The Use of Polysaccharide Matrices as a Basis for the Formation of Tellurium Nanoparticles with Different Morphologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Partial Alkaline Depolymerization of GM and κ-CG (General Methodology)
2.2.2. Generation of Telluride Anions from Tellurium Powder
2.2.3. Synthesis of Polysaccharide-Capped Tellurium Nanoparticles: General Procedure
3. Results
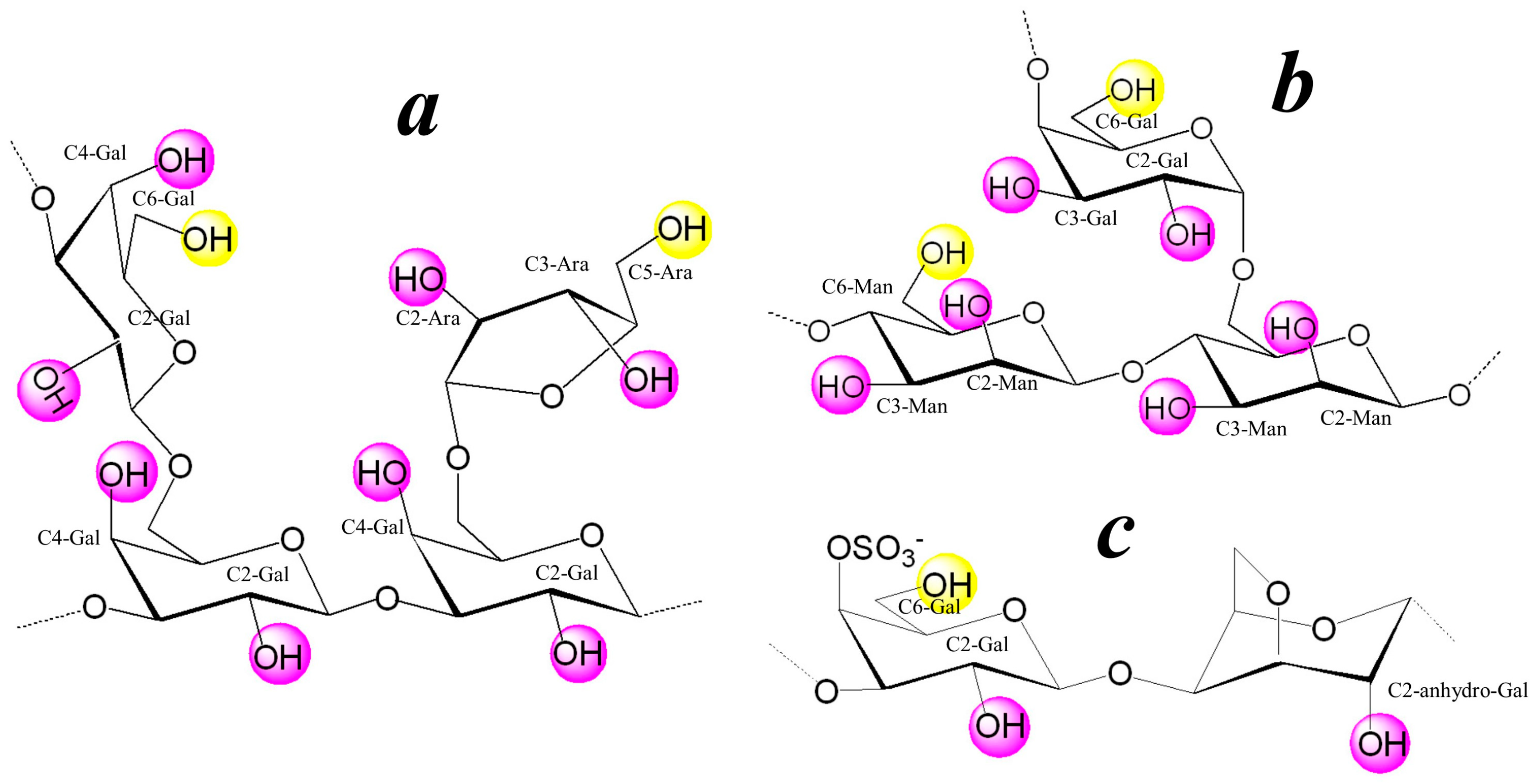
3.1. Structure of the Polysaccharide Macromolecules Ar-Gal, dP-GM, and dP-κ-CG
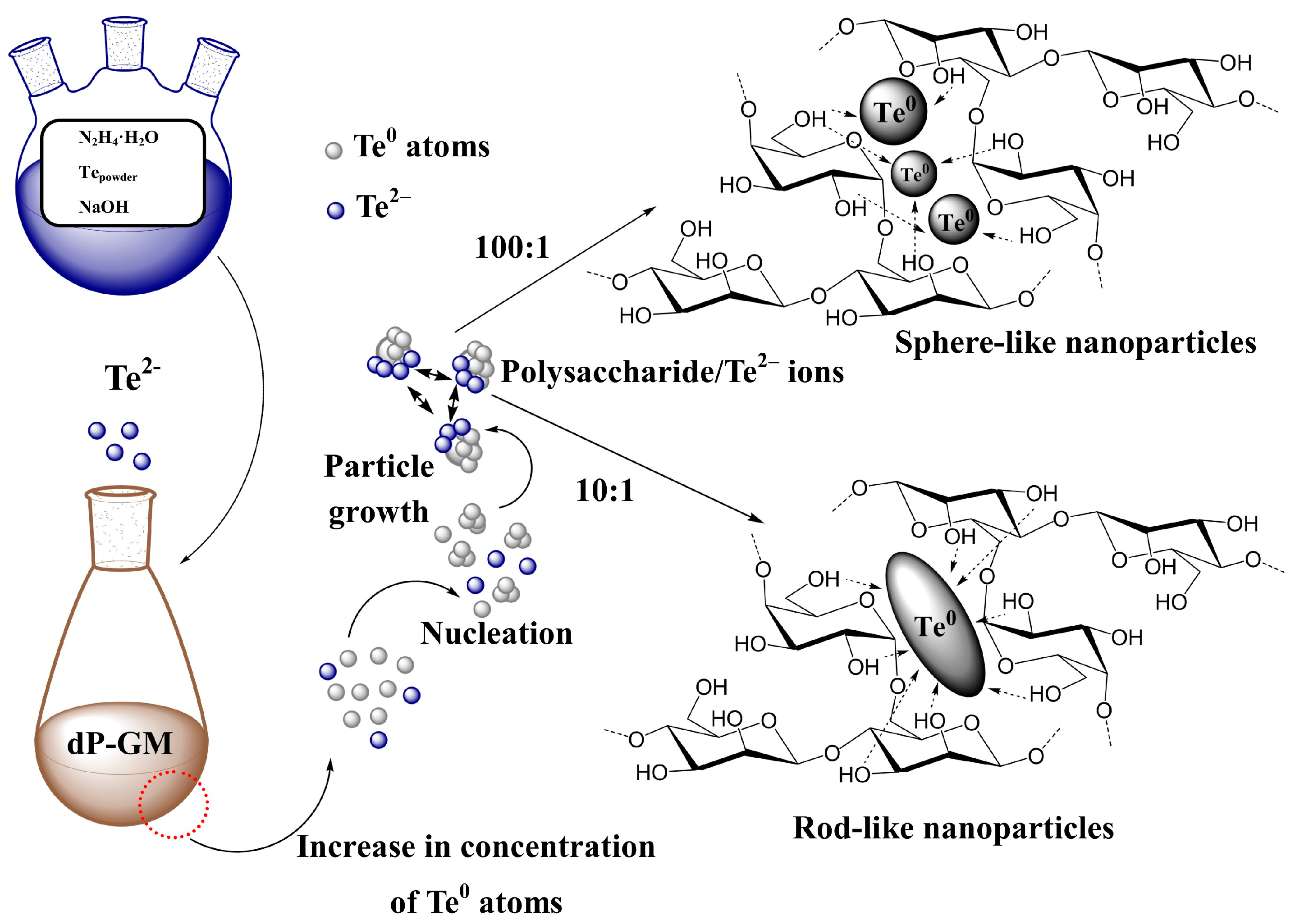
3.2. Synthesis of the Polysaccharides-Stabilized Te0NPs
3.3. Characterization of Structure and Nanomorphology of Polysaccharide-Stabilized Tellurium Nanoparticles
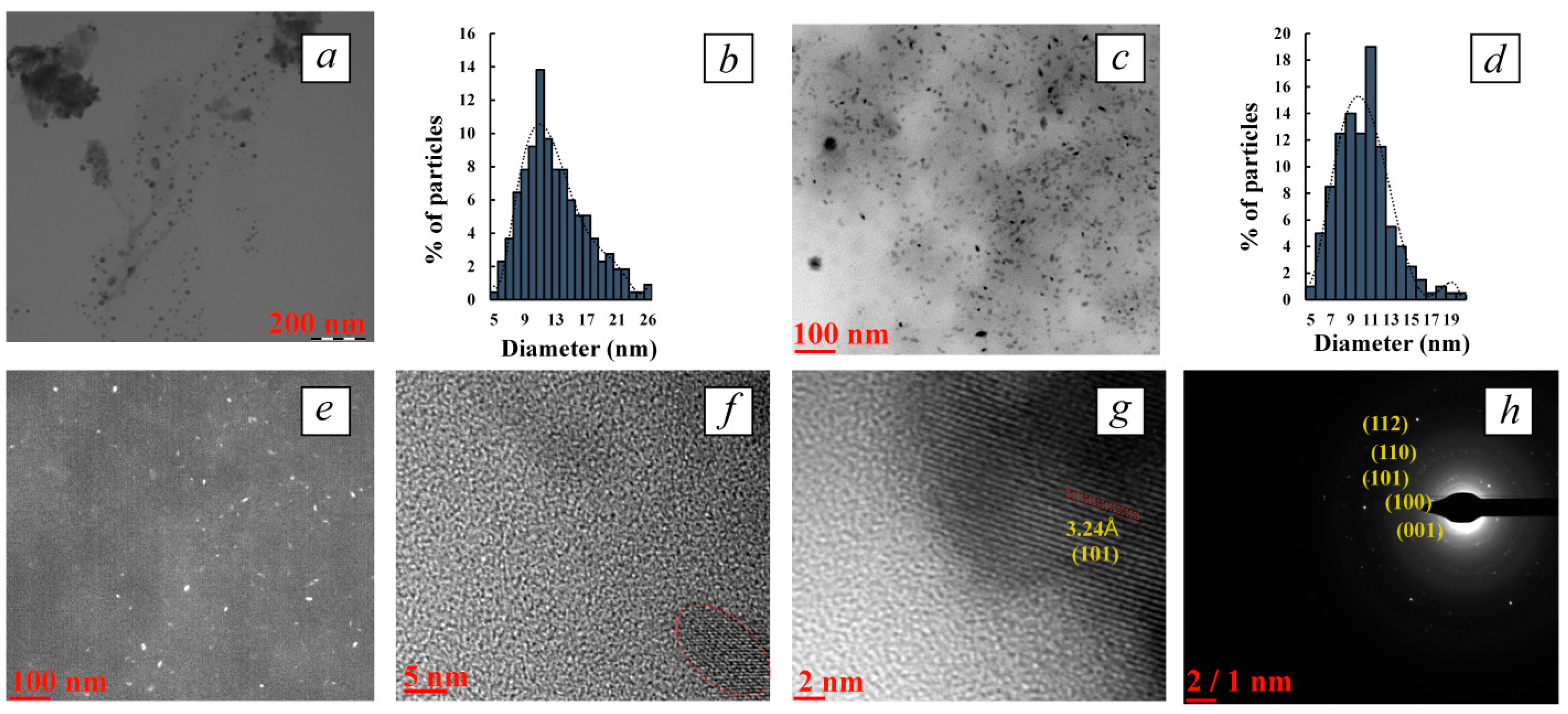
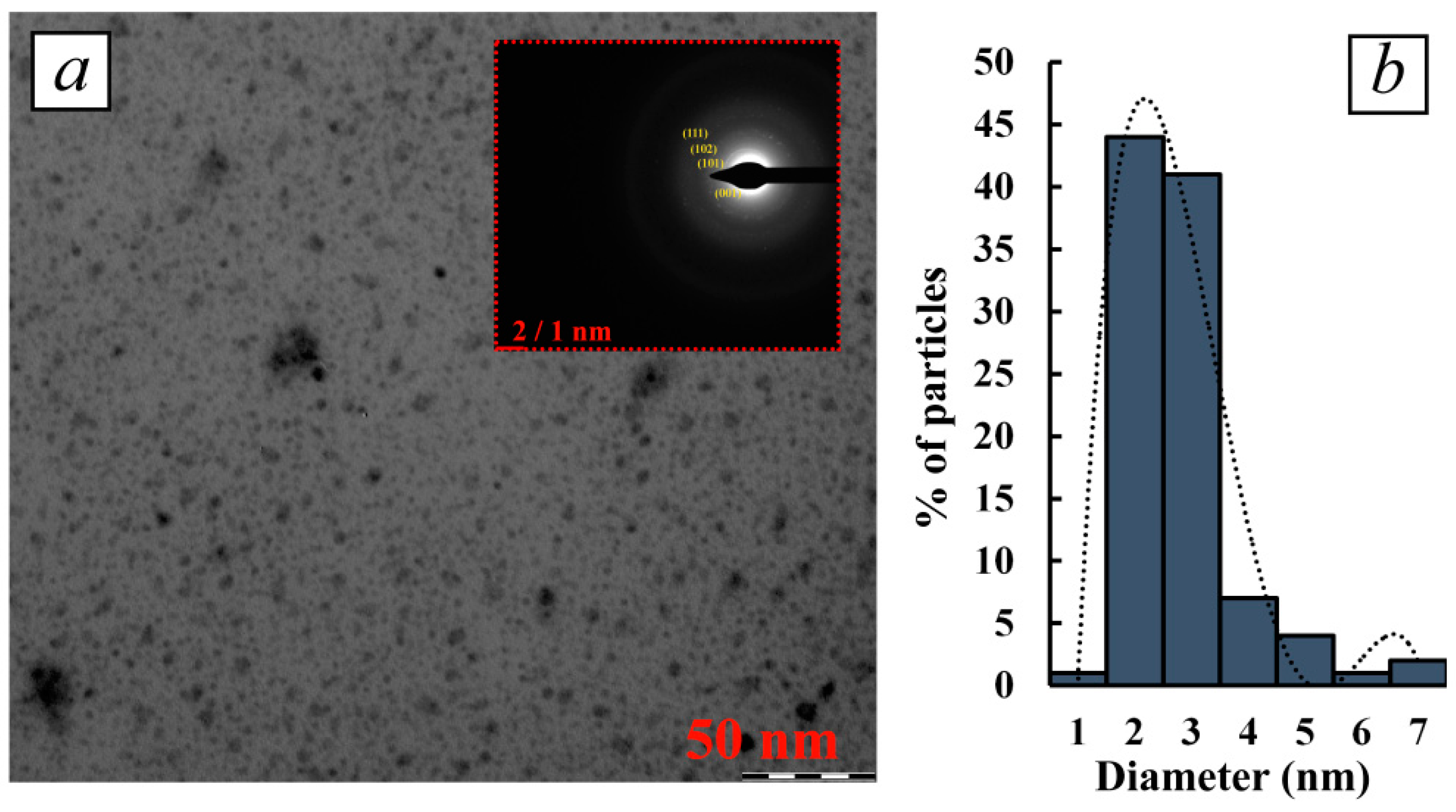
3.3.1. Determination of Nanoparticle Morphology and Characterization of their Structure by TEM and HR-TEM
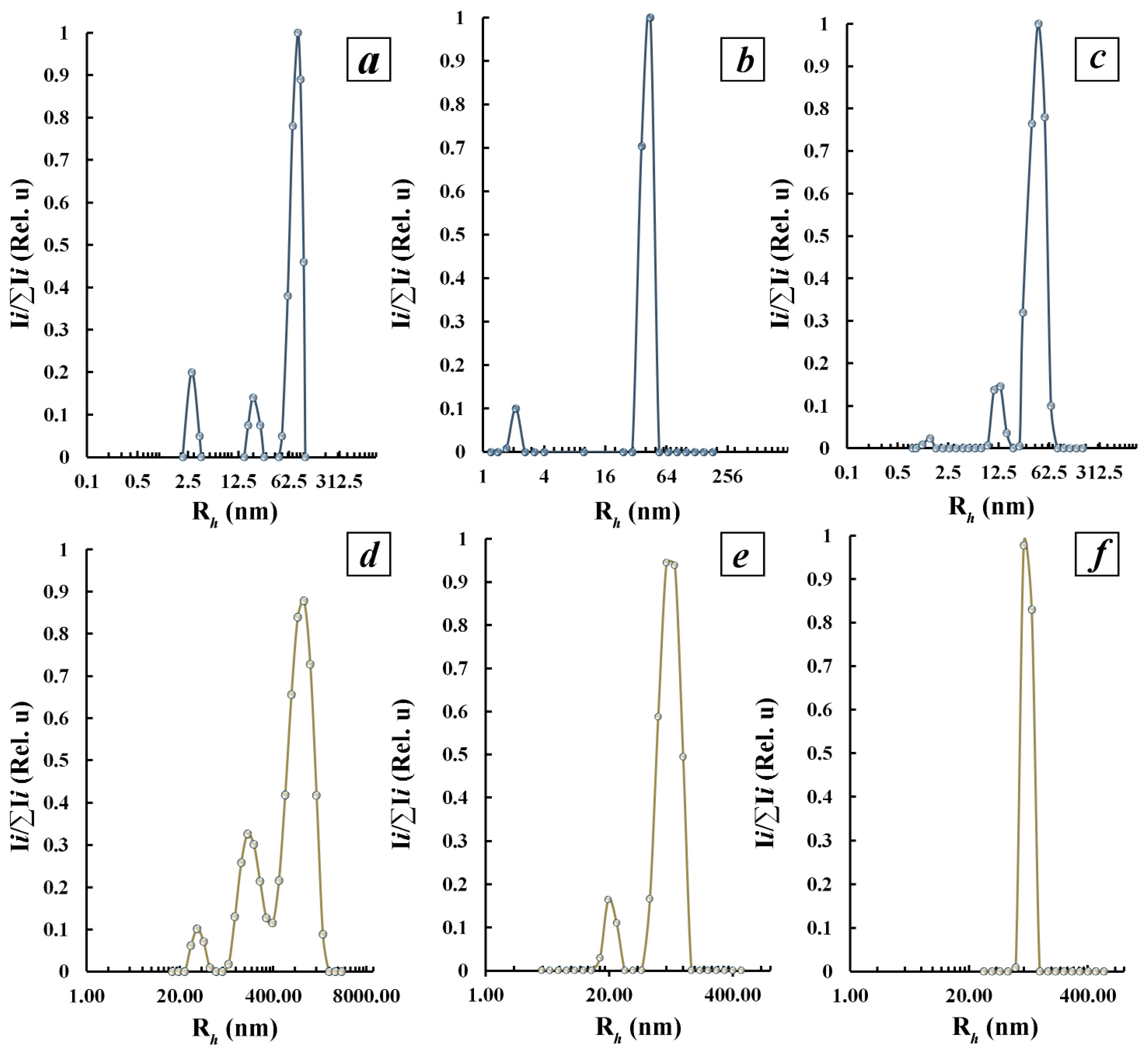
3.3.2. Characterization of Polysaccharide-Stabilized Tellurium Nanoparticles in Aqueous Solutions by Dynamic Light Scattering Method
3.3.3. Optical Absorption Spectroscopy of Polysaccharide-Stabilized Tellurium Nanoparticles with Different Morphologies
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina-Cruz, D.; Tien-Street, W.; Vernet-Crua, A.; Zhang, B.; Huang, X.; Murali, A.; Chen, J.; Liu, Y.; Garcia-Martin, J.M.; Cholula-Díaz, J.L.; et al. Tellurium, the Forgotten Element: A Review of the Properties, Processes, and Biomedical Applications of the Bulk and Nanoscale Metalloid. In Racing for the Surface; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 723–783. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Qin, R. Probing structural chirality of crystals using high-order harmonic generation in solids. Phys. Rev. A 2019, 101, 053423. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.; Zhang, Y. Tellurium Nanotubes and Chemical Analogues from Preparation to Application: A Minor Review. Nanomaterials 2022, 12, 2151. [Google Scholar] [CrossRef]
- Yi, S.; Zhu, Z.; Cai, X.; Jia, Y.; Cho, J.H. The Nature of Bonding in Bulk Tellurium Composed of One-Dimensional Helical Chains. Inorg. Chem. 2018, 57, 5083–5088. [Google Scholar] [CrossRef] [PubMed]
