Blends of Carboxymethyl Cellulose and Cottonseed Protein as Biodegradable Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
2.3. Fourier Transform Infrared (FT-IR)
2.4. Mechanical Properties of the Films
2.5. Water Vapor Permeability (WVP)
2.6. Opacity
2.7. Thermal Analysis
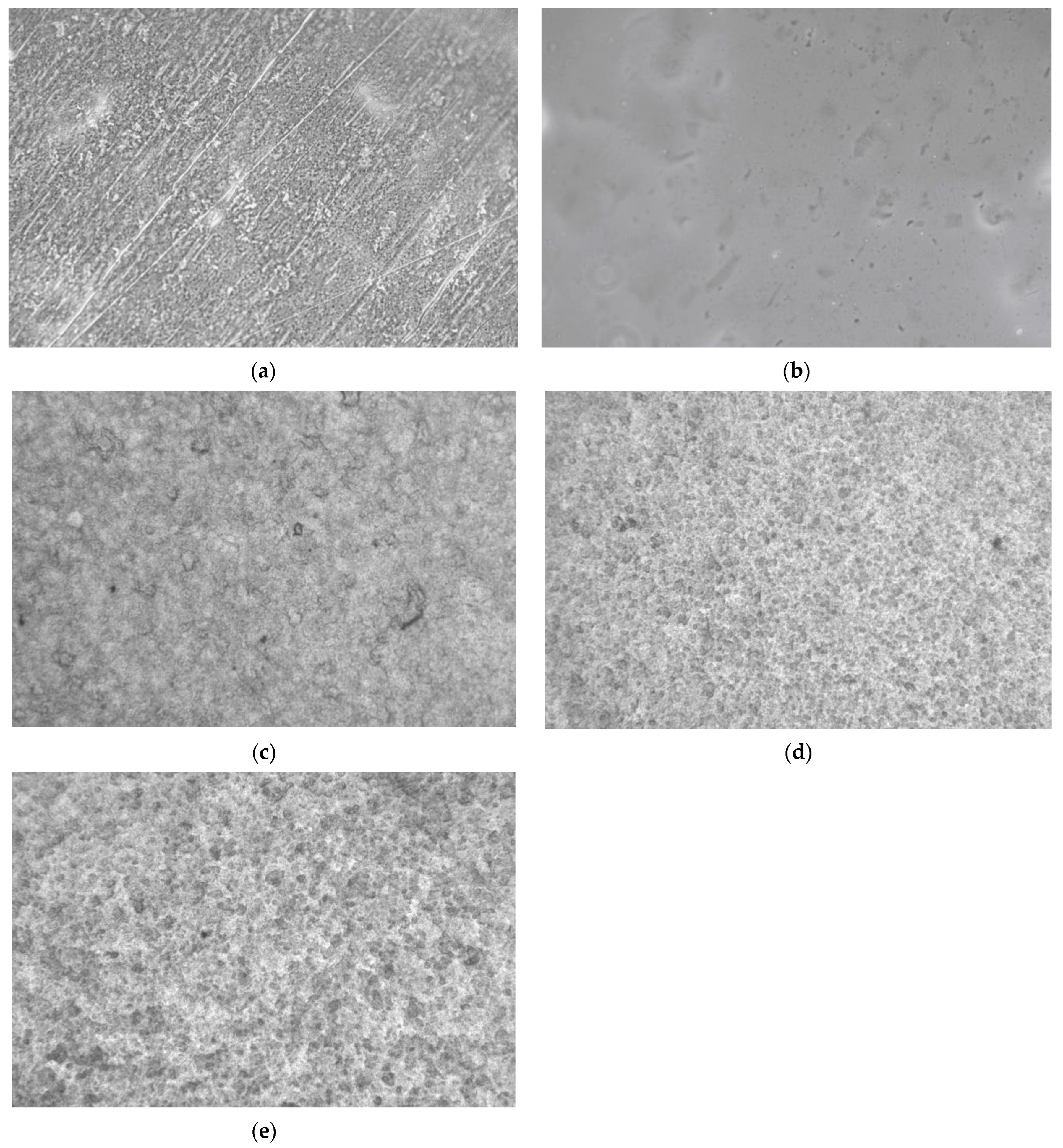
2.8. Optical Microscopy
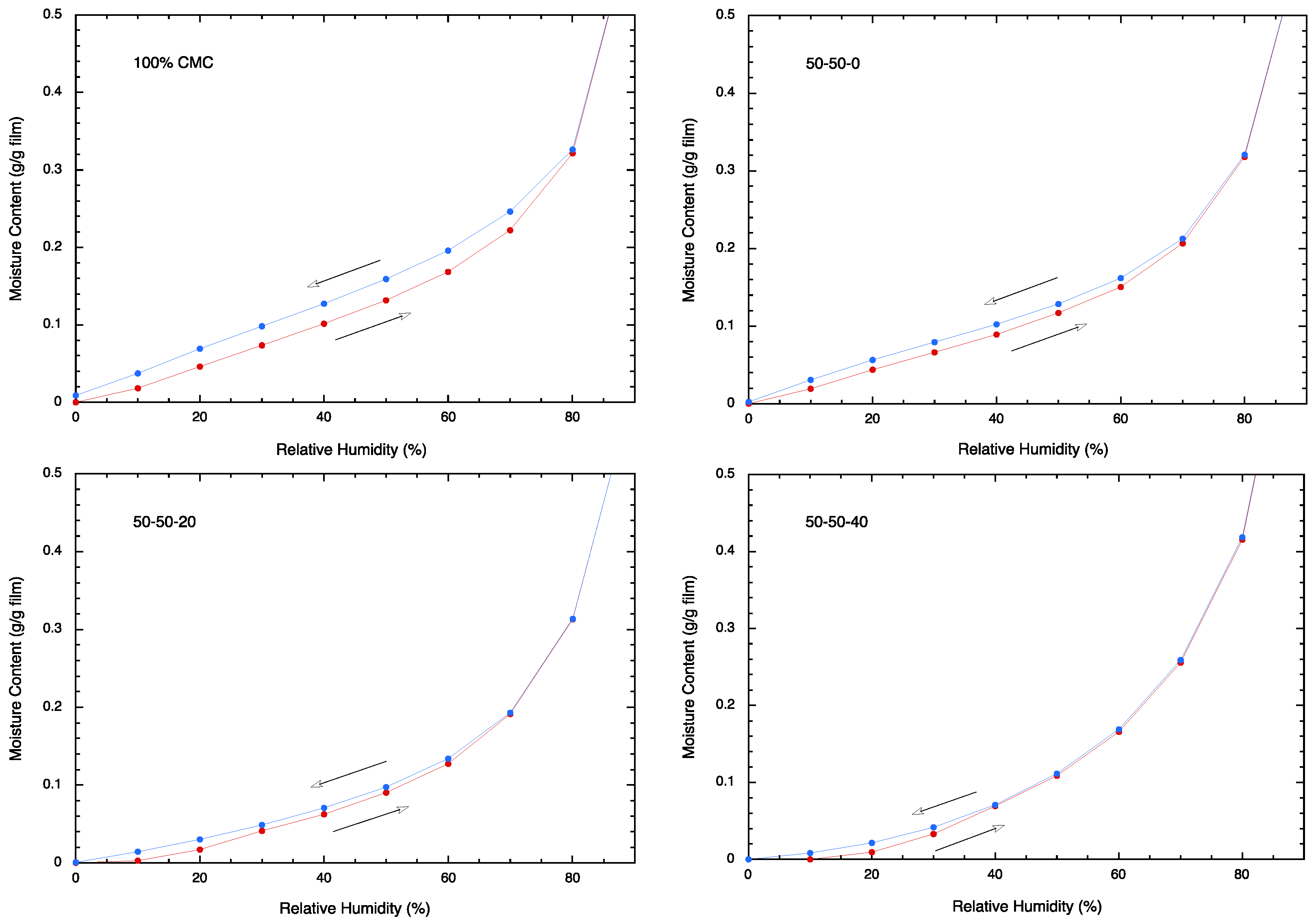
2.9. Moisture Sorption Analysis
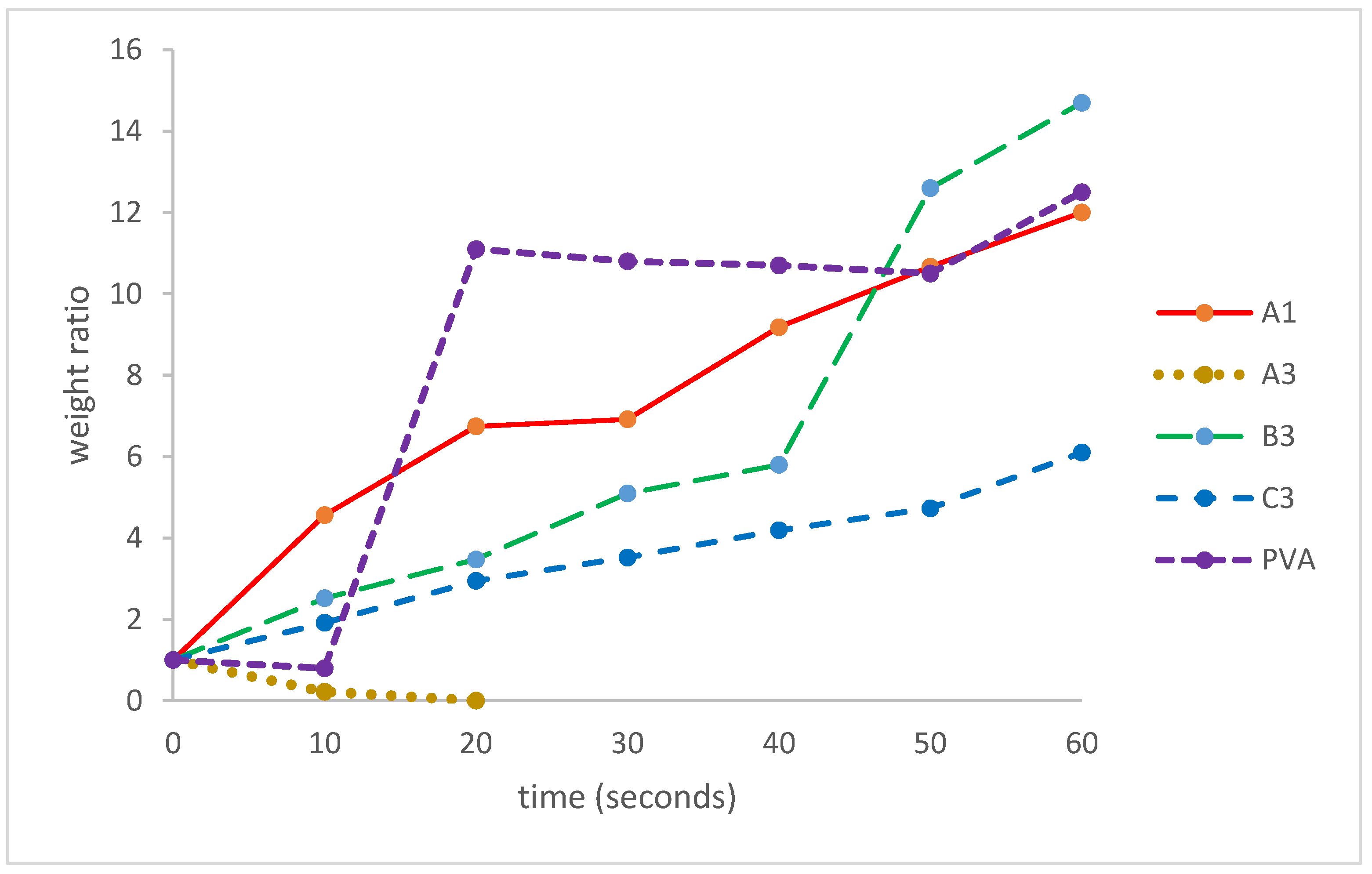
2.10. Swelling Test
3. Results and Discussion
3.1. Mechanical Properties
3.2. Water Vapor Permeation (WVP) and Opacity
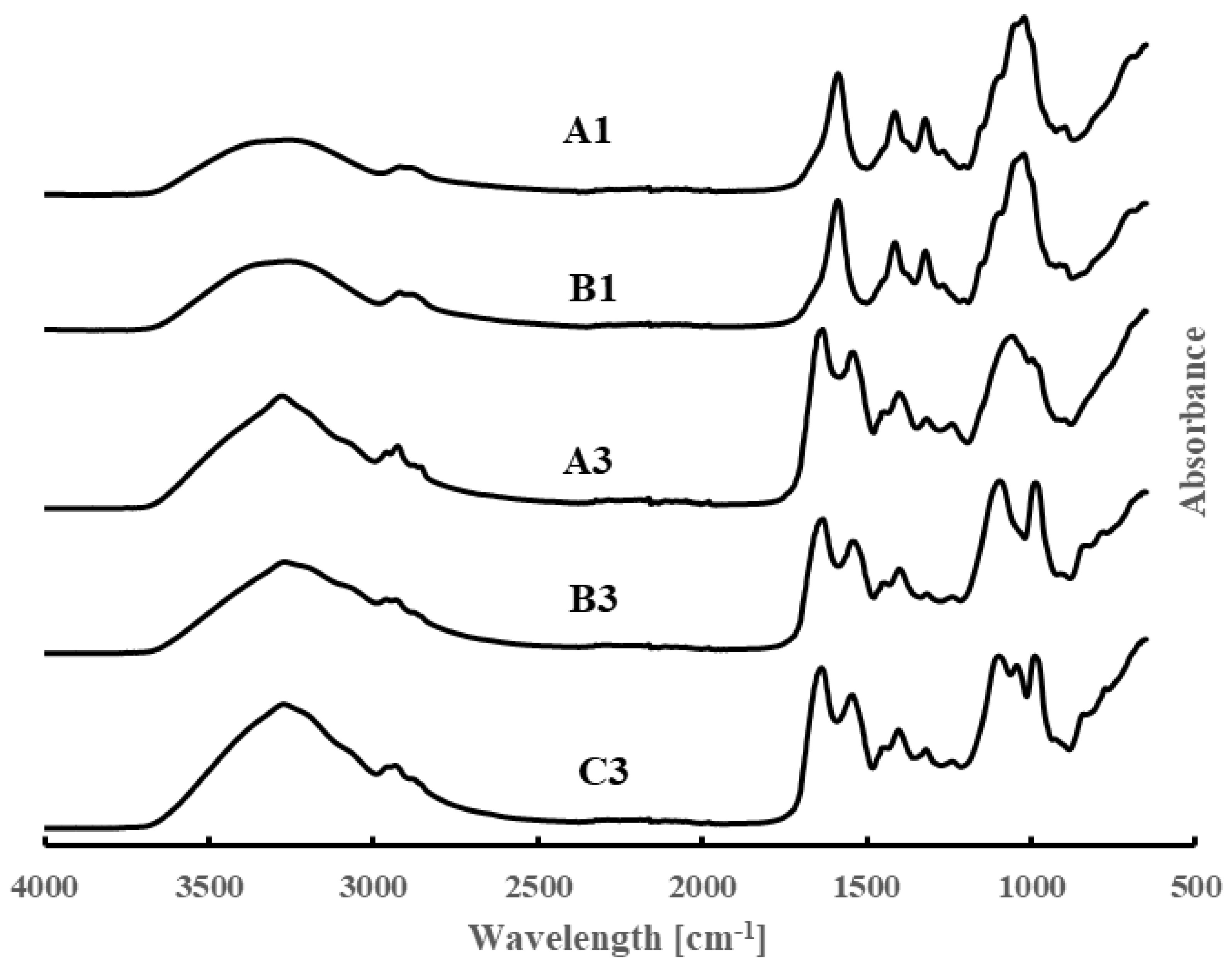
3.3. FT-IR Data
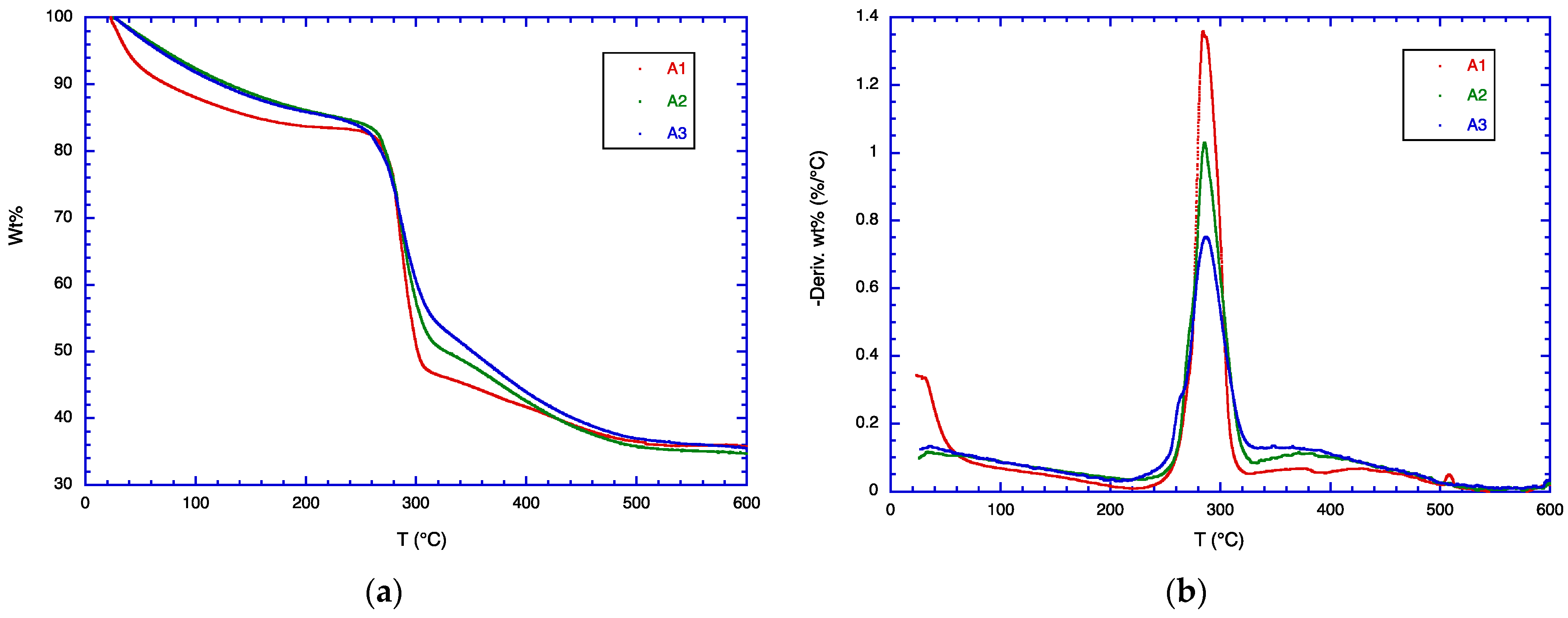
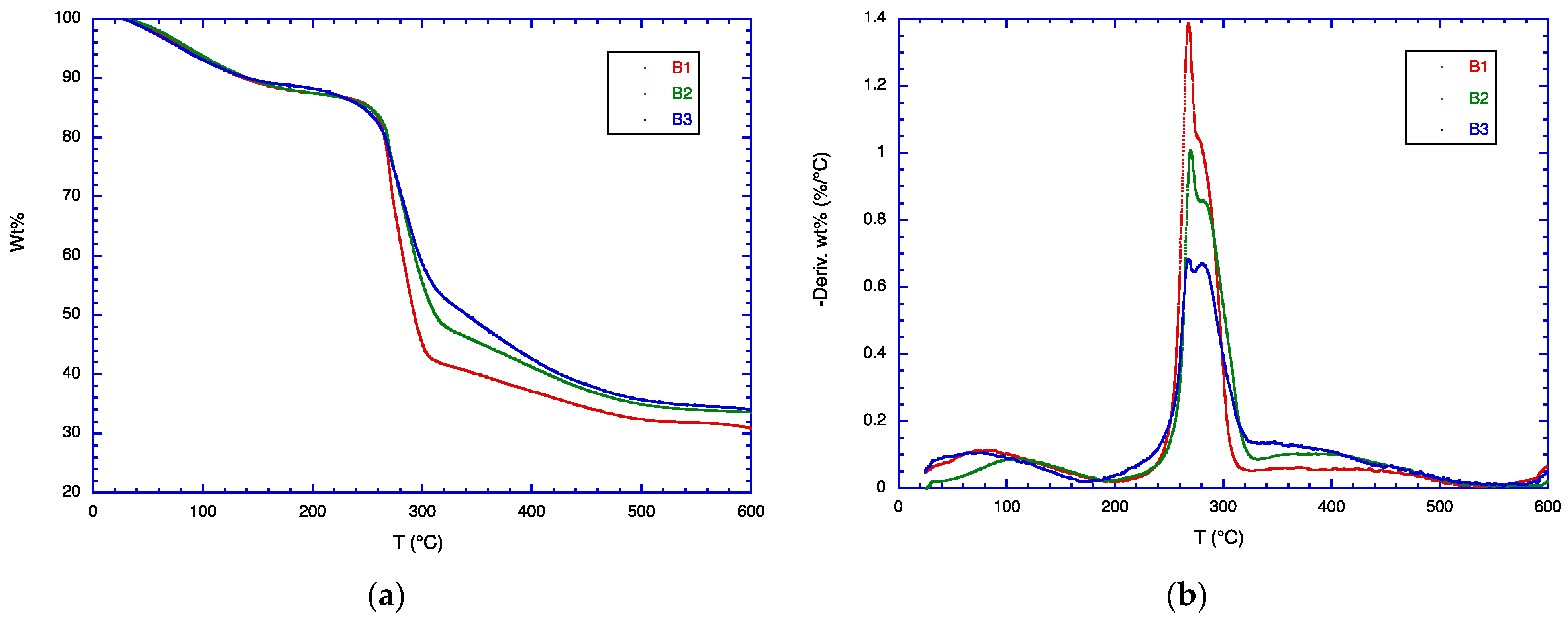
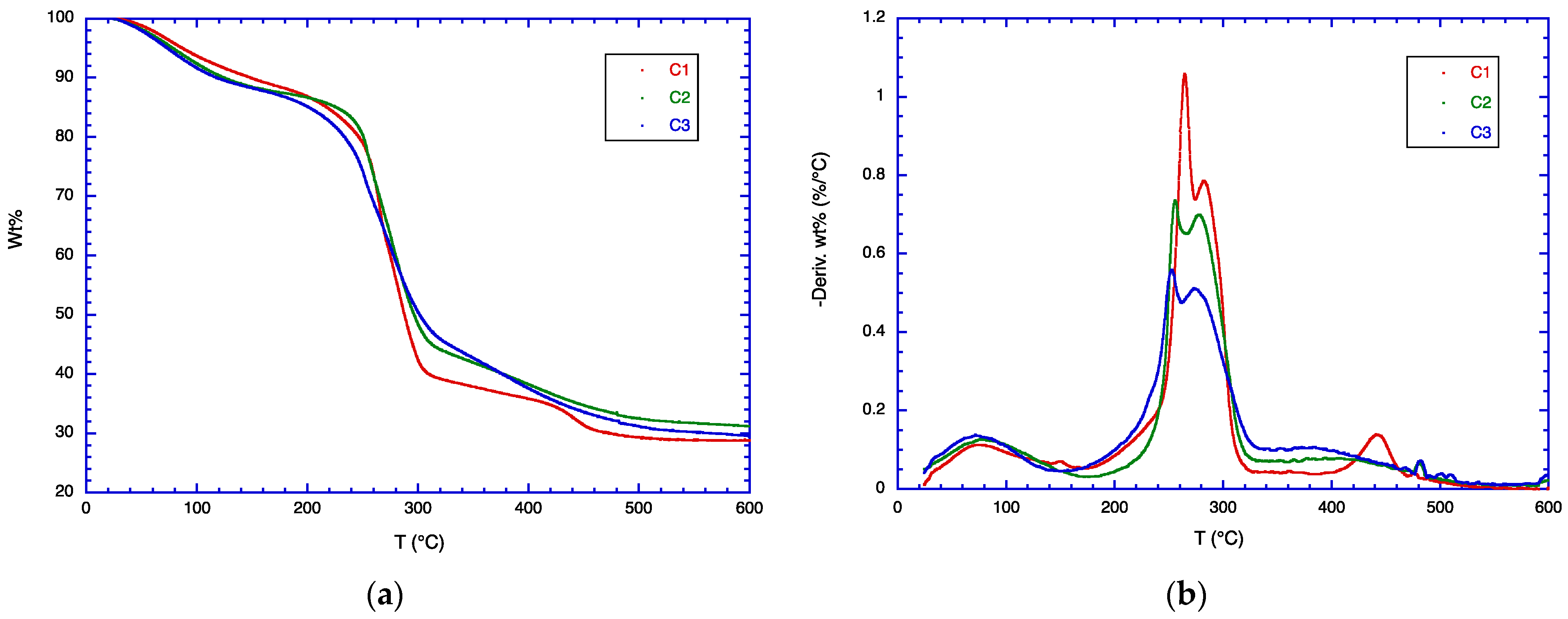
3.4. TGA Analysis
3.5. Optical Microscopy
