan | 64.65 | 221 | 324 | 420 | - |
| C | FA-SA-Tan | 61.53 | 200 | 338 | 425 | - |
| F | FA-TA-Tan | 61.26 | 216 | 360 | 423 | - |
| G | FA-TA | 64.98 | 192 | 228 | 364 | 435 |
| H | FA-Glyox-Tan | 58.74 | 418 | - | - | - |
| Additional | Tannins | 56.54 | 253 | - | - | - |
| Treatment | Sample | % MLTGA |
|---|---|---|
| A | Beech | 88.75 |
| B | FA-AA-Tan-Beech | 79.04 |
| C | FA-SA-Tan Beech | 77.13 |
| D | FA-AA Beech | 85.30 |
| E | FA-SA Beech | 83.69 |
| F | FA-TA-Tan-Beech | 76.63 |
| G | FA-TA Beech | 79.64 |
| H | FA-Glyox-Tan-Beech | 74.44 |
| Additional | Beech-Tan | 82.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarok, M.; Azadeh, E.; Obounou Akong, F.; Dumarçay, S.; Gérardin, P.; Gérardin-Charbonnier, C. Effect of Tannins Addition on Thermal Stability of Furfurylated Wood. Polymers 2023, 15, 2044. https://doi.org/10.3390/polym15092044
Mubarok M, Azadeh E, Obounou Akong F, Dumarçay S, Gérardin P, Gérardin-Charbonnier C. Effect of Tannins Addition on Thermal Stability of Furfurylated Wood. Polymers. 2023; 15(9):2044. https://doi.org/10.3390/polym15092044
Chicago/Turabian StyleMubarok, Mahdi, Elham Azadeh, Firmin Obounou Akong, Stéphane Dumarçay, Philippe Gérardin, and Christine Gérardin-Charbonnier. 2023. "Effect of Tannins Addition on Thermal Stability of Furfurylated Wood" Polymers 15, no. 9: 2044. https://doi.org/10.3390/polym15092044
APA StyleMubarok, M., Azadeh, E., Obounou Akong, F., Dumarçay, S., Gérardin, P., & Gérardin-Charbonnier, C. (2023). Effect of Tannins Addition on Thermal Stability of Furfurylated Wood. Polymers, 15(9), 2044. https://doi.org/10.3390/polym15092044