Preparation of Single-Helical Curdlan Hydrogel and Its Activation with Coagulation Factor G
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Curdlan Hydrogels with a Single-Helical Skeleton and a Random Skeleton
2.3. Diffuse Reflectance Infrared Fourier Transform Spectroscopy
2.4. X-ray Diffractometry Analysis
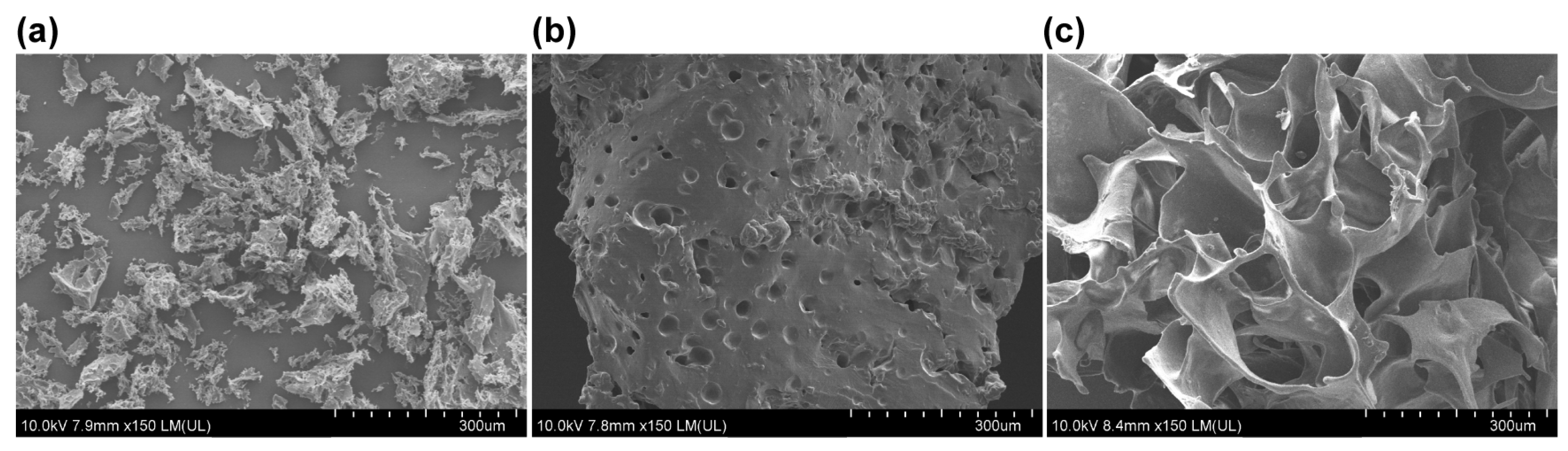
2.5. Scanning Electron Microscopy Analysis
2.6. Swelling Experiments of S Gel and C Gel
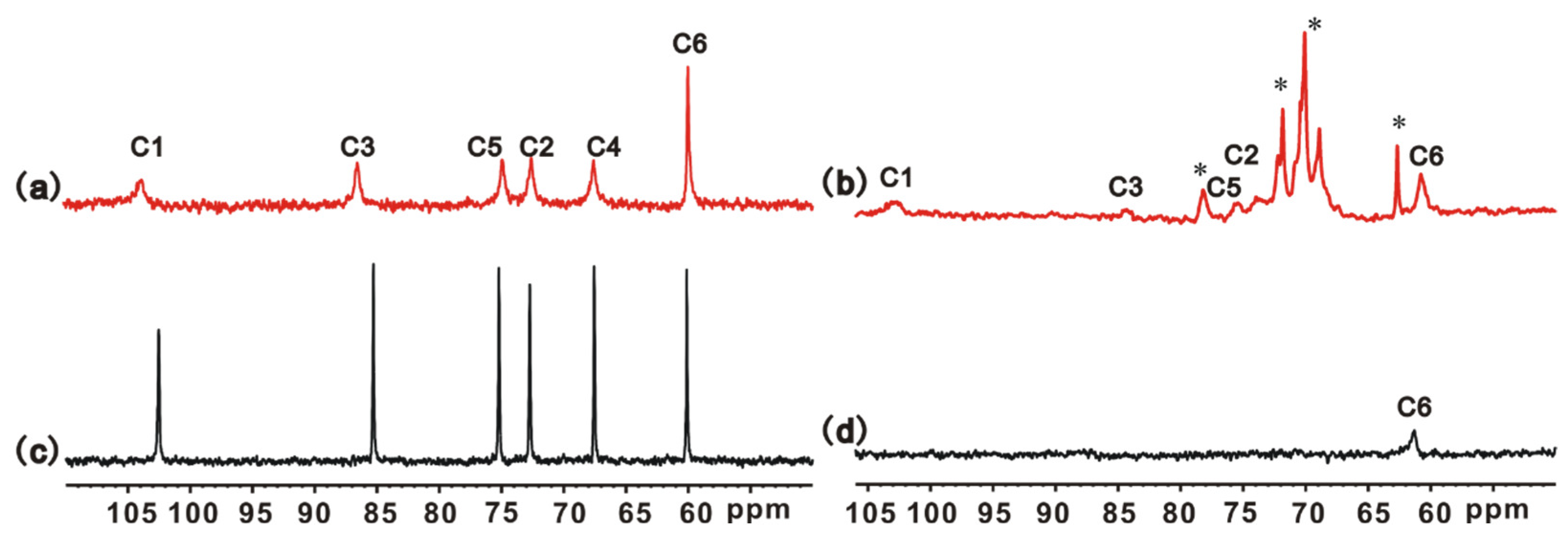
2.7. NMR Experiments
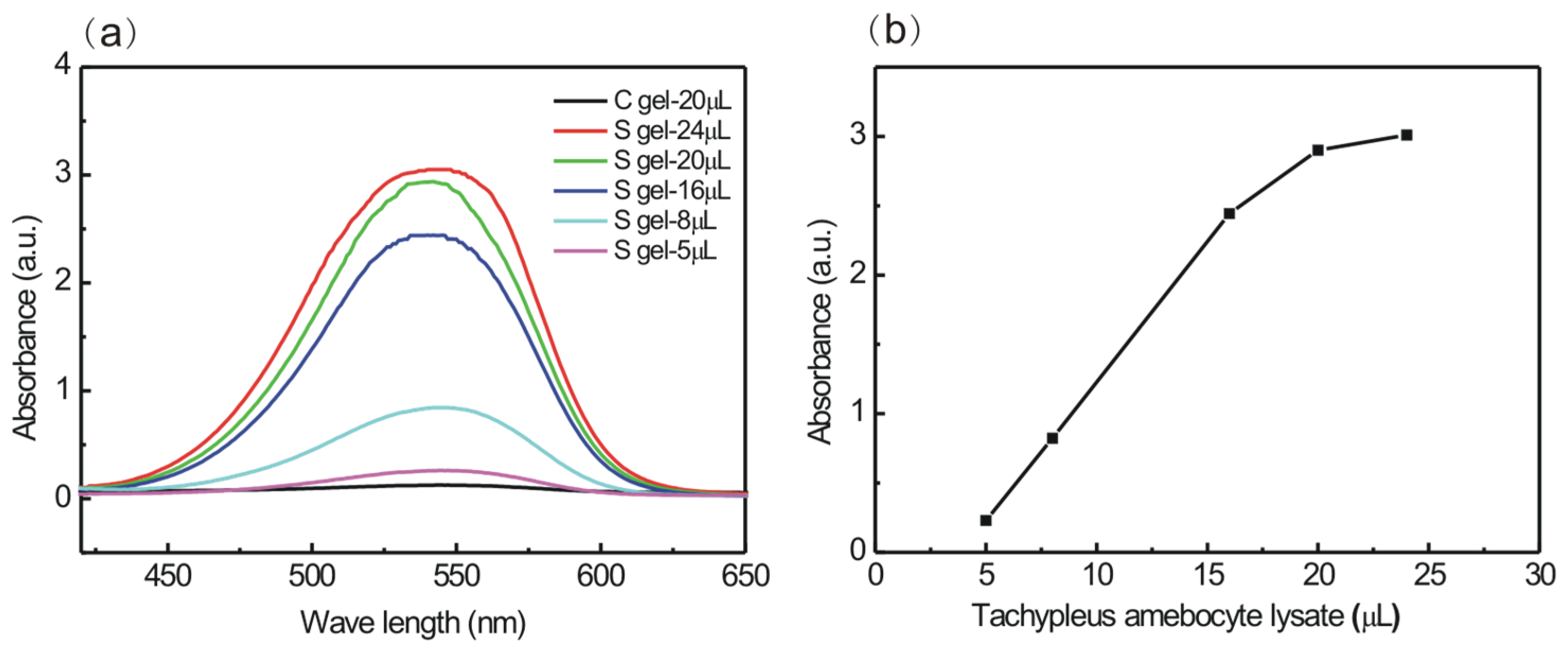
2.8. Activation of Factor G from Tachypleus Amebocyte Lysate
3. Results
3.1. Structure Characterizations of S Gel
3.2. Swelling Properties of S Gel and C Gel
3.3. Actiation of Factor G from Tachypleus Amebocyte Lysate by S Gel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, Q.M.; Ganbold, T.; Bao, M.M.; Xiao, H.; Han, S.Q.; Baigude, H. Tumor targeted siRNA delivery by adenosine receptor-specific curdlan nanoparticles. Int. J. Biol. Macromol. 2023, 253, 126845. [Google Scholar] [CrossRef]
- Zelechowska, P.; Rózalska, S.; Wiktorska, M.; Brzezinska-Blaszczyk, E.; Agier, J. Curdlan stimulates tissue mast cells to synthesize pro-inflammatory mediators, generate ROS, and migrate via Dectin-1 receptor. Cell Immunol. 2020, 351, 104079. [Google Scholar] [CrossRef]
- Chen, Y.P.; Wang, F.S. Review on the preparation, biological activities and applications of curdlan and its derivatives. Eur. Polym. J. 2020, 141, 110096. [Google Scholar] [CrossRef]
- Jin, Y.M.; Li, P.L.; Wang, F.S. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Fujimori, K.; Hirose, S.; Masada, M. Growth and β-Glucan 10C3K Production by a Mutant of Alcaligenes faecalis var. myxogenes in Defined Medium. Agric. Biol. Chem. 1966, 30, 764–769. [Google Scholar] [CrossRef]
- Funami, T.; Yotsuzuka, F.; Yada, H.; Nakao, Y. Thermo-irreversible characteristics of curdlan gels in a model reduced fat pork sausage. J. Food Sci. 1998, 63, 575–579. [Google Scholar] [CrossRef]