- Grillo, S.; Pulci, O.; Marri, I. Evolution of the Electronic and Optical Properties of Meta-Stable Allotropic Forms of 2D Tellurium for Increasing Number of Layers. Nanomaterials 2022, 12, 2503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, L.; Wang, K.; Liu, H.; Zhang, J.; Yan, S. Progress in the Synthesis and Application of Tellurium Nanomaterials. Nanomaterials 2023, 13, 2057. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, B.K. Semiconductor characteristics of tellurium and its implementations. Phys. Sci. Rev. 2022, 8, 4659–4687. [Google Scholar] [CrossRef]
- Kramer, A.; Van de Put, M.L.; Hinkle, C.L.; Vandenbergh, W.G. Tellurium as a successor of silicon for extremely scaled nanowires: A first-principles study. npj 2D Mater. Appl. 2020, 4, 10. [Google Scholar] [CrossRef]
- Park, H.; Son, W.; Lee, S.H.; Kim, S.; Lee, J.J.; Cho, W.; Choi, H.H.; Kim, J.H. Aqueous chemical synthesis of tellurium nanowires using a polymeric template for thermoelectric materials. CrystEngComm 2015, 17, 1092–1097. [Google Scholar] [CrossRef]
- Dasika, P.; Samantaray, D.; Murali, K.; Abraham, N.; Watanbe, K.; Taniguchi, T.; Ravishankar, N. Contact-barrier free, high mobility, dual-gated junctionless transistor using tellurium nanowire. Adv. Func. Mat. 2021, 31, 2006278. [Google Scholar] [CrossRef]
- Chaves, A.; Azadani, J.G.; Alsalman, H.; da Costa, D.R.; Frisenda, R.; Chaves, A.J.; Song, S.H.; Kim, Y.D.; He, D.; Zhou, J.; et al. Bandgap engineering of two-dimensional semiconductor materials. npj 2D Mater. Appl. 2020, 4, 29. [Google Scholar] [CrossRef]
- Wang, H.; Chai, L.; Xie, Z.; Zhang, H. Recent Advance of Tellurium for Biomedical Applications. Chem. Res. Chin. Univ. 2020, 36, 551–559. [Google Scholar] [CrossRef]
- Zare, B.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shakibaie, M.; Rezaie, S.; Shahverdi, A.R. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mat. Res. Bull. 2012, 47, 3719–3725. [Google Scholar] [CrossRef]
