Antimicrobial Solid Starch–Iodine Complex via Reactive Extrusion and Its Application in PLA-PBAT Blown Films
Abstract
1. Introduction
- (1)
- Active packaging: as an additive for film to be used in active packaging, ensuring food safety.
- (2)
- Air filtration systems: as an additive to make foams or filters to be used in air filtration systems for deactivating airborne viruses, including SARS-CoV-2.
- (3)
- Polymer enhancement: in pellet form as an additive to provide antibacterial and antiviral properties in other plastics and polymers.
2. Experimental Section
2.1. Materials
2.2. Preparation of MTPS–Iodine Pellets
2.3. Preparation of PLA-PBAT-Starch–Iodine Films
3. Characterization and Analysis
3.1. Thermogravimetric Analysis (TGA)
3.2. Antimicrobial Properties: Antifungal Test
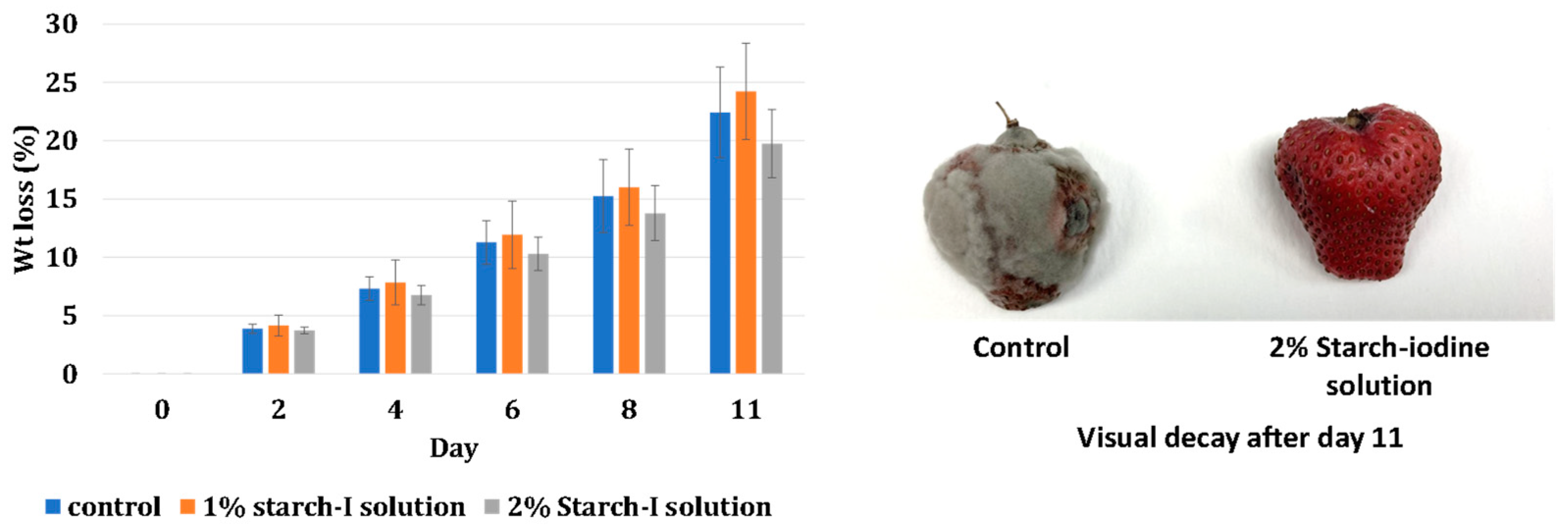
Visual Decay and Weight Loss of Strawberries
3.3. Antimicrobial Properties
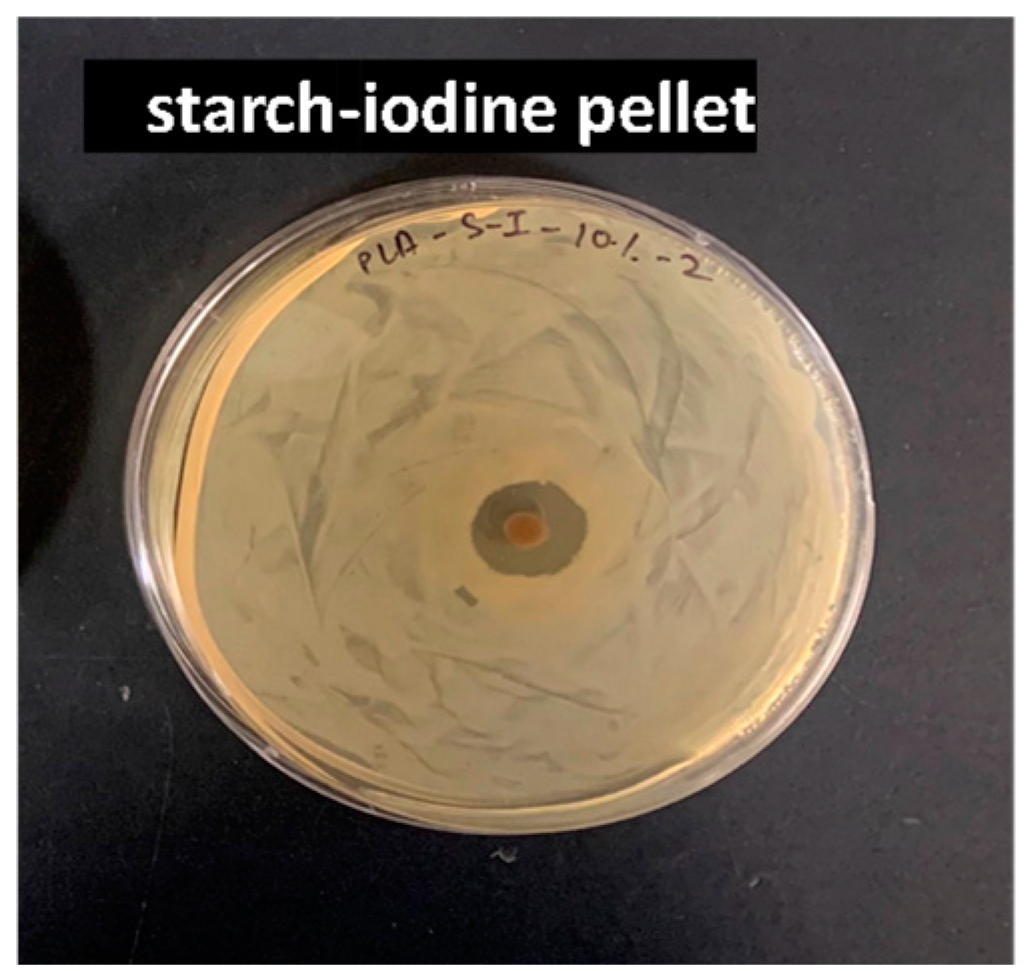
3.3.1. Antibacterial (Kirby–Bauer) Test
3.3.2. Antimicrobial Properties: Antibacterial (Direct Inoculation) Test
3.4. Mechanical Properties
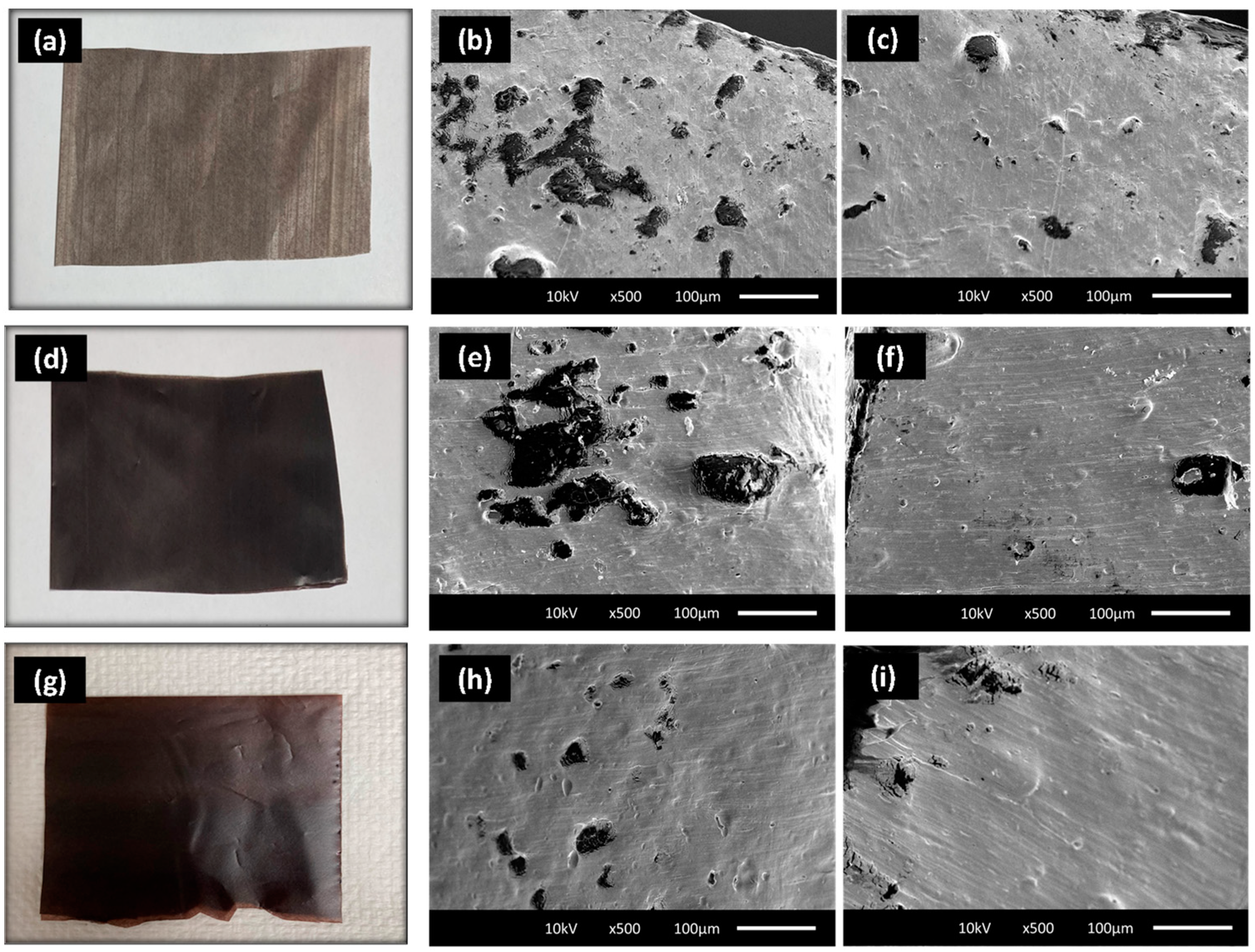
3.5. Scanning Electron Microscopy
4. Results and Discussion
4.1. Iodine Content Evaluation and Thermal Stability of the Blends
4.2. Antifungal Properties: Effect on Weight Loss and Visual Decay
4.3. Antimicrobial Activity Studies on Pellets and Films
4.4. Mechanical Properties and Morphology of the Films