- Young, S.H.; Jacobs, R.R. Sodium hydroxide-induced conformational change in schizophyllan detected by the fluorescence dye, aniline blue. Carbohydr. Res. 1998, 310, 91–99. [Google Scholar] [CrossRef]
- Tanaka, S.; Aketagawa, J.; Takahashi, S.; Shibata, Y.; Tsumuraya, Y.; Hashimoto, Y. Inhibition of high-molecular-weight-(1-3)-beta-D-glucan-dependent activation of a limulus coagulation factor G by laminaran oligosaccharides and curdlan degradation products. Carbohydr. Res. 1993, 244, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.A.; Lafleur, M. Comparison of the structure and the transport properties of low-set and high-set curdlan hydrogels. J. Colloid Interface Sci. 2011, 357, 419–427. [Google Scholar] [CrossRef]
- Konno, A.; Harada, T. Thermal properties of curdlan in aqueous suspension and curdlan gel. Food Hydrocoll. 1991, 5, 427–434. [Google Scholar] [CrossRef]
- Hirashima, M.; Takaya, T.; Nishinari, K. DSC and rheological studies on aqueous dispersions of curdlan. Thermochim. Acta 1997, 306, 109–114. [Google Scholar] [CrossRef]
- Saito, H. Conformation and dynamic of (1- 3)-β-D-glucans in the solid and gel state-high-resolution solid-dtate C-13 NMR specroscopic study. ACS Sym. Ser. 1992, 489, 296–310. [Google Scholar]
- Saito, H.; Yoshioka, Y.; Uehara, N.; Aketagawa, J.; Tanaka, S.; Shibata, Y. Relationship between conformation and biological response for (1-3)-β-d-glucans in the activation of coagulation Factor G from limulus amebocyte lysate and host-mediated antitumor activity. Demonstration of single-helix conformation as a stimulant. Carbohydr. Res. 1991, 217, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.A.; Lafleur, M. From curdlan powder to the triple helix gel structure: An attenuated total reflection-infrared study of the gelation process. Appl. Spectrosc. 2007, 61, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Jiang, M.F.; Wu, K.; Yang, H.; Ni, X.W.; Yan, W.L.; Phillips, G.O.; Jiang, F.T. Investigation on curdlan dissociation by heating in water. Food Hydrocoll. 2017, 70, 57–64. [Google Scholar] [CrossRef]
