Preparation of Polydopamine Functionalized HNIW Crystals and Application in Solid Propellants
Abstract
:1. Introduction
2. Experimental
2.1. Materials
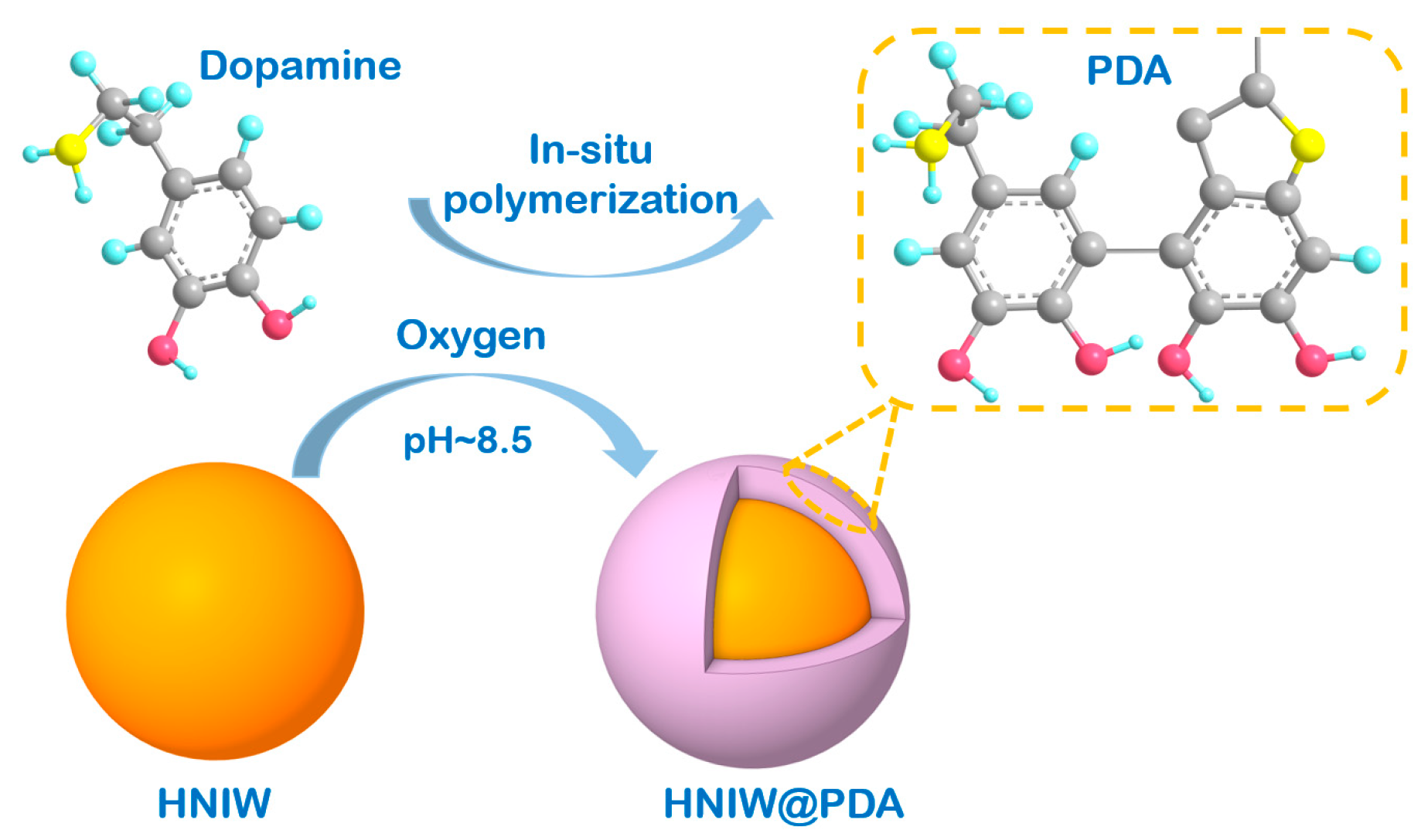
2.2. Preparations of PDA and HNIW@PDA
2.3. Preparation of Solid Propellants
3. Results and Discussions
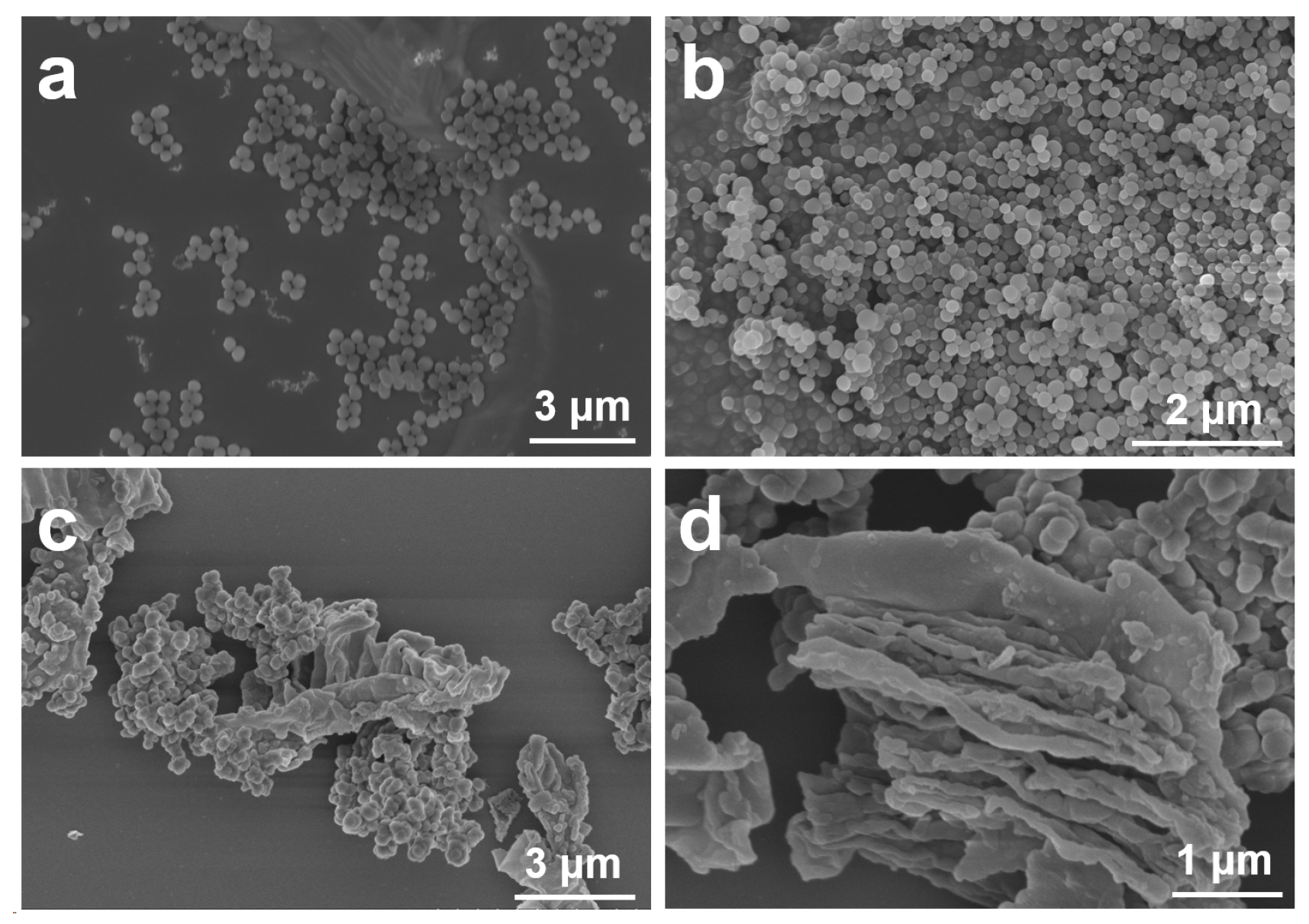
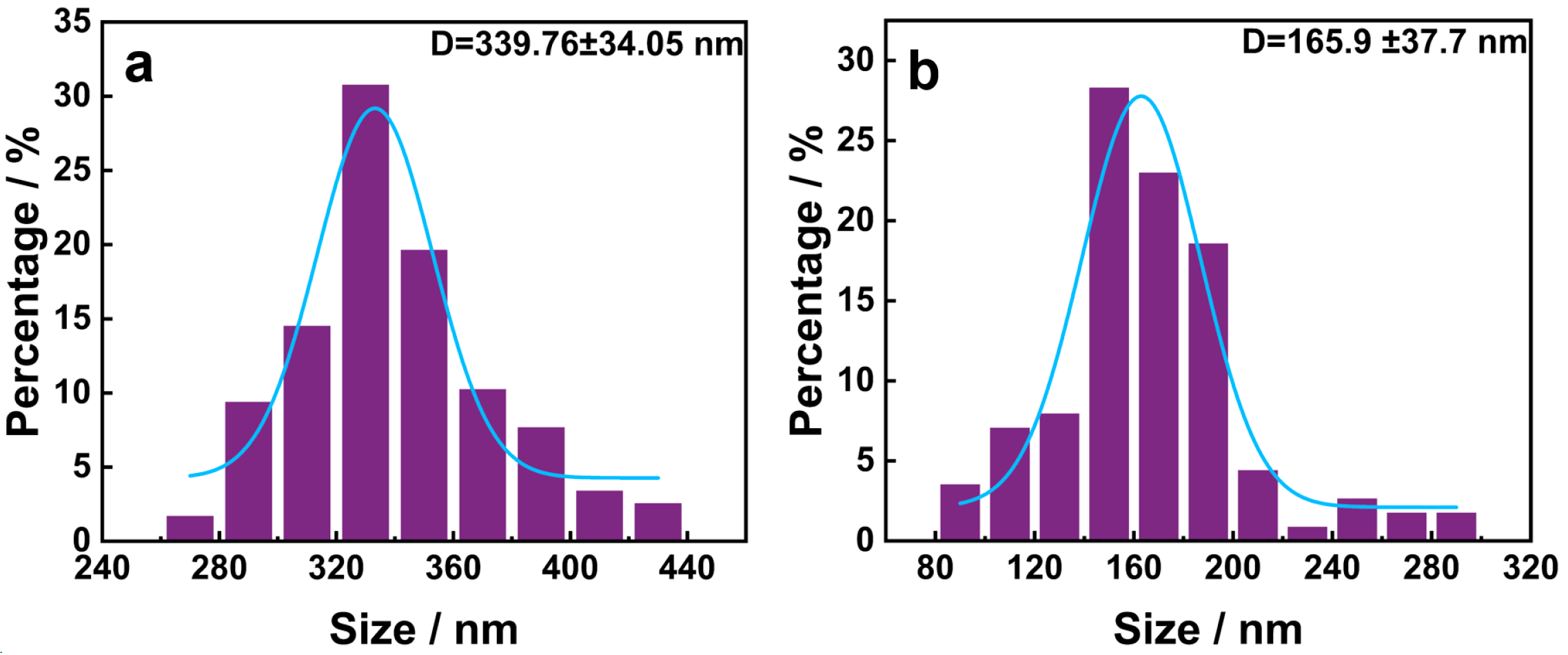
3.1. Morphology and Structure of PDA