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jandhyala, H. Top 5 trends shaping the antimicrobial packaging market. Packaging Digest, 16 March 2020. [Google Scholar]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A Review on Antimicrobial Packaging for Extending the Shelf Life of Food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Mathur, R.; Kharkwal, H.; Malhotra, B.; Keshwani, A. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Moulay, S. Molecular iodine/polymer complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity against SARS-CoV-2, the Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Landsman, T.; Touchet, T.; Hasan, S.; Smith, C.; Russell, B.; Rivera, J.; Maitland, D.; Cosgriff-Hernandez, E. A shape memory foam composite with enhanced fluid uptake and bactericidal properties as a hemostatic agent. Acta Biomater. 2017, 47, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lundin, J.G.; McGann, C.L.; Weise, N.K.; Estrella, L.A.; Balow, R.B.; Streifel, B.C.; Wynne, J.H. Iodine binding and release from antimicrobial hemostatic polymer foams. React. Funct. Polym. 2019, 135, 44–51. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Valentini, P.; Mussino, F.; Pompa, P.P.; Athanassiou, A.; Bayer, I.S. Antibacterial bioelastomers with sustained povidone-iodine release. Chem. Eng. J. 2018, 347, 19–26. [Google Scholar] [CrossRef]
- Greff, R.J.; Askill, I.N.; Lee, C.C. EP1028848A1—Prepolymer Compositions Comprising an Antimicrobial Agent—Google Patents. EP1028848A1, 23 August 2000. Available online: https://patents.google.com/patent/EP1028848A1/en (accessed on 30 April 2022).
