Supercritical Impregnation of PETG with Olea europaea Leaf Extract: Influence of Operational Parameters on Expansion Degree, Antioxidant and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Enhanced Solvent Extraction of Olea europaea Leaves
2.2.2. Supercritical Solvent Impregnation
2.2.3. Expansion Degree (% EXP)
2.2.4. Loading of Olea europaea Leaf Extract (% L)
2.2.5. Scanning Electron Microscopy
2.2.6. Antioxidant Activity
2.2.7. Mechanical Properties
2.2.8. Design of Experiments (DOE)
3. Results and Discussion
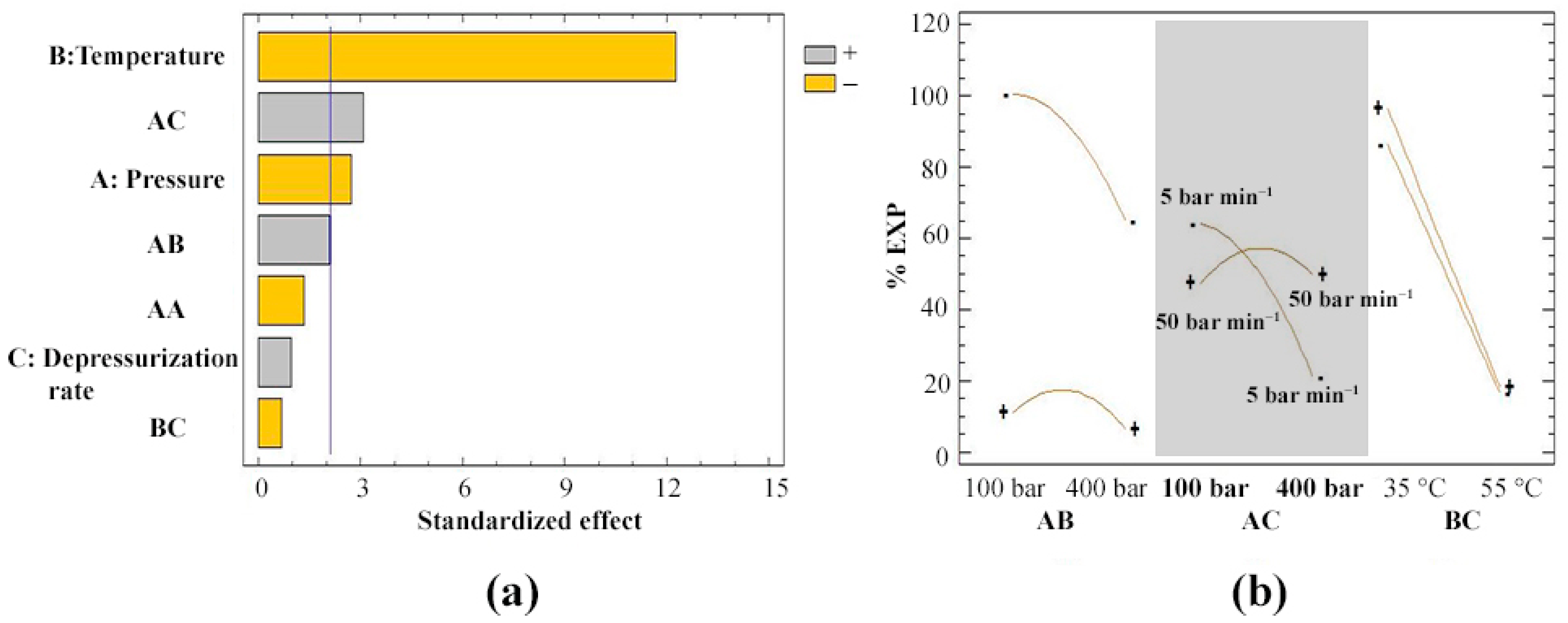
3.1. Expansion Degree of Impregnated Filaments
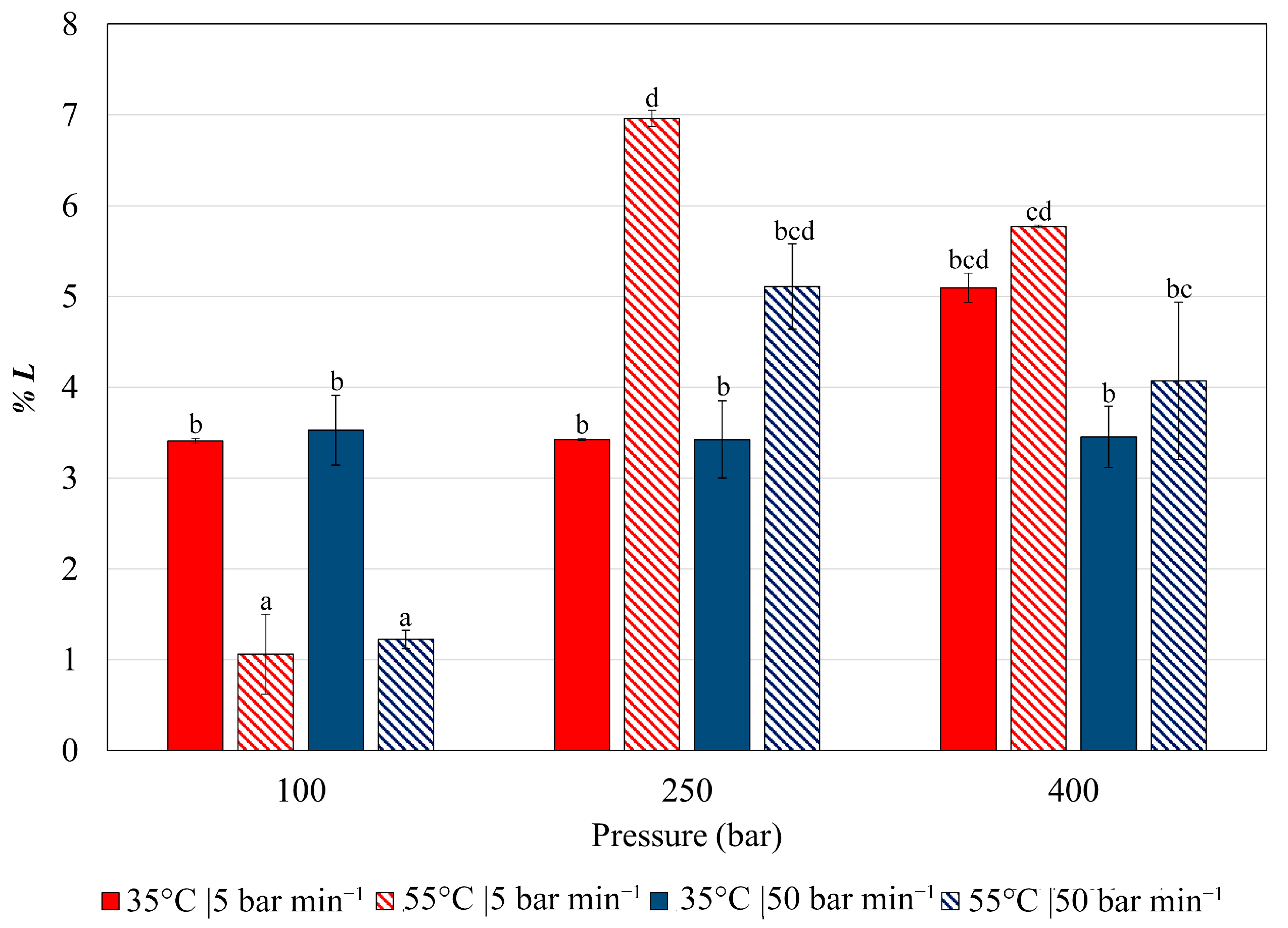
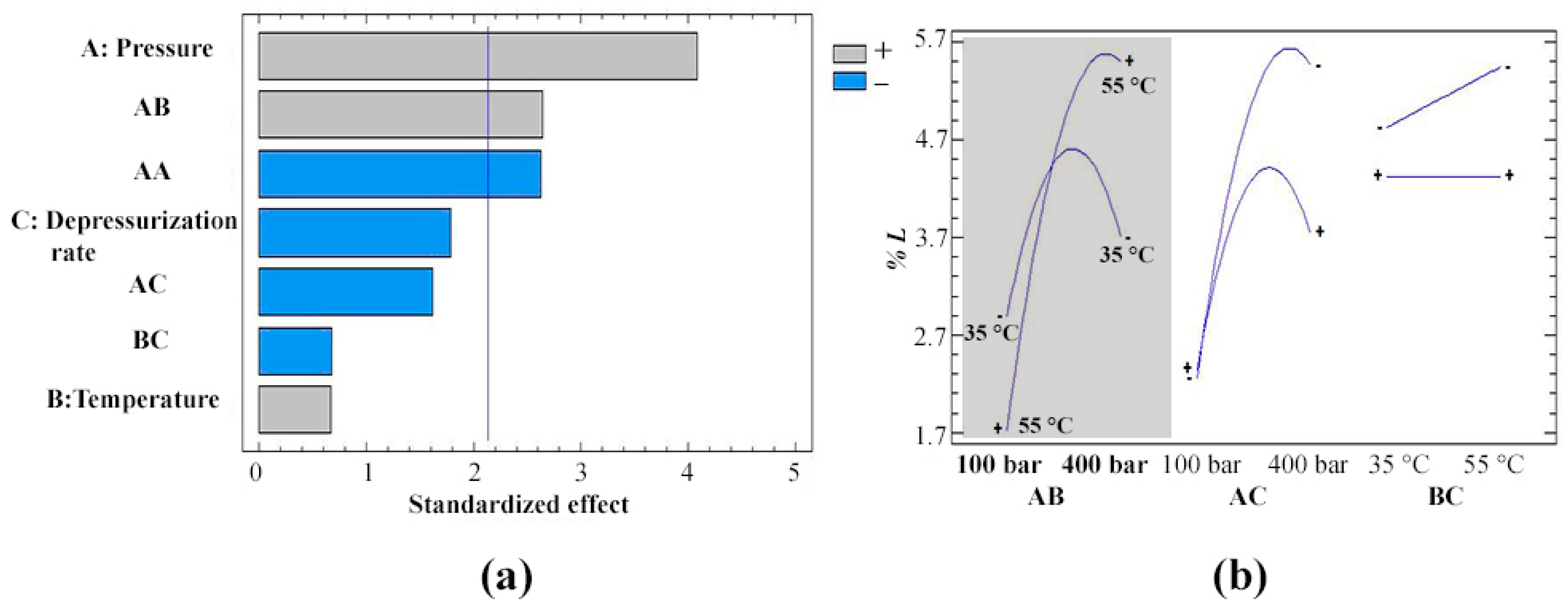
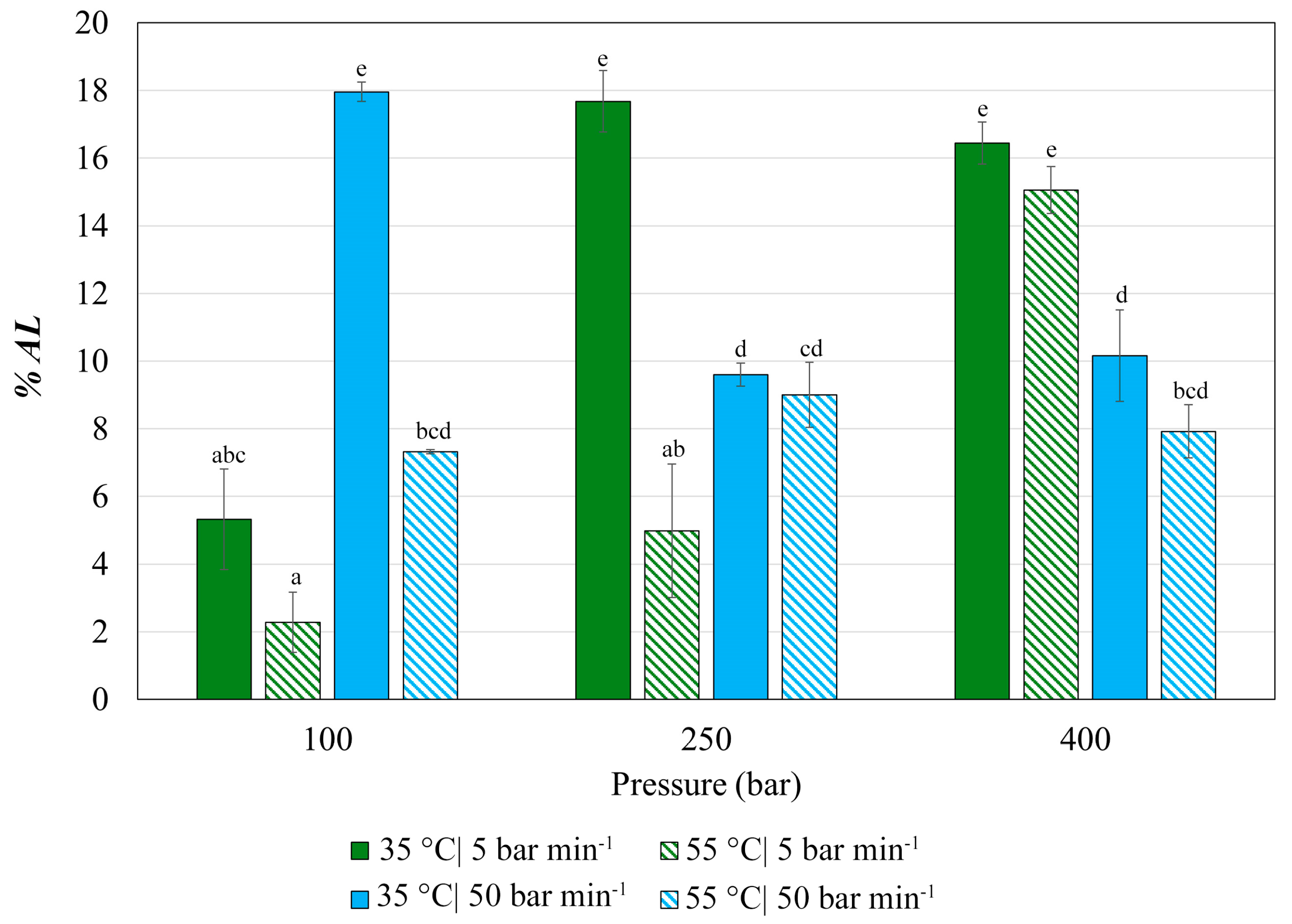
3.2. Impregnation of Olea europaea Leaf Extract into PETG Filaments
3.3. Antioxidant Activity
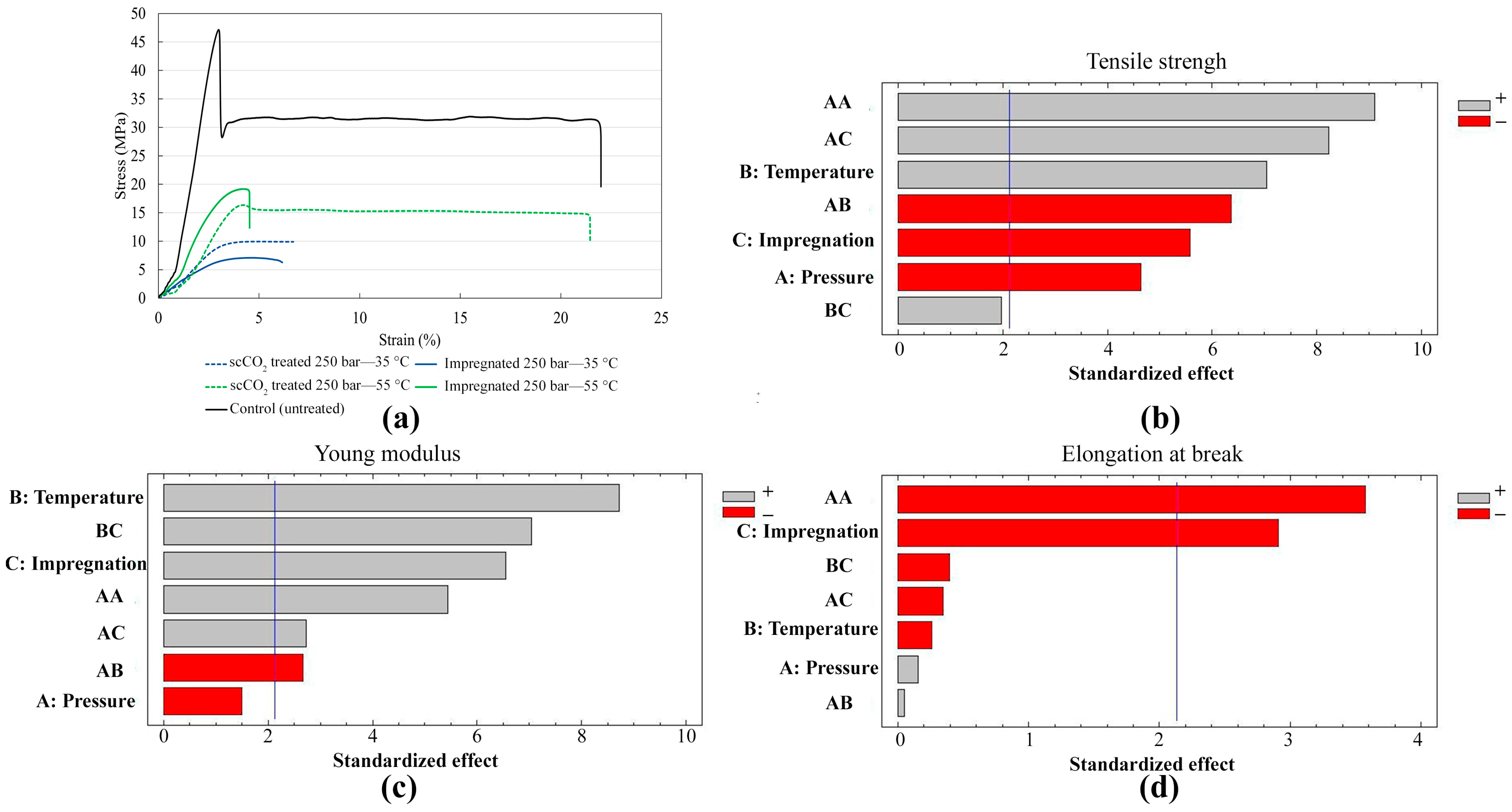
3.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-Based Biomaterials for Pharmaceutical and Biomedical Applications: A Focus on Topical Drug Administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Yan, C.; Kleiner, C.; Tabigue, A.; Shah, V.; Sacks, G.; Shah, D.; DeStefano, V. PETG: Applications in Modern Medicine. Eng. Regen. 2024, 5, 45–55. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Chen, S.; Sun, Y.; Wang, X.; Wang, Y. Construction of a Three-Dimensional Network in Branched PETG to Prepare PETG Composite Microcellular Foam with Outstanding Mechanical Properties at Low Temperatures. Eur. Polym. J. 2024, 202, 112643. [Google Scholar] [CrossRef]
- Soleyman, E.; Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. Cold Programming of Ordered Porous PETG 4D Printed by Material Extrusion. Arch. Civil Mech. Eng. 2024, 24, 67. [Google Scholar] [CrossRef]
- Soleyman, E.; Rahmatabadi, D.; Soltanmohammadi, K.; Aberoumand, M.; Ghasemi, I.; Abrinia, K.; Baniassadi, M.; Wang, K.; Baghani, M. Shape Memory Performance of PETG 4D Printed Parts under Compression in Cold, Warm, and Hot Programming. Smart Mater. Struct. 2022, 31, 085002. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Soleyman, E.; Bashi, M.F.M.; Aberoumand, M.; Soltanmohammadi, K.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. 4D Printing and Annealing of PETG Composites Reinforced with Short Carbon Fibers. Phys. Scr. 2024, 99, 055957. [Google Scholar] [CrossRef]