- Abed, N.N.; Abou El-Enain, I.M.M.; El-Husseiny Helal, E.; Yosri, M. Novel biosynthesis of tellurium nanoparticles and investigation of their activity against common pathogenic bacteria. J. Taibah Univ. Med. Sci. 2022, 18, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Ren, Q.; Wu, Y.; Wu, C.; Cheng, Y. Investigation into the Antibacterial Mechanism of Biogenic Tellurium Nanoparticles and Precursor Tellurite. Int. J. Mol. Sci. 2022, 23, 11697. [Google Scholar] [CrossRef]
- Sosedova, L.M.; Rukavishnikov, V.S.; Sukhov, B.G.; Borovskii, G.B.; Titov, E.A.; Novikov, M.A.; Vokina, V.A.; Yakimova, N.L.; Lesnichaya, M.V.; Kon’kova, T.V.; et al. Synthesis of Chalcogen-Containing Nanocomposites of Selenium and Tellurium with Arabinogalactan and a Study of Their Toxic and Antimicrobial Properties. Nanotechnol. Russ. 2018, 13, 290–294. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Haider, S.I.; Solangi, A.R.; Memon, A.F. Toxicity of tellurium and its compounds. Phys. Sci. Rev. 2023, 8, 4375–4390. [Google Scholar] [CrossRef]
- Castro, L.; Li, J.; González, F.; Muñoz, J.A.; Blázquez, M.L. Green synthesis of tellurium nanoparticles by tellurate and tellurite reduction using Aeromonas hydrophila under different aeration conditions. Hydrometallurgy 2020, 196, 105415. [Google Scholar] [CrossRef]
- Beleneva, I.A.; Kharchenko, U.V.; Kukhlevsky, A.D. Boroda, Biogenic synthesis of selenium and tellurium nanoparticles by marine bacteria and their biological activity. World J. Microbiol. Biotechnol. 2022, 38, 178. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Medina-Cruz, D.; Vernet-Crua, A.; Truong, L.B.; Sotelo, E.; Mostafavi, E.; González, M.U.; García-Martín, J.M.; Cholula-Díaz, J.L.; Webster, T.J. Pepper-Mediated Green Synthesis of Selenium and Tellurium Nanoparticles with Antibacterial and Anticancer Potential. J. Funct. Biomater. 2023, 14, 24. [Google Scholar] [CrossRef]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. 2018, 17, 87–97. [Google Scholar]
- Medina Cruz, D.; Tien-Street, W.; Zhang, B.; Huang, X.; Vernet Crua, A.; Nieto-Argüello, A.; Cholula-Díaz, J.L.; Martínez, L.; Huttel, Y.; Ujué González, M.; et al. Citric Juice-mediated Synthesis of Tellurium Nanoparticles with Antimicrobial and Anticancer Properties. Green Chem. 2019, 21, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Medina-Cruz, D.; Vernet-Crua, A.; Mostafavi, E.; González, M.U.; Martínez, L.; Iii, A.D.J.; Kusper, M.; Sotelo, E.; Gao, M.; Geoffrion, L.D.; et al. Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects. Nanomaterials 2021, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Xu, J.; Hu, W.; Yang, J.L.; Yu, S.H. Systematic Synthesis of Tellurium Nanostructures and Their Optical Properties: From Nanoparticles to Nanorods, Nanowires, and Nanotubes. ChemNanoMat 2016, 2, 167–170. [Google Scholar] [CrossRef]
- Guisbiers, G.; Mimun, L.C.; Mendoza-Cruz, R.; Nash, K.L. Synthesis of tunable tellurium nanoparticles. Semicond. Sci. Technol. 2017, 32, 04LT01. [Google Scholar] [CrossRef]
- Gutiérrez-Lazos, C.D.; Solís-Pomar, F.; Meléndrez, M.F.; Espinoza-Rivas, A.M.; Pérez-Guzmán, M.A.; Ortega-Amaya, R.; Ortega-López, M.; Pérez-Tijerina, E. A simple method for the deposition of nanostructured tellurium synthesized in ammonia solution. Appl. Nanosci. 2016, 6, 1053–1057. [Google Scholar] [CrossRef]
- He, W.; Krejci, A.; Lin, J.; Osmulski, M.E.; Dickerson, J.H. A facile synthesis of Te nanoparticles with binary size distribution by green chemistry. Nanoscale 2011, 3, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Schmalz, H.; Xu, Y.; Miyajima, N.; Drechsler, M.; Moeller, M.W.; Schacher, F.; Müller, A.H. Room-Temperature Growth of Uniform Tellurium Nanorods and the Assembly of Tellurium or Fe3O4 Nanoparticles on the Nanorods. Adv. Mater. 2008, 20, 947–952. [Google Scholar] [CrossRef]
- Zhmurova, A.V.; Prozorova, G.F.; Korzhova, S.A.; Pozdnyakov, A.S.; Zvereva, M.V. Synthesis and DC Electrical Conductivity of Nanocomposites Based on Poly(1-vinyl-1,2,4-triazole) and Thermoelectric Tellurium Nanoparticles. Materials 2023, 16, 4676. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Chunlin, X. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, M.V.; Aleksandrova, G.P. Application Potential of Natural Polysaccharides for the Synthesis of Biologically Active Nanobiocomposites (A Review). Russ. J. Genl. Chem. 2023, 93, 347–370. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Kusuma, H.S. Polysaccharides for inorganic nanomaterials synthesis. Green. Sustain. Process Chem. Environ. Eng. Sci. 2021, 8, 201–225. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Zhang, X.; Lin, J.; Wang, M.H. In situ, synthesis of chitosan fabricated tellurium nanoparticles for improved antimicrobial and anticancer applications. Int. J. Biol. Macromol. 2024, 258, 128778. [Google Scholar] [CrossRef] [PubMed]
- Leijiao, L.; Shestakova, L.; Ding, J.; Sun, T.; Li, W.; Ostrovsky, Y. Tellurium-based chitosan hydrogel as an efficient NIR-induced antibacterial platform. Emerg. Cardiol. Cardiovasc. Risks 2023, 7, 2033–2040. [Google Scholar] [CrossRef]
- Vahidi, H.; Kobarfard, F.; Alizadeh, A.; Saravanan, M.; Barabadi, H. Green nanotechnology-based tellurium nanoparticles: Exploration of their antioxidant, antibacterial, antifungal and cytotoxic potentials against cancerous and normal cells compared to potassium tellurite. Inorg. Chem. Comm. 2021, 124, 108385. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, F.; Komarneni, S. A Green Chemical Approach to the Synthesis of Tellurium Nanowires. Langmuir 2005, 21, 6002–6005. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, N.N.; Medvedeva, E.N.; Ivanova, N.V.; Malkov, Y.A.; Babkin, V.A. Polysaccharides from Larch Biomass; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Lesnichaya, M.V.; Aleksandrova, G.P.; Sukhov, B.G.; Rokhin, A.V. Molecular-weight characteristics of galactomannan and carrageenan. Chem. Nat. Compd. 2013, 49, 405–410. [Google Scholar] [CrossRef]
- Gasilova, E.R.; Toropova, A.A.; Bushin, S.V.; Khripunov, A.K.; Grischenko, L.A.; Aleksandrova, G.P. Light Scattering from Aqueous Solutions of Colloid Metal Nanoparticles Stabilized by Natural Polysaccharide Arabinogalactan. J. Phys. Chem. B 2010, 114, 4204–4212. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.E.; Anderson, R.L.; Stone, B.A. Form and function of arabinogalactans and arabinogalactan-proteins. Phytochem 1979, 18, 521–540. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications-A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Aziza, M.; Givernaud, T.; Chikhaoui-khay, M.; Bennasser, L. Seasonal variation of the growth, chemical composition and carrageenan extracted from Hypnea musciformis (Wulfen) Lamouroux harvested along the Atlantic coast of Morocco. Sci. Res. Essay 2008, 2, 509–514. [Google Scholar]