- Li, S.-H.; Wang, Y.; Gao, H.-B.; Zhao, K.; Hou, Y.-C.; Sun, W. Experimental study on the toxicity of povidone-iodine solution in brain tissues of rabbits. Int. J. Clin. Exp. Med. 2015, 8, 14863–14870. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4658857/ (accessed on 28 March 2022). [PubMed]
- Klimaviciute, R.; Bendoraitiene, J.; Rutkaite, R.; Siugzdaite, J.; Zemaitaitis, A. Preparation, stability, and antimicrobial activity of cationic cross-linked starch-iodine complexes. Int. J. Biol. Macromol. 2012, 51, 800–807. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z. Biofunctionalization of textile materials. 3. fabrication of poly(lactide)-potassium iodide composites with antifungal properties. Coatings 2020, 10, 593. [Google Scholar] [CrossRef]
- Makhayeva Danelya, N.; Galiya, S. Advances in antimicrobial polymeric iodophors. Eur. Polym. J. 2023, 201, 112573. [Google Scholar] [CrossRef]
- Saenger, W. The Structure of the Blue Starch-Iodine Complex. Naturwissenschaften 1984, 71, 31–36. Available online: https://link.springer.com/article/10.1007/BF00365977 (accessed on 28 March 2022). [CrossRef]
- Pesek, S.; Silaghi-Dumitrescu, R. The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2024, 29, 641. [Google Scholar] [CrossRef] [PubMed]
- Starch and Iodine—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Bookshelves/Biological_Chemistry/Supplemental_Modules_(Biological_Chemistry)/Carbohydrates/Case_Studies/Starch_and_Iodine (accessed on 28 March 2022).