- Katschnig, M.; Wallner, J.; Janics, T.; Burgstaller, C.; Zemann, W.; Holzer, C. Biofunctional Glycol-Modified Polyethylene Terephthalate and Thermoplastic Polyurethane Implants by Extrusion-Based Additive Manufacturing for Medical 3D Maxillofacial Defect Reconstruction. Polymers 2020, 12, 1751. [Google Scholar] [CrossRef]
- Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Hou, Y.; Huang, B.; Strashnov, I.; Grieve, B.D.; Bártolo, P. The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering. Polymers 2020, 12, 3045. [Google Scholar] [CrossRef]
- Arany, P.; Papp, I.; Zichar, M.; Csontos, M.; Elek, J.; Regdon, G.; Budai, I.; Béres, M.; Gesztelyi, R.; Fehér, P.; et al. In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants. Molecules 2020, 25, 5889. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, C.; Verano-Naranjo, L.; Toro-Barrios, N.; Pereyra, C.; Mantell, C.; Casas, L. Structural Modification of Polymers Functionalized with Mango Leaf Extract by Supercritical Impregnation: Approaching of Further Food and Biomedical Applications. Polymers 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Alogla, A. Enhancing Antioxidant Delivery through 3D Printing: A Pathway to Advanced Therapeutic Strategies. Front. Bioeng. Biotechnol. 2023, 11, 1256361. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.D.; Mosquera, J.E.; Martini, R.E.; Goñi, M.L.; Gañán, N.A. Supercritical CO2-Assisted Impregnation/Deposition of Polymeric Materials with Pharmaceutical, Nutraceutical, and Biomedical Applications: A Review (2015–2021). J. Supercrit. Fluids 2022, 191, 105763. [Google Scholar] [CrossRef]