- Sletmoen, M.; Stokke, B.T. Higher order structure of (1,3)-β-D-glucans and its influence on their biological activities and complexation abilities. Biopolym. Orig. Res. Biomol. 2008, 89, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.A.; BeMiller, J.N. (1-3)-beta-D-glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Ogawa, K.; Tsurugi, J.; Watanabe, T. The dependence of the conformation of a (1-3)-β-D-glucan on chain-length in alkaline solution. Carbohydr. Res. 1973, 29, 397–403. [Google Scholar] [CrossRef]
- Ogawa, K.; Ono, S.; Watanabe, T.; Tsurugi, J. Conformational behavior of a gel-forming (1-3)-β-D-glucan in alkaline solution. Carbohydr. Res. 1972, 23, 399–405. [Google Scholar] [CrossRef]
- Yan, X.S.; Liu, B.L.; Ru, G.Y.; Feng, J.W. Preparation and characterization of curdlan with unique single-helical conformation and its assembly with Congo Red. Carbohydr. Polym. 2021, 263, 117985. [Google Scholar] [CrossRef]
- Bouchut, A.; Cathala, B.; Moreau, C.; Lecourt, M.; Petit-Conil, M.; Pettignano, A.; Bernard, J.; Charlot, A.; Fleury, E. Cellulosic surfaces endowed with chemical reactivity by physical adsorption of functionalized polysaccharides. Cellulose 2023, 30, 8185–8203. [Google Scholar] [CrossRef]
- Hansen, T.; Vermeeren, P.; Haim, A.; van Dorp, M.J.H.; Codée, J.D.C.; Bickelhaupt, F.M.; Hamlin, T.A. Regioselectivity of Epoxide Ring-Openings via SN2 Reactions Under Basic and Acidic Conditions. Eur. J. Org. Chem. 2020, 2020, 3822–3828. [Google Scholar] [CrossRef]