3.6. Moisture Sorption Analysis
3.7. Swelling Test
3.8. Comments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millican, J.M.; Agarwal, S. Plastic Pollution: A Material Problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- Haward, M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat. Commun. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; Ghani, A.A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef] [PubMed]
- Feddersen, R.L.; Thorp, S.N. Sodium carboxymethylcellulose. In Industrial Gums, 3rd ed.; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 537–578. [Google Scholar]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Biswas, A.; Furtado, R.F.; Bastos, M.S.R.; Benevides, S.D.; Oliveira, M.A.; Boddu, V.; Cheng, H.N. Preparation and Characterization of Carboxymethyl Cellulose Films with Embedded Essential Oils. J. Material Sci. Res. 2018, 7, 16–25. [Google Scholar] [CrossRef]
- Li, M.; Wu, Q.; Song, K.; Cheng, H.N.; Suzuki, S.; Lei, T. Chitin Nanofibers as Reinforcing and Antimicrobial Agents in Carboxymethyl Cellulose Films: Influence of Partial Deacetylation. ACS Sustain. Chem. Eng. 2016, 4, 4385–4395. [Google Scholar] [CrossRef]
- Cheng, H.N.; He, Z.; Ford, C.; Wyckoff, W.; Wu, Q. A Review of Cottonseed Protein Chemistry and Non-Food Applications. Sus. Chem. 2020, 1, 256–274. [Google Scholar] [CrossRef]
- Yue, H.B.; Yin, G.; Cui, Y.D. Glandless Cottonseed Protein for Environmentally Friendly Bioplastics; InTech Open: London, UK, 2016. [Google Scholar] [CrossRef]