- Milovanovic, S.; Lukic, I.; Stamenic, M.; Kamiński, P.; Florkowski, G.; Tyśkiewicz, K.; Konkol, M. The Effect of Equipment Design and Process Scale-up on Supercritical CO2 Extraction: Case Study for Silybum Marianum Seeds. J. Supercrit. Fluids 2022, 188, 105676. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.; de Oliveira, P.V.; Pellenz, L.; de Aguiar, C.R.L.; da Silva Júnior, A.H. Supercritical Fluid Technology as a Sustainable Alternative Method for Textile Dyeing: An Approach on Waste, Energy, and CO2 Emission Reduction. J. Environ. Sci. 2024, 140, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Aslanbay Guler, B.; Tepe, U.; Imamoglu, E. Sustainable Point of View: Life Cycle Analysis for Green Extraction Technologies. ChemBioEng Rev. 2024, 11, 348–362. [Google Scholar] [CrossRef]
- Soergel, B.; Kriegler, E.; Weindl, I.; Rauner, S.; Dirnaichner, A.; Ruhe, C.; Hofmann, M.; Bauer, N.; Bertram, C.; Bodirsky, B.L.; et al. A Sustainable Development Pathway for Climate Action within the UN 2030 Agenda. Nat. Clim. Chang. 2021, 11, 656–664. [Google Scholar] [CrossRef]
- Jacobs, L.J.M.; Kemmere, M.F.; Keurentjes, J.T.F. Sustainable Polymer Foaming Using High Pressure Carbon Dioxide: A Review on Fundamentals, Processes and Applications. Green Chem. 2008, 10, 731–738. [Google Scholar] [CrossRef]
- Udenni Gunathilake, T.M.S.; Ching, Y.C.; Ching, K.Y.; Chuah, C.H.; Abdullah, L.C. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Silva, N.H.C.S.; Casas Cardoso, L.; MantellSerrano, C.; Martínez de la Ossa, E.J.; Freire, C.S.R.; Vilela, C. Biobased Films of Nanocellulose and Mango Leaf Extract for Active Food Packaging: Supercritical Impregnation versus Solvent Casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Olive Trees Cover 4.6 Million Hectares in the EU—Products Eurostat News—Eurostat. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20190301-1 (accessed on 16 February 2024).
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of Major Bioactive Compounds from Olive Leaf. LWT-Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Romero-García, J.M.; López-Linares, J.C.; Contreras, M.d.M.; Romero, I.; Castro, E. Exploitation of Olive Tree Pruning Biomass through Hydrothermal Pretreatments. Ind. Crops Prod. 2022, 176, 114425. [Google Scholar] [CrossRef]