- Koumoto, K.; Umeda, M.; Numata, M.; Matsumoto, T.; Sakurai, K.; Kunitake, T.; Shinkai, S. Low MW sulfated curdlan with improved water solubility forms macromolecular complexes with polycytidylic acid. Carbohydr. Res. 2004, 339, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Shen, M.Y.; Liu, S.C.; Jiang, L.; Song, Q.Q.; Xie, J.H. Gel properties and interactions of Mesona blumes polysaccharide-soy protein isolates mixed gel: The effect of salt addition. Carbohydr. Polym. 2018, 192, 193–201. [Google Scholar] [CrossRef]
- Wu, C.X.; Wang, X.Y.; Wang, J.J.; Zhang, Z.; Wang, Z.P.; Wang, Y.F.; Tang, S.Q. Tile-based self-assembly of a triple-helical polysaccharide into cell wall-like mesoporous nanocapsules. Nanoscale 2017, 9, 9938–9945. [Google Scholar] [CrossRef]
- Sakamoto, J.; Kita, R.; Duelamae, I.; Kunitake, M.; Hirano, M.; Yoshihara, D.; Yamamoto, T.; Noguchi, T.; Roy, B.; Shinkai, S. Cohelical Crossover Network by Supramolecular Polymerization of a 4,6-Acetalized β-1,3-Glucan Macromer. ACS Macro Lett. 2017, 6, 21–26. [Google Scholar] [CrossRef]
- Enomoto-Rogers, Y.; Kimura, S.; Iwata, T. Soft, tough, and flexible curdlan hydrogels and organogels fabricated by covalent cross-linking. Polymer 2016, 100, 143–148. [Google Scholar] [CrossRef]
- Gao, F.P.; Zhang, H.Z.; Liu, L.R.; Wang, Y.S.; Jiang, Q.; Yang, X.D.; Zhang, Q.Q. Preparation and physicochemical characteristics of self-assembled nanoparticles of deoxycholic acid modified-carboxymethyl curdlan conjugates. Carbohydr. Polym. 2008, 71, 606–613. [Google Scholar] [CrossRef]
- Hisamatsu, M.; Mishima, T.; Teranishi, K.; Yamada, T. The correlation between adhesion of schizophyllan to yeast glucan and its effect on regeneration of yeast protoplast. Carbohydr. Res. 1997, 298, 117–121. [Google Scholar] [CrossRef]
- Ochiai, T.; Terao, K.; Nakamura, Y.; Yoshikawa, C.; Sato, T. Rigid helical conformation of curdlan tris(phenylcarbamate) in solution. Polymer 2012, 53, 3946–3950. [Google Scholar] [CrossRef]
- Le, T.N.L.; Shiraki, T.; Dawn, A.; Tsuchiya, Y.; Tokunaga, D.; Tamaru, S.; Enomoto, N.; Hojo, J.; Shinkai, S. A pH-responsive carboxylic β-1,3-glucan polysaccharide for complexation with polymeric guests. Org. Biomol. Chem. 2011, 9, 4266–4275. [Google Scholar]