- Aleksandrova, G.P.; Boymirzaev, A.S.; Lesnichaya, M.V.; Sukhov, B.G.; Trofimov, B.A. Metal-polymer nanobiocomposites with galactose-containing stabilizing matrices: Dimensional effect in changes of molar mass parameters. Russ. J. Gen. Chem. 2015, 85, 488–496. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, Y.; Oda, K.; Kato, K. Acceptor activity of subcellular membranes for two terminal sugars, galactose and sialic acid. J. Biochem. 1977, 81, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Schlepper-Schäfer, J.; Hülsmann, D.; Djovkar, A.; Meyer, H.E.; Herbertz, L.; Kolb, H.; Kolb-Bachofen, V. Endocytosis via galactose receptors in vivo. Ligand size directs uptake by hepatocytes and/or liver macrophages. Exp. Cell Res. 1986, 165, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, S.; Jose, S. An Innovative Approach in Developing Sustained Release Matrix Tablets using Isolated and Characterized Novel Ceasalpinia sappan L. Seed Galactomannan. Indian. J. Pharm. Ed. Res. 2019, 53, 239–245. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Geronço, M.S.; da Silveira Ramos, I.F.; da Silva Filho, E.C.; dos Santos Rizzo, M.; Ribeiro, A.B.; da Costa, M.P. Are Structurally Modified Galactomannan Derivatives Biologically Active? Polysaccharides 2021, 2, 1–15. [Google Scholar] [CrossRef]
- Grishchenko, L.A.; Parshina, L.N.; Larina, L.I.; Belovezhetz, L.A.; Trofimov, B.A. Arabinogalactan propargyl ethers in the A3-coupling reaction with aldehydes and secondary cyclic amines. Carb. Pol. 2023, 300, 120239. [Google Scholar] [CrossRef]
- Deryagina, E.N.; Russavskaya, N.V.; Papernaya, L.K.; Levanova, E.P.; Sukhomazova, E.N.; Korchevin, N.A. Synthesis of organochalcogen compounds in basic reducing systems. Russ. Chem. Bull. 2005, 54, 2473–2483. [Google Scholar]
- Whitehead, C.B.; Özkar, S.; Finke, R.G. LaMer’s 1950 Model for Particle Formation of Instantaneous Nucleation and Diffusion-Controlled Growth: A Historical Look at the Model’s Origins, Assumptions, Equations, and Underlying Sulfur Sol Formation Kinetics Data. Chem. Mater. 2019, 31, 7116–7132. [Google Scholar] [CrossRef]