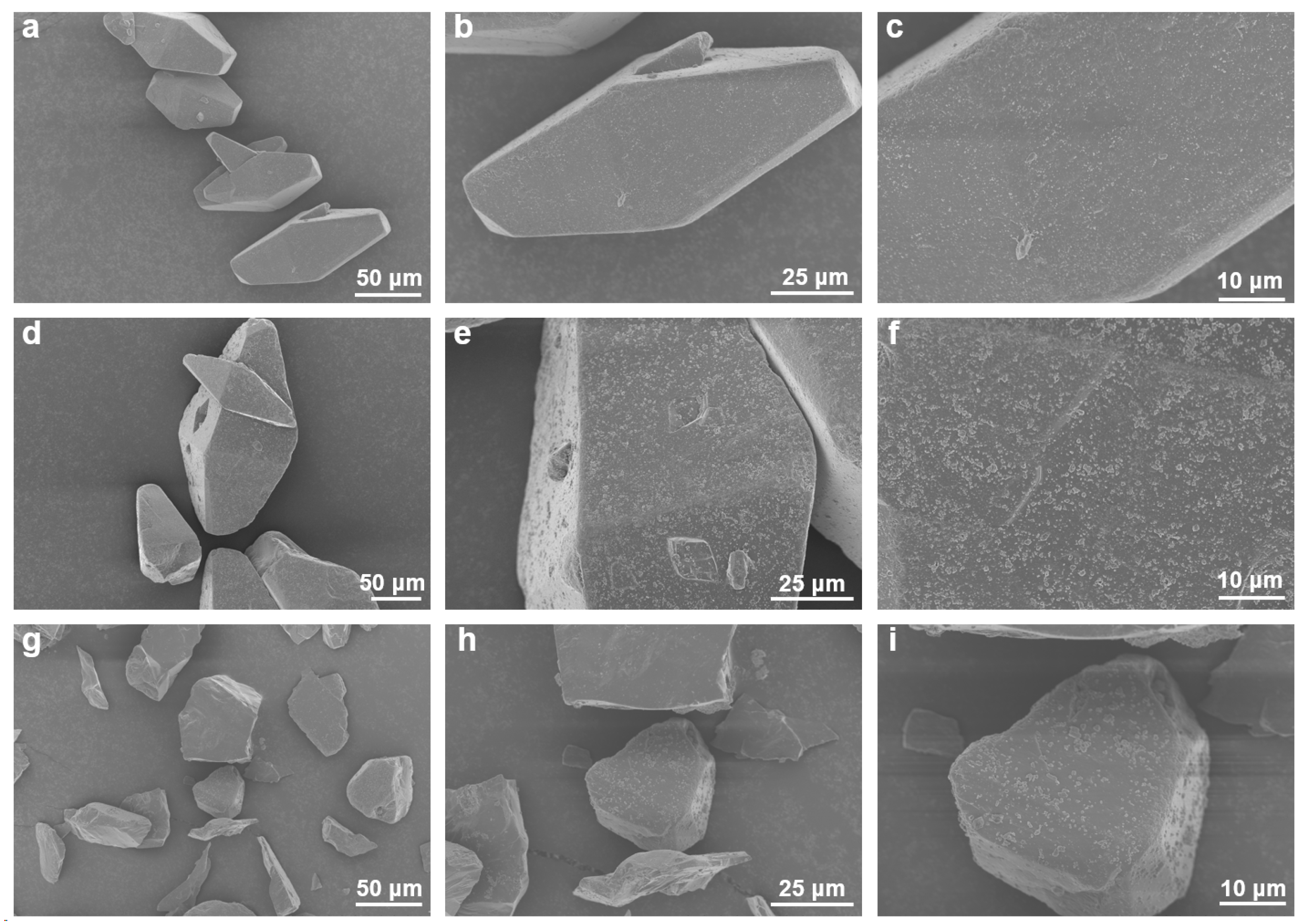
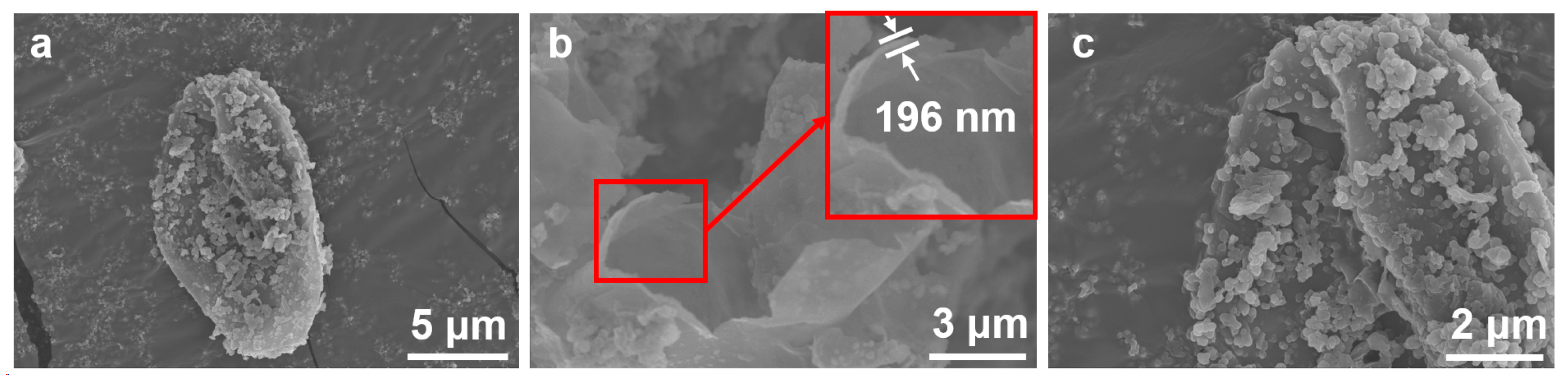
3.2. Microscopic Morphology and Structure of HNIW@PDA
3.2.1. Microscopic Morphology of HNIW@PDA
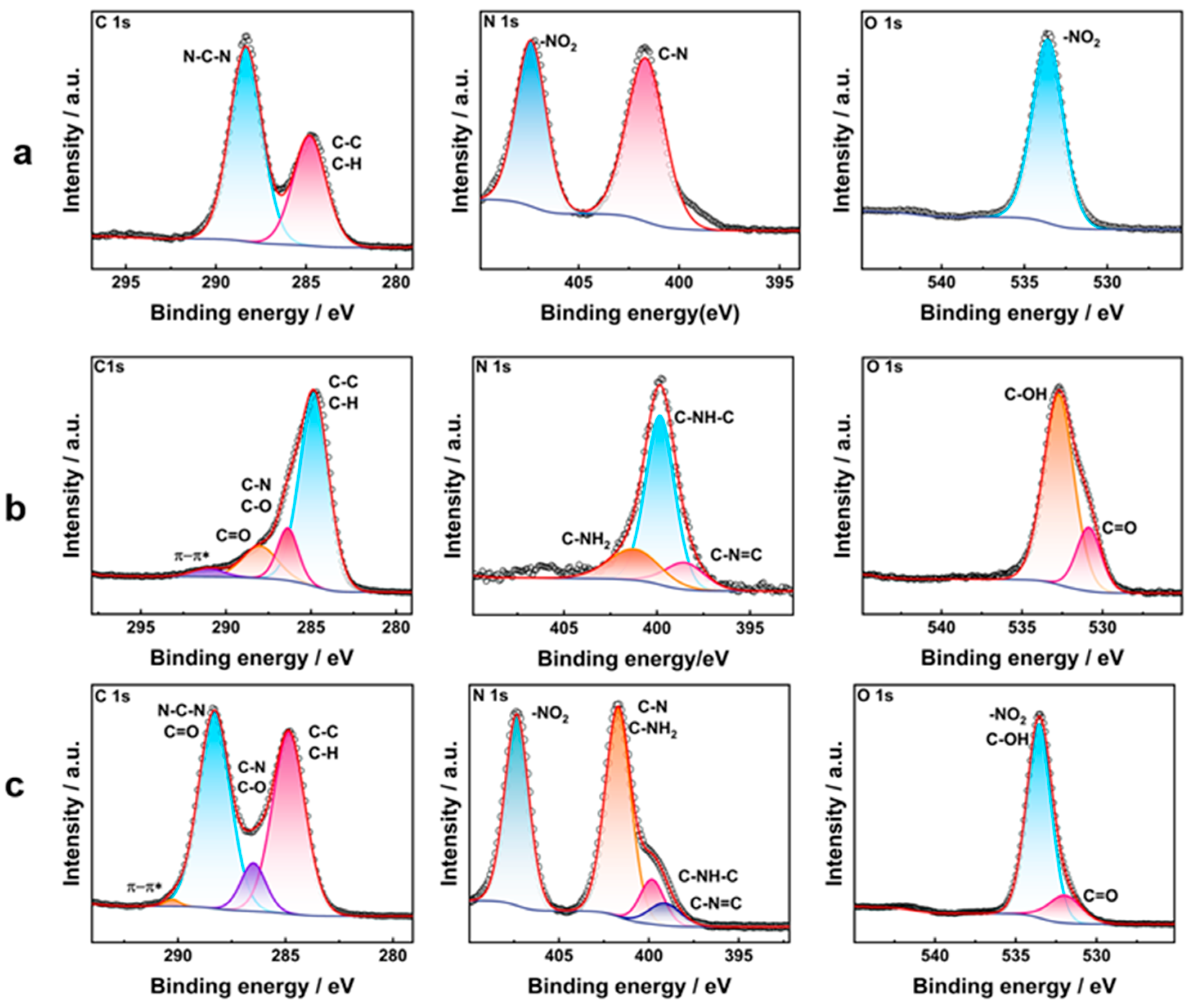
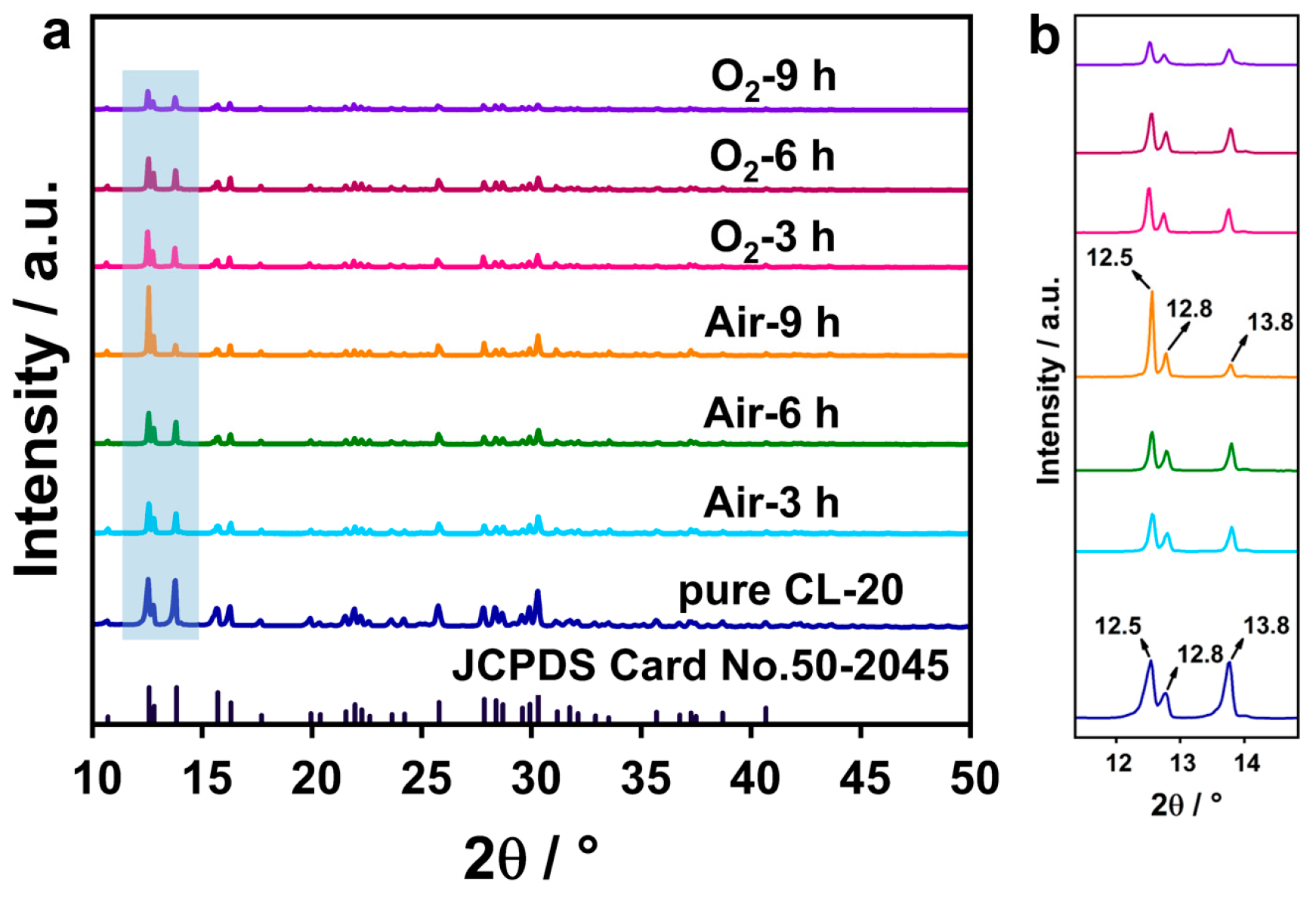
3.2.2. Structure of HNIW@PDA
3.2.3. Properties of HNIW@PDA
3.3. Properties of Solid Propellants
3.3.1. Mechanical Performance