- Tran, M.; Castro, J.; O’Brien, K.R.; Pham, C.; Bird, T.H.; Iovine, P.M. Release Kinetics and Antimicrobial Properties of Iodinated Species Liberated from Physically and Chemically Modified Starch Granules. Starch/Staerke 2020, 72, 1–7. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Ulmer, H.; Klay, N.; van Dijl, J.M. New In Situ-Generated Polymer-Iodine Complexes with Broad-Spectrum Antimicrobial Activity. Microbiol. Spectr. 2022, 10, e0055022. [Google Scholar] [CrossRef]
- Kulkarni, A.; Narayan, R. Effects of Modified Thermoplastic Starch on Crystallization Kinetics and Barrier Properties of PLA. Polymers 2021, 13, 4125. [Google Scholar] [CrossRef]
- Raquez, J.M.; Nabar, Y.; Narayan, R.; Dubois, P. In Situ Compatibilization of Maleated Thermoplastic Starch/Polyester Melt-Blends by Reactive Extrusion. Polym. Eng. Sci. 2008, 48, 1747–1754. [Google Scholar] [CrossRef]
- Gajdosova, V.; Strachota, B.; Strachota, A.; Michalkova, D.; Krejcikova, S.; Fulin, P.; Nyc, O.; Brinek, A.; Zemek, M.; Slouf, M. Biodegradable Thermoplastic Starch/Polycaprolactone Blends with Co-Continuous Morphology Suitable for Local Release of Antibiotics. Materials 2022, 15, 1101. [Google Scholar] [CrossRef]
- Hablot, E.; Dewasthale, S.; Zhao, Y.; Zhiguan, Y.; Shi, X.; Graiver, D.; Narayan, R. Reactive extrusion of glycerylated starch and starch-polyester graft copolymers. Eur. Polym. J. 2013, 49, 873–881. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydr. Polym. 