- Yue, H.B.; Cui, Y.D.; Shuttleworth, P.S.; Clark, J.H. Preparation and characterisation of bioplastics made from cottonseed protein. Green Chem. 2012, 14, 2009–2016. [Google Scholar] [CrossRef]
- Yue, H.B.; Cui, Y.D.; Yin, G.Q.; Jia, Z.Y.; Liao, L.W. Environment-Friendly Cottonseed Protein Bioplastics: Preparation and Properties. Adv. Mater. Res. 2011, 311–313, 1518–1521. [Google Scholar] [CrossRef]
- Grevellec, J.; Marquié, C.; Ferry, L.; Crespy, A.; Vialettes, V. Processability of Cottonseed Proteins into Biodegradable Materials. Biomacromol. 2001, 2, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; Dowd, M.K.; He, Z. Investigation of modified cottonseed protein adhesives for wood composites. Ind. Crops Prod. 2013, 46, 399–403. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Soy and cottonseed protein blends as wood adhesives. Ind. Crops Prod. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Selling, G.W.; Hojilla-Evangelista, M.P.; Hay, W.T.; Utt, K.D.; Grose, G.D. Preparation and properties of solution cast films from pilot-scale cottonseed protein isolate. Ind. Crops Prod. 2022, 178, 114615. [Google Scholar] [CrossRef]
- Biswas, A.; Cheng, H.N.; Kuzniar, G.; He, Z.; Furtado, R.F.; Alves, C.R.; Sharma, B.K. Cottonseed Protein-Poly(Lactic Acid) Bilayer Films for Packaging Applications. Polymers 2023, 15, 1425. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Xu, J.; Zhong, F.; Rotello, V.M. Strategies for Fabricating Protein Films for Biomaterial Applications. Adv. Sus. Syst. 2021, 5, 2000167. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Dowd, M.K. Comparison of adhesive properties of water- and phosphate-buffer-washed cottonseed meals with cottonseed protein isolates on bonding maple and poplar veneers. Int. J. Adhe. Adhes. 2014, 50, 102–106. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; Chapital, D.C.; Dowd, M.K. Sequential Fractionation of Cottonseed Meal to Improve Its Wood Adhesive Properties. J. Am. Oil Chem. Soc. 2014, 91, 151–158. [Google Scholar] [CrossRef]
- Market and Market. Water Soluble Films Market. Available online: https://www.marketsandmarkets.com/Market-Reports/water-soluble-film-market-31753669.html (accessed on 30 April 2024).
- ASTM D-882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E96-00; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM D1746; Standard Test Method for Transparency of Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 1999.