- Marquina, J.; Colinet, M.J.; Pablo-romero, M.D.P. Measures to Promote Olive Grove Biomass in Spain and Andalusia: An Opportunity for Economic Recovery against COVID-19. Sustainability 2021, 13, 11318. [Google Scholar] [CrossRef]
- Donner, M.; Erraach, Y.; López-i-Gelats, F.; Manuel-i-Martin, J.; Yatribi, T.; Radić, I.; El Hadad-Gauthier, F. Circular Bioeconomy for Olive Oil Waste and By-Product Valorisation: Actors’ Strategies and Conditions in the Mediterranean Area. J. Environ. Manag. 2022, 321, 115836. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef] [PubMed]
- Sezer, F.; Deniz, S.; Sevim, D.; Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of Olive Leaf Extract Polyphenols Loaded by Supercritical Solvent Impregnation into PET/PP Food Packaging Films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Food Packaging Films to Provide Antioxidant Properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Olive Leaf Extract to Obtain Bioactive Films Effective in Cherry Tomato Preservation. Food Packag. Shelf Life 2019, 21, 100338–100354. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J.; Bigger, S.W. Effect of Supercritical CO2 and Olive Leaf Extract on the Structural, Thermal and Mechanical Properties of an Impregnated Food Packaging Film. J. Supercrit. Fluids 2019, 145, 181–191. [Google Scholar] [CrossRef]
- Machado, N.D.; Cejudo-Bastante, C.; Goñi, M.L.; Gañán, N.A.; Casas-Cardoso, L. Screening of the Supercritical Impregnation of Olea Europaea Leaves Extract into Filaments of Thermoplastic Polyurethane (TPU) and Polylactic Acid (PLA) Intended for Biomedical Applications. Antioxidants 2022, 11, 1170. [Google Scholar] [CrossRef]
- Sethuramiah, A.; Kumar, R. Statistics and Experimental Design in Perspective. In Modeling of Chemical Wear; Elsevier: Amsterdam, The Netherlands, 2016; pp. 129–159. [Google Scholar] [CrossRef]