2020, 239, 116231. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Tango, C.N.; Chelliah, R.; Oh, D.H. Development of antimicrobial edible coating based on modified chitosan for the improvement of strawberries shelf life. Food Sci. Biotechnol. 2019, 28, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.S.; Stark, N.M.; Bergholz, T.M.; Kraft, A.L. Spin coating method improved the performance characteristics of films obtained from poly(lactic acid) and cellulose nanocrystals. Sustain. Mater. Technol. 2020, 26, e00212. [Google Scholar] [CrossRef]
- ISO 22196:2011; Plastics-Measurement of Antibacterial Activity on Plastics Surfaces. ISO: Geneva, Switzerland, 2007.
- ASTM D882-10; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D1922; Standard Test Method for Propagation Tear Resistance of Plastic Film and Thin Sheeting by Pendulum Method. ASTM International: West Conshohocken, PA, USA, 2023.
- He, X.; Zhang, F.; Li, C.; Ding, W.; Jin, Y.; Tang, L.; Huang, R. Effect of Starch Plasticization on Morphological, Mechanical, Crystalline, Thermal, and Optical Behavior of Poly(butylene adipate-co-terephthalate)/Thermoplastic Starch Composite Films. Polymers 2024, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.M.C.; Correia, P.R.C.; Druzian, J.I.; Fakhouri, F.M.; Fialho, R.L.L.; de Albuquerque, E.C.M.C. PBAT/TPS Composite Films Reinforced with Starch Nanoparticles Produced by Ultrasound. Int. J. Polym. Sci. 2017, 2017, 4308261. [Google Scholar] [CrossRef]