- Tabari, M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.G.; Silva, K.S.; Mauro, M.A. Evaluation of Interactions between Carboxymethylcellulose and Soy Protein Isolate and their Effects on the Preparation and Characterization of Composite Edible Films. Food Biophys. 2021, 16, 214–228. [Google Scholar] [CrossRef]
- Wang, X.Y.; Su, J.F. Biodegradation behaviors of soy protein isolate/carboxymethyl cellulose blend films. Mater. Sci. Technol. 2014, 30, 534–539. [Google Scholar] [CrossRef]
- Han, J.; Shin, S.-H.; Park, K.-M.; Kim, K.M. Characterization of physical, mechanical, and antioxidant properties of soy protein-based bioplastic films containing carboxymethylcellulose and catechin. Food Sci. Biotechnol. 2015, 24, 939–945. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Suriyatem, R. Moisture sorption characteristic of soy protein isolate/carboxymethyl cellulose blended film. Ital. J. Food Sci. 2012, 24, 154. [Google Scholar]
- Mondal, M.I.H.; Yeasmin, M.S.; Rahman, M.S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; Biswas, A. Chemical Modification of Cotton-Based Natural Materials: Products from Carboxymethylation. Carbohydr. Polym. 2011, 84, 1004–1010. [Google Scholar] [CrossRef]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling behavior of carboxymethylcellulose hydrogels in relation to crosslinking, pH, and charge density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Rani, Z.; Ridwanto, R.; Miswanda, D.; Pulungan, A.F. Isolation and characterization of glycerol by transesterification of used cooking oil. Rasayan J. Chem. 2023, 16, 648–652. [Google Scholar] [CrossRef]
- Joshi, R.; Joshi, R.; Amanah, H.Z.; Faqeerzada, M.A.; Jayapal, P.K.; Kim, G.; Baek, I.; Park, E.S.; Masithoh, R.E.; Cho, B.K. Quantitative analysis of glycerol concentration in red wine using Fourier transform infrared spectroscopy and chemometrics analysis. Korean J. Agric. Sci. 2021, 48, 299–310. [Google Scholar]
- So, J.; Chung, Y.; Sholl, D.S.; Sievers, C. In-situ ATR-IR Study of Surface Reaction during Aqueous Phase Reforming of Glycerol, Sorbitol and Glucose over Pt/γ-Al2O3. Mol. Catal. 2019, 475, 110423. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y. Fourier Transform Infrared Spectroscopic Analysis in Applied Cotton Fiber and Cottonseed Research: A Review. J. Cotton Sci. 2021, 25, 167–183. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shi, R.; Ji, Q.; Wang, B.; Quan, F.; Xia, Y. Effect of Na+ and Ca2+ on the Thermal Degradation of Carboxymethylcellulose in Air. Polym. Polym. Compos. 2017, 25, 309–314. [Google Scholar] [CrossRef]
- Ahmad, N.; Wahab, R.; Omar, S.Y.A. Thermal decomposition kinetics of sodium carboxymethyl cellulose: Model-free methods. Eur. J. Chem. 2014, 5, 247–251. [Google Scholar] [CrossRef]
- Cheng, H.N.; Kilgore, K.; Ford, C.; Fortier, C.; Dowd, M.K.; He, Z. Cottonseed protein-based wood adhesive reinforced with nanocellulose. J. Adhes. Sci. Technol. 2019, 33, 1357–1368. [Google Scholar] [CrossRef]
- Rahman, L.; Goswami, J. Poly(Vinyl Alcohol) as Sustainable and Eco-Friendly Packaging: A Review. J. Package Technol. Res. 2023, 7, 1–10. [Google Scholar] [CrossRef]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging ap-plications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Mori, R. Replacing all petroleum-based chemical products with natural biomass-based chemical products: A tutorial review. RSC Sustain. 2023, 1, 179–212. [Google Scholar] [CrossRef]
- Cheng, H.N.; Gross, R.A. The Pursuit for Green Sustainable Chemistry in Polymer Research. ACS Symp. Ser. 2023, 1450, 1–14. [Google Scholar]
- Chemanalyst. Polyvinyl Alcohol Price Trend and Forecast. Available online: https://www.chemanalyst.com/ (accessed on 30 April 2024).
- Business Analytiq. Carboxymethyl Cellulose Price Index. Available online: https://businessanalytiq.com/procurementanalytics/index/carboxymethyl-cellulose-price-index/ (accessed on 30 April 2024).
- USDA National Grain and Oilseed Processor Feedstuff Report. Available online: https://www.ams.usda.gov/mnreports/ams_3511.pdf (accessed on 30 April 2024).
- Halake, K.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.C.; Kim, Y.J.; Kim, S.; Kim, H.J.; Ahn, S.; An, S.Y.; et al. Recent application developments of water-soluble synthetic polymers. J. Ind. Eng. Chem. 2014, 20, 3913–3918. [Google Scholar] [CrossRef]
- Wang, X. Review of characterization methods for water-soluble polymers used in oil sand and heavy oil industrial applications. Env. Rev. 2016, 24, 460–470. [Google Scholar] [CrossRef]
- Vandermeulen, G.W.M.; Boarino, A.; Klok, H.-A. Biodegradation of water-soluble and water-dispersible polymers for agricultural, consumer, and industrial applications—Challenges and opportunities for sustainable materials solutions. J. Polym. Sci. 2022, 60, 1797–1813. [Google Scholar] [CrossRef]
- Cotton Incorporated. Cottonseed: An and Crop. Available online: https://cottontoday.cottoninc.com/our-sustainability-story/circularity/cottonseed-an-and-crop/ (accessed on 25 May 2024).