- Handa, Y.P.; Wong, B.; Zhang, Z.; Kumar, V.; Eddy, S.; Khemani, K. Some Thermodynamic and Kinetic Properties of the System PETG-CO2, and Morphological Characteristics of the CO2-Blown PETG Foams. Polym. Eng. Sci. 1999, 39, 55–61. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of Polymers with Supercritical Fluids and Perspectives on the Current Knowledge Gaps and Challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Angelopoulou, A.G.; Panayiotou, C. Foaming of Polymers with Supercritical CO2: An Experimental and Theoretical Study. Polymer 2007, 48, 5928–5939. [Google Scholar] [CrossRef]
- White, L.J.; Hutter, V.; Tai, H.; Howdle, S.M.; Shakesheff, K.M. The Effect of Processing Variables on Morphological and Mechanical Properties of Supercritical CO2 Foamed Scaffolds for Tissue Engineering. Acta Biomater. 2012, 8, 61–71. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Rocha-Pimienta, J.; Martillanes, S.; Garrido, M.; Espino, J.; Delgado-Adámez, J. Chlorophyll Pigments of Olive Leaves and Green Tea Extracts Differentially Affect Their Antioxidant and Anticancer Properties. Molecules 2023, 28, 2779. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Sanz, M.T.; Blanco, B.; Beltrán, S.; Niknam, S.M. Freeze Dried Extract from Olive Leaves: Valorisation, Extraction Kinetics and Extract Characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez De La Ossa-Fernández, E.J. Supercritical Impregnation of Antioxidant Mango Polyphenols into a Multilayer PET/PP Food-Grade Film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; De La Ossa, E.M. Use of High Pressure Techniques to Produce Mangifera Indica L. Leaf Extracts Enriched in Potent Antioxidant Phenolic Compounds. Innov. Food Sci. Emerg. Technol. 2015, 29, 94–106. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.; Moon, Y.; Kwak, S.; Kang, C.G.; Park, C.; Jo, J.H.; Kim, S.W.; Pal, K.; Kang, D.H.; et al. Enhancement of the Water Solubility and Antioxidant Capacities of Mangiferin by Transglucosylation Using a Cyclodextrin Glycosyltransferase. Enzym. Microb. Technol. 2022, 159, 110065. [Google Scholar] [CrossRef]