- Aitboulahsen, M.; Zantar, S.; Laglaoui, A.; Chairi, H.; Arakrak, A.; Bakkali, M.; Zerrouk, M.H. Gelatin-Based Edible Coating Combined with Mentha pulegium Essential Oil as Bioactive Packaging for Strawberries. J. Food Qual. 2018, 2018, 8408915. [Google Scholar] [CrossRef]
- Abu Salha, B.; Gedanken, A. Extending the Shelf Life of Strawberries by the Sonochemical Coating of their Surface with Nanoparticles of an Edible Anti-Bacterial Compound. Appl. Nano 2021, 2, 14–24. [Google Scholar] [CrossRef]
- Yang, S.; Shang, P.; Zhang, K.; Wang, J.; Zhang, B.; Gao, X.; Waterhouse, G.I.N.; Xie, J.; Zhang, L.; Xu, J. PBAT/PLA food packaging film containing sodium dehydroacetate-loaded diatomite as an antibacterial agent: Fabrication, water-gas regulation and long-acting antimicrobial mechanism. Food Chem. 2024, 446, 138880. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of antibacterial poly(lactide)/poly(butylene adipate-co-terephthalate) composite films incorporated with grapefruit seed extract. Int. J. Biol. Macromol. 2018, 120, 846–852. [Google Scholar] [CrossRef]
- Pan, H.; Ju, D.; Zhao, Y.; Wang, Z.; Yang, H.; Zhang, H.; Dong, L. Mechanical properties, hydrophobic properties and thermal stability of the biodegradable poly(butylene adipate-co-terephthalate)/maleated thermoplastic starch blown films. Fibers Polym. 2016, 17, 1540–1549. [Google Scholar] [CrossRef]