- Sczostak, A. Cotton Linters: An Alternative Cellulosic Raw Material. Macromol. Symp. 2009, 280, 45–53. [Google Scholar] [CrossRef]
| Sample Code | CMC | CSM | Glycerol |
|---|---|---|---|
| A1 | 100 | 0 | 0 |
| A2 | 75 | 25 | 0 |
| A3 | 50 | 50 | 0 |
| B1 | 100 | 0 | 20 |
| B2 | 75 | 25 | 20 |
| B3 | 50 | 50 | 20 |
| C1 | 100 | 0 | 40 |
| C2 | 75 | 25 | 40 |
| C3 | 50 | 50 | 40 |
| C4 | 25 | 75 | 40 |
| C5 | 0 | 100 | 40 |
| Code | Weight Ratio CMC:CSM:Glycerol | Thickness (mm) * | YM (MPa) * | TS (MPa) * | EB (%) * |
|---|---|---|---|---|---|
| A1 | 100:0:0 | 0.0751 ± 0.0053 b | 3909 ± 178 a | 56 ± 4 a | 5 ± 0 d |
| A2 | 75:25:0 | 0.0798 ± 0.0037 b | 3911 ± 105 a | 50 ± 6 a | 3 ± 1 d |
| A3 | 50:50:0 | 0.0524 ± 0.0027 d | 3710 ± 143 a | 33 ± 3 b,c | 1 ± 0 d |
| B1 | 100:0:20 | 0.0725 ± 0.0133 b,c | 2080 ± 361 b | 40 ± 6 b | 12 ± 2 c |
| B2 | 75:25:20 | 0.0731 ± 0.0130 b,c | 1159 ± 116 c | 27 ± 1 c | 21 ± 4 b,c |
| B3 | 50:50:20 | 0.0719 ± 0.0037 b,c | 1763 ± 102 b | 24 ± 1 c | 3 ± 0 d |
| C1 | 100:0:40 | 0.0806 ± 0.0110 a,b | 660 ± 52 d | 23 ± 3 c | 34 ± 3 a,b |
| C2 | 75:25:40 | 0.0812 ± 0.0114 a,b | 309 ± 29 e | 20 ± 1 c | 51 ± 2 a |
| C3 | 50:50:40 | 0.0993 ± 0.0122 a | 164 ± 44 f | 12 ± 2 d | 50 ± 5 a |
| C4 | 25:75:40 | 0.0720 ± 0.0042 b,c | 65 ± 7 g | 6 ± 1 e | 54 ± 9 a |
| C5 | 0:100:40 | 0.0744 ± 0.0083 b | 51 ± 8 g | 2 ± 1 e | 51 ± 11 a |
| Code | Weight Ratio CMC:CSM:Glycerol | Thickness (mm) * | WVP (mg-m/kPa-d-m2) * | Thickness (mm) * | Opacity (A/mm) * |
|---|---|---|---|---|---|
| A1 | 100:0:0 | 0.0547 ± 0.0027 d | 0.894 ± 0.067 a | 0.0668 ± 0.0191 a,b,c,d | 1.2 ± 0.5 d |
| A2 | 75:25:0 | 0.0683 ± 0.0070 a,b,c | 0.459 ± 0.048 b | 0.0877 ± 0.0056 a | 18.0 ± 1.3 c |
| A3 | 50:50:0 | 0.0560 ± 0.0022 d | 0.310 ± 0.017 d,e,f | 0.0607 ± 0.0072 c,d | 28.0 ± 1.9 a |
| B1 | 100:0:20 | 0.0620 ± 0.0055 b,c,d | 0.371 ± 0.016 c | 0.0703 ± 0.0281 a,b,c,d | 0.9 ± 0.2 d |
| B2 | 75:25:20 | 0.0596 ± 0.0213 a.b.c.d | 0.320 ± 0.041 c,d,e,f | 0.0803 ± 0.0095 a,b | 17.6 ± 0.8 c |
| B3 | 50:50:20 | 0.0683 ± 0.0047 a,b,c | 0.180 ± 0.014 g,h | 0.0841 ± 0.0130 a,b | 21.0 ± 3.5 b,c |
| C1 | 100:0:40 | 0.0905 ± 0.0209 a | 0.325 ± 0.049 c,d,e,f | 0.0727 ± 0.0111 a,b,c | 1.0 ± 0.3 d |
| C2 | 75:25:40 | 0.0721 ± 0.0106 a,b,c | 0.269 ± 0.037 d,e,f,g | 0.0809 ± 0.0260 a,b | 19.7 ± 5.0 b,c |
| C3 | 50:50:40 | 0.0683 ± 0.0192 a,b,c,d | 0.227 ± 0.033 f,g,h | 0.0711 ± 0.0155 a,b,c,d | 23.5 ± 5.3 a,b |
| C4 | 25:75:40 | 0.0767 ± 0.0160 a.b.c | 0.296 ± 0.019 d,e,f | 0.0692 ± 0.0069 b,c,d | 21.5 ± 1.9 b |
| C5 | 0:100:40 | 0.0750 ± 0.0326 a,b,c,d | 0.319 ± 0.048 c,d,e,f | 0.0593 ± 0.0014 c,d | 23.3 ± 6.2 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.N.; Biswas, A.; Kuzniar, G.; Kim, S.; Liu, Z.; He, Z. Blends of Carboxymethyl Cellulose and Cottonseed Protein as Biodegradable Films. Polymers 2024, 16, 1554. https://doi.org/10.3390/polym16111554
Cheng HN, Biswas A, Kuzniar G, Kim S, Liu Z, He Z. Blends of Carboxymethyl Cellulose and Cottonseed Protein as Biodegradable Films. Polymers. 2024; 16(11):1554. https://doi.org/10.3390/polym16111554
Chicago/Turabian StyleCheng, Huai N., Atanu Biswas, Gary Kuzniar, Sanghoon Kim, Zengshe Liu, and Zhongqi He. 2024. "Blends of Carboxymethyl Cellulose and Cottonseed Protein as Biodegradable Films" Polymers 16, no. 11: 1554. https://doi.org/10.3390/polym16111554