- Acosta, J.; Sevilla, I.; Salomón, S.; Nuevas, L.; Romero, A.; Amaro, D. Determination of Mangiferin Solubility in Solvents Used in the Biopharmaceutical Industry. J. Pharm. Pharmacogn. Res. 2016, 4, 49–53. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Study on Oleuropein Extraction from Olive Tree (Olea Europaea) Leaves by Means of SFE: Comparison of Water and Ethanol as Co-Solvent. Sep. Sci. Technol. 2012, 47, 2391–2398. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and Antioxidant Evaluation of Lipophilic Oleuropein Aglycone Derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.S.; Dias, A.L.B.; Rodrigues, K.P.; Hatami, T.; Mei, L.H.I.; Martínez, J.; Viganó, J. Supercritical Fluid Adsorption of Natural Extracts: Technical, Practical, and Theoretical Aspects. J. CO2 Util. 2022, 56, 101865. [Google Scholar] [CrossRef]
- Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Pla Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Impregnation of Mango Leaf Extract into a Polyester Textile Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Araujo, E.J.S.; Scopel, E.; Rezende, C.A.; Martínez, J. Supercritical Impregnation of Polyphenols from Passion Fruit Residue in Corn Starch Aerogels: Effect of Operational Parameters. J. Food Eng. 2023, 343, 111394. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; José Galotto, M.; Guarda, A.; Julio, R. Supercritical Impregnation for Food Applications: A Review of the Effect of the Operational Variables on the Active Compound Loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef]
- Valor, D.; García-Casas, I.; Montes, A.; Danese, E.; Pereyra, C.; de la Ossa, E.M. Supercritical Impregnation of Mangifera Indica Leaves Extracts into Porous Conductive PLGA-PEDOT Scaffolds. Polymers 2023, 16, 133. [Google Scholar] [CrossRef]