- Chhabra, R.; Basavar, M.G. Colloidal Dispersions. In Coulson and Richardson’s Chemical Engineering, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 693–737. [Google Scholar] [CrossRef]
- García, Á.G.; Nagelkerke, M.M.B.; Tuinier, R.; Vis, M. Polymer-mediated colloidal stability: On the transition between adsorption and depletion. Adv. Coll. Interf. Sci. 2020, 275, 102077. [Google Scholar] [CrossRef]
- Ortega-Vinuesa, J.L.; Martín-Rodríguez, A.; Hidalgo-Álvarez, R. Colloidal Stability of Polymer Colloids with Different Interfacial Properties: Mechanisms. J. Coll. Interf. Sci. 1996, 184, 259–267. [Google Scholar] [CrossRef]
- Xue, X.; Penn, R.L.; Leite, E.R.; Huang, F.; Lin, Z. Crystal growth by oriented attachment: Kinetic models and control factors. CrystEngComm 2014, 16, 1419–1429. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Zeichner, G.R.; Schowalter, W.R. Effects of hydrodynamic and colloidal forces on the coagulation of dispersions. J. Colloid. Interf. Sci. 1979, 71, 237–253. [Google Scholar] [CrossRef]
- Gasilova, E.R.; Matveeva, G.N.; Aleksandrova, G.P.; Sukhov, B.G.; Trofimov, B.A. Colloidal Aggregates of Pd Nanoparticles Supported by Larch Arabinogalactan. J. Phys. Chem. B 2013, 117, 2134–2141. [Google Scholar] [CrossRef]
- Burchard, W. Physical Techniques for the Study of Food Biopolymers; Simon, B.R.-M., Ed.; Springer: Greer, SC, USA, 1994; pp. 151–213. [Google Scholar]
- Ma, C.; Yan, J.; Huang, Y.; Wang, C.; Yang, G. The optical duality of tellurium nanoparticles for broadband solar energy harvesting and efficient photothermal conversion. Sci. Adv. 2018, 4, eaas9894. [Google Scholar] [CrossRef]
- Abo Elsoud, M.; Al-Hagar, O.E.A.; Abdelkhalek, E.S.; Sidkey, N.M. Synthesis and investigations on tellurium myconanoparticles. Biotechnol. Rep. 2018, 18, e00247. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Zhang, Y.; Teng, R.; Huang, T.; Chen, C.; Lu, G. Controlled synthesis of tellurium nanowires and nanotubes via a facile, efficient, and relatively green solution phase method. J. Mat. Chem. A 2013, 1, 15046–15052. [Google Scholar] [CrossRef]