- Xie, J.H.; Zhang, F.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Q.; Xu, X.J.; Zhang, L.N. Effect of Heating on Chain Conformation of Branched β-Glucan in Water. J. Phys. Chem. B 2013, 117, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.J.; Katsuraya, K.; Inazu, T.; Kaneko, Y.; Mimura, T.; Uryu, T. NMR spectroscopic detection of interactions between a HIV protein sequence and a highly anti-HIV active curdlan sulfate. J. Am. Chem. Soc. 2000, 122, 12536–12541. [Google Scholar] [CrossRef]
- Tamura, H.; Tanaka, S.; Oda, T.; Uemura, Y.; Aketagawa, J.; Hashimoto, Y. Purification and characterization of a (1-3)-beta-D-glucan-binding protein from horseshoe crab (Tachyleus tridentatus) amoebocytes. Carbohydr. Res. 1996, 295, 103–116. [Google Scholar]
- Nagi, N.; Ohno, N.; Adachi, Y.; Aketagawa, J.; Tamura, H.; Shibata, Y.; Tanaka, S.; Yadomae, T. Application of limulus test (G pathway) for the detection of different conformers of (1-3) (1-->3)-beta-D-glucans. Biol. Pharm. Bull. 1993, 16, 822–828. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, G.; Yan, X.; Wang, H.; Feng, J. Preparation of Single-Helical Curdlan Hydrogel and Its Activation with Coagulation Factor G. Polymers 2024, 16, 1323. https://doi.org/10.3390/polym16101323
Ru G, Yan X, Wang H, Feng J. Preparation of Single-Helical Curdlan Hydrogel and Its Activation with Coagulation Factor G. Polymers. 2024; 16(10):1323. https://doi.org/10.3390/polym16101323
Chicago/Turabian StyleRu, Geying, Xiaoshuang Yan, Huijuan Wang, and Jiwen Feng. 2024. "Preparation of Single-Helical Curdlan Hydrogel and Its Activation with Coagulation Factor G" Polymers 16, no. 10: 1323. https://doi.org/10.3390/polym16101323
APA StyleRu, G., Yan, X., Wang, H., & Feng, J. (2024). Preparation of Single-Helical Curdlan Hydrogel and Its Activation with Coagulation Factor G. Polymers, 16(10), 1323. https://doi.org/10.3390/polym16101323