- Hosford, W.F. Tensile Testing. In Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2005; pp. 39–52. [Google Scholar]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Physical, Thermal, and Mechanical Properties of Polymers. In Biosurfaces: A Materials Science and Engineering Perspective; Wiley: Hoboken, NJ, USA, 2014; pp. 329–344. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Martini, R.E.; Andreatta, A.E. Carvone-Loaded LDPE Films for Active Packaging: Effect of Supercritical CO2-Assisted Impregnation on Loading, Mechanical and Transport Properties of the Films. J. Supercrit. Fluids 2018, 133, 278–290. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of Processing Conditions on the Physical, Chemical and Transport Properties of Polylactic Acid Films Containing Thymol Incorporated by Supercritical Impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Miranda-Villa, P.P.; Gañán, N.A.; Martini, R.E.; Goñi, M.L. Supercritical CO2-Assisted Impregnation of Polylactic Acid Films with R-Carvone: Effect of Processing on Loading, Mass Transfer Kinetics, and Final Properties. J. CO2 Util. 2022, 61, 102029. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-Loaded LLDPE Films with Antioxidant Activity by Supercritical Carbon Dioxide Impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Kováčová, M.; Kozakovičová, J.; Procházka, M.; Janigová, I.; Vysopal, M.; Černičková, I.; Krajčovič, J.; Špitalský, Z. Novel Hybrid PETG Composites for 3D Printing. Appl. Sci. 2020, 10, 3062. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Ahzi, S.; Barth, N.; Kochkodan, V.; Khraisheh, M.; Koç, M. A Review on the Modeling of the Elastic Modulus and Yield Stress of Polymers and Polymer Nanocomposites: Effect of Temperature, Loading Rate and Porosity. Polymers 2022, 14, 360. [Google Scholar] [CrossRef]
- Sousa, A.M.; Amaro, A.M.; Piedade, A.P. 3D Printing of Polymeric Bioresorbable Stents: A Strategy to Improve Both Cellular Compatibility and Mechanical Properties. Polymers 2022, 14, 1099. [Google Scholar] [CrossRef]




| Factor | Variable | Levels | Responsive Variables |
|---|---|---|---|
| A | Pressure (bar) | 100, 250, 400 | % L, % AL, and % EXP |
| B | Temperature (°C) | 35, 55 | |
| C | Depressurization rate (bar min−1) | 5, 50 | |