- Rozentsveig, I.B.; Nikonova, V.S.; Manuilov, V.V.; Ushakov, I.A.; Borodina, T.N.; Smirnov, V.I.; Korchevin, N.A. Heterocyclization of Bis(2-chloroprop-2-en-1-yl) sulfide in Hydrazine Hydrate–KOH: Synthesis of Thiophene and Pyrrole Derivatives. Molecules 2022, 27, 6785. [Google Scholar] [CrossRef]
- Levanova, E.P.; Vilms, A.I.; Bezborodov, V.A.; Babenko, I.A.; Sosnovskaya, N.G.; Istomina, N.V.; Albanov, A.I.; Russavskaya, N.V.; Rozentsveig, I.B. Synthesis of polydentate chalcogen-containing ligands using the system hydrazine hydrate–base. Russ. J. Gen. Chem. 2017, 87, 396–401. [Google Scholar] [CrossRef]
- Lesnichaya, M.V.; Sukhov, B.G.; Shendrik, R.Y.; Sapozhnikov, A.N.; Trofimov, B.A. Synthesis of Water-Soluble Silver Selenide Quantum Dots Luminescing within the Transparency Window of Biological Tissues. Russ. J. Gen. Chem. 2018, 88, 284–287. [Google Scholar] [CrossRef]
- Lesnichaya, M.V.; Zhmurova, A.V.; Sapozhnikov, A.N. Synthesis and Characterization of Water-Soluble Arabinogalactan-Stabilized Bismuth Telluride Nanoparticles. Russ. J. Gen. Chem. 2021, 91, 1379–1386. [Google Scholar] [CrossRef]
- Zvereva, M.V.; Zhmurova, A.V. Synthesis, Structure, and Spectral Properties of ZnTe-Containing Nanocomposites Based on Arabinogalactan. Russ. J. Gen. Chem. 2022, 92, 1995–2004. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvereva, M. The Use of Polysaccharide Matrices as a Basis for the Formation of Tellurium Nanoparticles with Different Morphologies. Polymers 2024, 16, 1482. https://doi.org/10.3390/polym16111482
Zvereva M. The Use of Polysaccharide Matrices as a Basis for the Formation of Tellurium Nanoparticles with Different Morphologies. Polymers. 2024; 16(11):1482. https://doi.org/10.3390/polym16111482
Chicago/Turabian StyleZvereva, Marina. 2024. "The Use of Polysaccharide Matrices as a Basis for the Formation of Tellurium Nanoparticles with Different Morphologies" Polymers 16, no. 11: 1482. https://doi.org/10.3390/polym16111482
APA StyleZvereva, M. (2024). The Use of Polysaccharide Matrices as a Basis for the Formation of Tellurium Nanoparticles with Different Morphologies. Polymers, 16(11), 1482. https://doi.org/10.3390/polym16111482