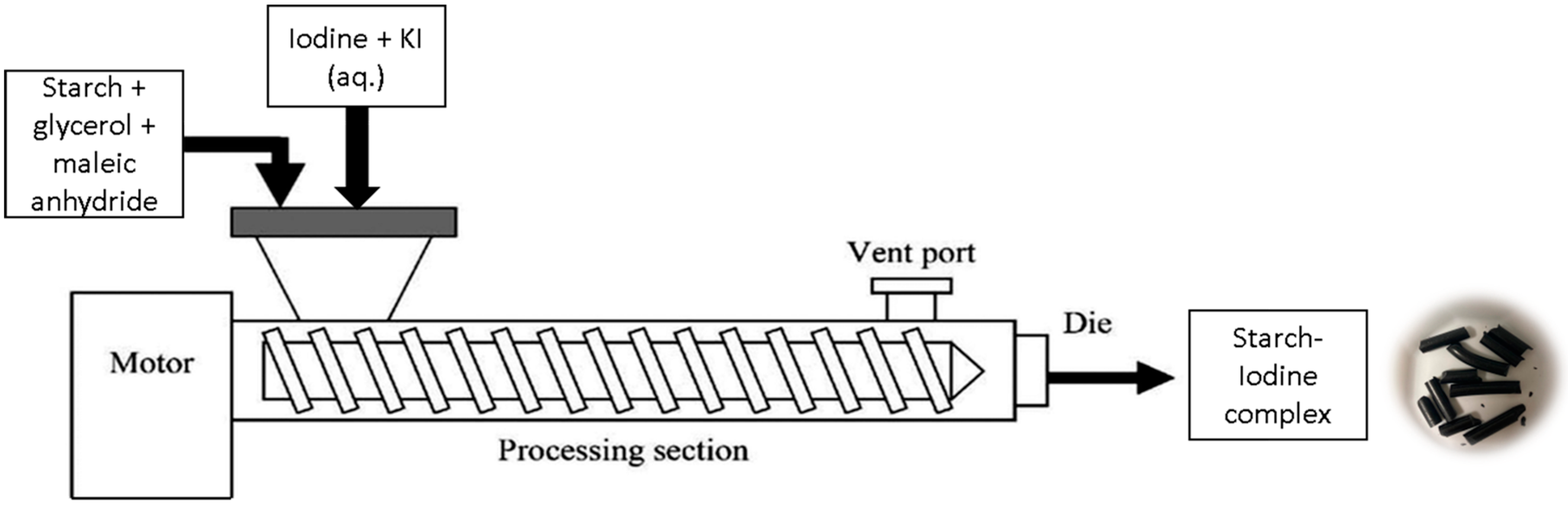
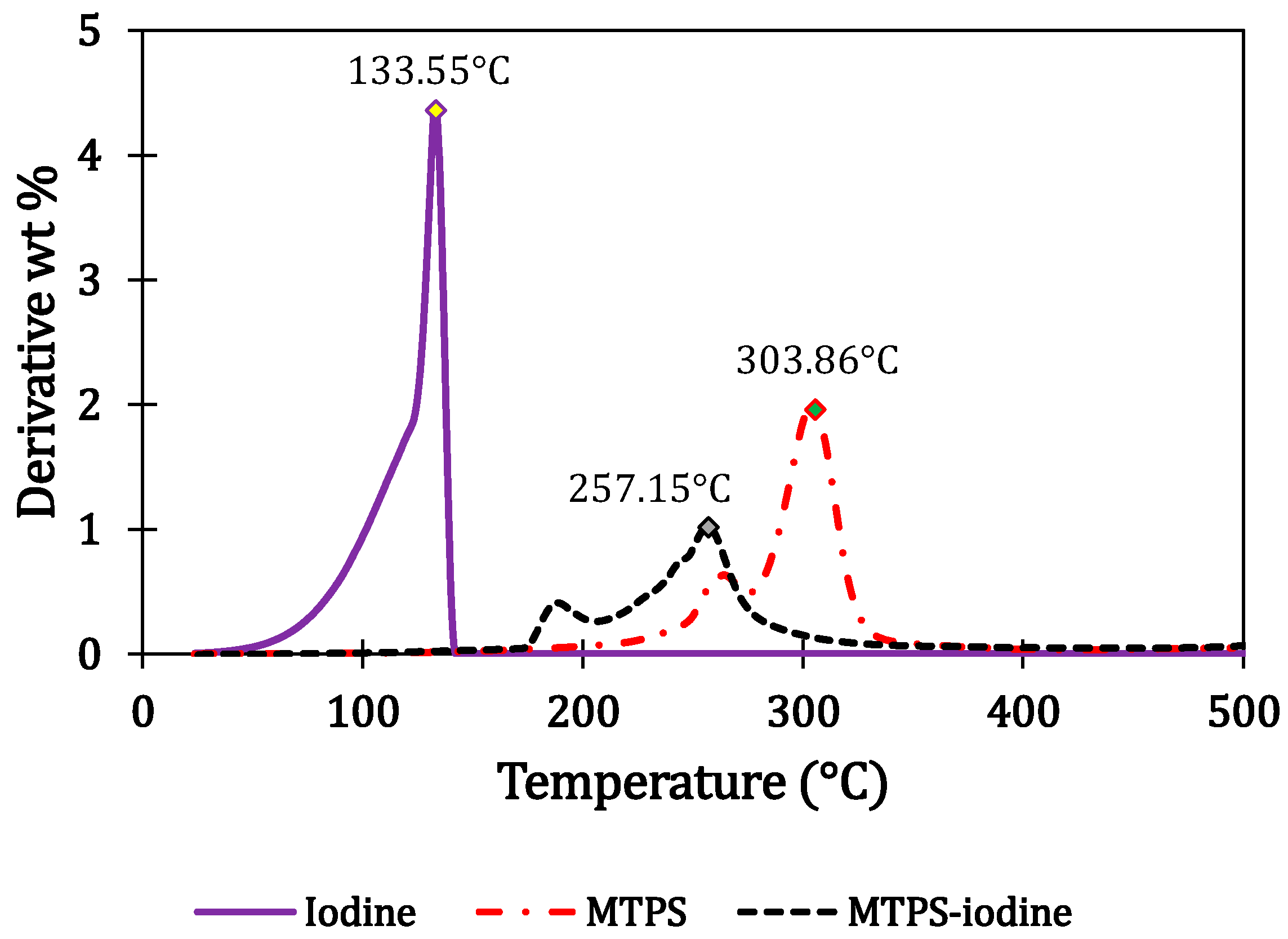
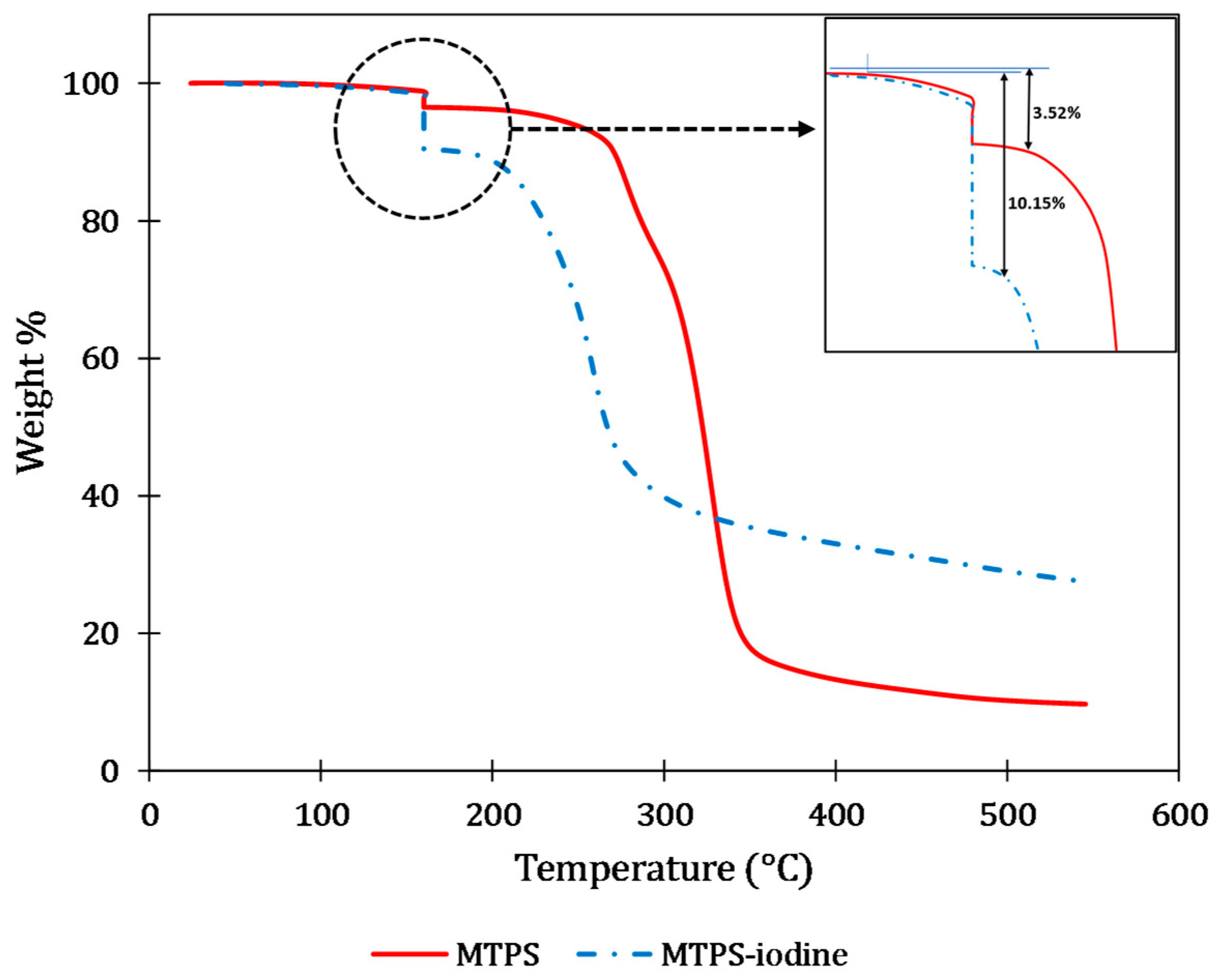
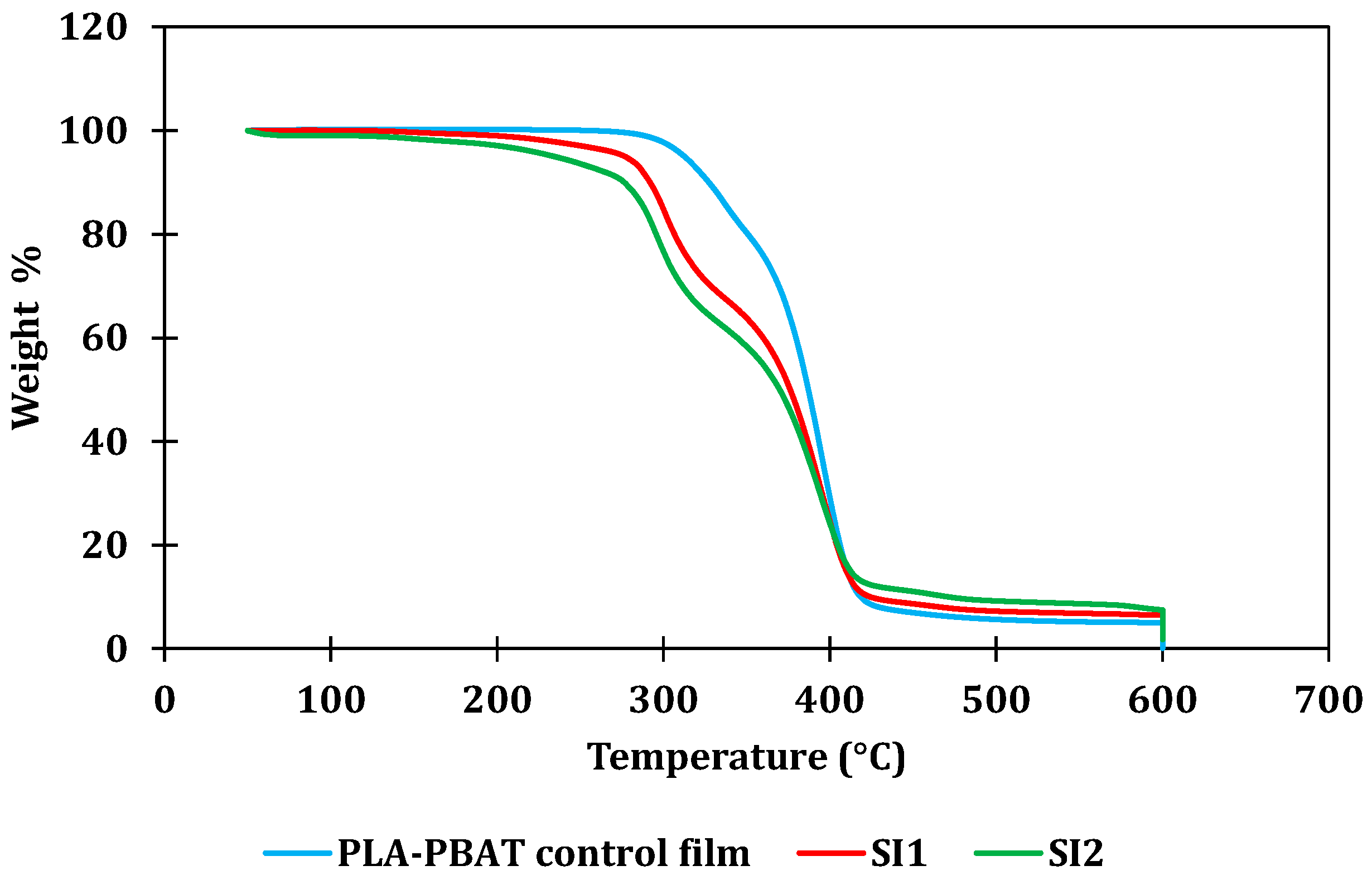


| (a) | |||||||||
| Feed Zone Temp (°C) | Zone 2 Temp (°C) | Zone 3 Temp (°C) | Zone 4 Temp (°C) | Zone 5 Temp (°C) | Zone 6 Temp (°C) | Zone 7 Temp (°C) | Zone 8 Temp (°C) | Zone 9 Temp (°C) | Die Temp (°C) |
| 60 | 80 | 100 | 110 | 120 | 120 | 130 | 140 | 140 | 130 |
| (b) | |||||||||
| Upper Die Temp (°C) | Lower Die Temp (°C) | Die Adapter Temp (°C) | Zone 3 (°C) | Zone 2 (°C) | Zone 1 (°C) | Screw RPM | Upper Pull Rate (ft/min) | Lower Pull Rate (ft/min) | Motor Amps |
| 170 | 170 | 165 | 160 | 154 | 148 | 100 | 10 | 10.5 | 77 |
| Formulation Code | Iodine Content | Film Thickness |
|---|---|---|
| SI1 | 0.7% | 1 mil (25.4 μm) |
| SI2 | 0.7% | 2 mil (50.8 μm) |
| SI3 | 1.3% | 2 mil (50.8 μm) |
| PLA-PBAT film | - | 1 mil (25.4 μm) |
| AM film | Silver Antimicrobial present | 1 mil (25.4 μm) |
| Temperature (°C) | ||
|---|---|---|
| T5% | T50% | |
| Control PLA-PBAT film | 312.46 | 386.96 |
| SI1 (10% MB) | 276.62 | 376.12 |
| SI2 (18% MB) | 234.15 | 369.65 |
| Tensile Strength (MPa) | Extension at Break (%) | Tear Strength (N) | ||||
| Machine Direction (MD) | Transverse Direction (TD) | Machine Direction (MD) | Transverse Direction (TD) | Machine Direction (MD) | Transverse Direction (TD) | |