| A | Pressure (bar) | 100, 250, 400 | TS, E, and ε |
| B | Temperature (°C) | 35, 55 | |
| C | Extract impregnation | −1 (no), +1 (yes) |
| Variables | scCO2-Treated | Impregnated with Olea europaea Extract | |||||
|---|---|---|---|---|---|---|---|
| Pres. (bar) | Temp. (°C) | TS (MPa) | E (MPa) | ε (%) | TS (MPa) | E (MPa) | ε (%) |
| 100 | 35 | 34.6 ± 0.4 | 603.0 ± 43.7 | 6.0 ± 0.6 | 6.9 ± 0.5 | 202.0 ± 21.5 | 5.9 ± 1.3 |
| 55 | 52.3 ± 2.3 | 1000.8 ± 67.3 | 3.8 ± 0.3 | 29.9 ± 1.3 | 1960.2 ± 248.8 | 4.0 ± 0.6 | |
| 250 | 35 | 11.4 ± 0.7 | 172.0 ± 16.8 | 42.0 ± 18.6 | 7.4 ± 0.6 | 283.4 ± 50.2 | 5.0 ± 1.2 |
| 55 | 16.7 ± 1.0 | 268.0 ± 27.6 | 38.5 ± 26.1 | 19.8 ± 0.5 | 953.2 ± 89.8 | 3.6 ± 0.4 | |
| 400 | 35 | 22.9 ± 1.7 | 420.8 ± 67.2 | 6.2 ± 0.4 | 23.1 ± 19 | 588.1 ± 60.1 | 7.0 ± 1.5 |
| 55 | 19.9 ± 1.8 | 329.2 ± 52.1 | 9.3 ± 3.5 | 22.4 ± 6.9 | 1875.2 ± 102.9 | 0.1 ± 0.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, N.D.; Mosquera, J.E.; Cejudo-Bastante, C.; Goñi, M.L.; Martini, R.E.; Gañán, N.A.; Mantell-Serrano, C.; Casas-Cardoso, L. Supercritical Impregnation of PETG with Olea europaea Leaf Extract: Influence of Operational Parameters on Expansion Degree, Antioxidant and Mechanical Properties. Polymers 2024, 16, 1567. https://doi.org/10.3390/polym16111567
Machado ND, Mosquera JE, Cejudo-Bastante C, Goñi ML, Martini RE, Gañán NA, Mantell-Serrano C, Casas-Cardoso L. Supercritical Impregnation of PETG with Olea europaea Leaf Extract: Influence of Operational Parameters on Expansion Degree, Antioxidant and Mechanical Properties. Polymers. 2024; 16(11):1567. https://doi.org/10.3390/polym16111567
Chicago/Turabian StyleMachado, Noelia D., José E. Mosquera, Cristina Cejudo-Bastante, María L. Goñi, Raquel E. Martini, Nicolás A. Gañán, Casimiro Mantell-Serrano, and Lourdes Casas-Cardoso. 2024. "Supercritical Impregnation of PETG with Olea europaea Leaf Extract: Influence of Operational Parameters on Expansion Degree, Antioxidant and Mechanical Properties" Polymers 16, no. 11: 1567. https://doi.org/10.3390/polym16111567