| Control PLA-PBAT | 28 ± 3 | 30 ± 3 a | 188 ± 67 a | 464 ± 50 a | 1.47 ± 0.14 a | 0.45 ± 0.04 a |
| SI1 | 26 ± 2 | 11 ± 2 b | 200 ± 40 a | 115 ± 42 b | 0.92 ± 0.28 b | 0.76 ± 0.10 b |
| SI2 | 27 ± 1 | 12 ± 1 b | 291 ± 10 b | 120 ± 64 b | 0.80 ± 0.17 b | 0.66 ± 0.12 b |
| SI3 | 23 ± 3 | 11 ± 1 b | 185 ± 74 a | 96 ± 15 b | 0.64 ± 0.15 b | 0.90 ± 0.19 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.; Sharma, D.; Ermlich, A.; Manjure, S.; Narayan, R.; Bergholz, T.M. Antimicrobial Solid Starch–Iodine Complex via Reactive Extrusion and Its Application in PLA-PBAT Blown Films. Polymers 2024, 16, 1487. https://doi.org/10.3390/polym16111487
Kulkarni A, Sharma D, Ermlich A, Manjure S, Narayan R, Bergholz TM. Antimicrobial Solid Starch–Iodine Complex via Reactive Extrusion and Its Application in PLA-PBAT Blown Films. Polymers. 2024; 16(11):1487. https://doi.org/10.3390/polym16111487
Chicago/Turabian StyleKulkarni, Apoorva, Dimple Sharma, Alexander Ermlich, Shilpa Manjure, Ramani Narayan, and Teresa M. Bergholz. 2024. "Antimicrobial Solid Starch–Iodine Complex via Reactive Extrusion and Its Application in PLA-PBAT Blown Films" Polymers 16, no. 11: 1487. https://doi.org/10.3390/polym16111487
APA StyleKulkarni, A., Sharma, D., Ermlich, A., Manjure, S., Narayan, R., & Bergholz, T. M. (2024). Antimicrobial Solid Starch–Iodine Complex via Reactive Extrusion and Its Application in PLA-PBAT Blown Films. Polymers, 16(11), 1487. https://doi.org/10.3390/polym16111487