3.3.2. Energy Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.A.; Ashutosh, P.; Vargeese, A.A. Decomposition mechanism of hexanitrohexaazaisowurtzitane (CL-20) by coupled computational and experimental study. J. Phys. Chem. A 2019, 123, 4014–4020. [Google Scholar] [CrossRef] [PubMed]
- Sinditskii, V.P.; Yudin, N.V.; Fedorchenko, S.I.; Egorshev, V.Y.; Kostin, N.A.; Gezalyan, L.V.; Zhang, J.G. Thermal decomposition behavior of CL-20 co-crystals. Thermochim. Acta 2020, 691, 178703. [Google Scholar] [CrossRef]
- Agrawal, J.P.; Dodke, V.S. Some novel high energy materials for improved performance. Z. Anorg. Allg. Chem. 2021, 647, 1856–1882. [Google Scholar] [CrossRef]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High power explosive with good sensitivity: A 2: 1 cocrystal of CL-20: HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Ghosh, M.; Venkatesan, V.; Mandave, S.; Banerjee, S.; Sikder, N.; Sikder, A.K.; Bhattacharya, B. Probing crystal growth of ε-and α-CL-20 polymorphs via metastable phase transition using microscopy and vibrational spectroscopy. Cryst. Growth Des. 2014, 14, 5053–5063. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Qin, Z.; Wang, C.; Xu, Y.; Sun, Z.; Xu, S.; Yi, J.; Zhao, F. Catalytic activity of K2Ba[Ni (NO2)6] on the thermolysis and laser ignition of CL-20, FOX-7 and TKX-50. J. Phys. Chem. Solids 2022, 161, 110411. [Google Scholar] [CrossRef]
- Bayat, Y.; Zeynali, V. Preparation and characterization of nano-CL-20 explosive. J. Energ. Mater. 2011, 29, 281–291. [Google Scholar] [CrossRef]
- McBain, A.; Vuppuluri, V.; Gunduz, I.E.; Groven, L.J.; Son, S.F. Laser ignition of CL-20 (hexanitrohexaazaisowurtzitane) cocrystals. Combust. Flame 2018, 188, 104–115. [Google Scholar] [CrossRef]
- Atamanov, M.; Lyu, J.Y.; Chen, S.; Yan, Q.L. Preparation of CNTs coated with polydopamine-Ni complexes and their catalytic effects on the decomposition of CL-20. ACS Omega 2021, 6, 22866–22875. [Google Scholar] [CrossRef] [PubMed]
- Naik, N.H.; Gore, G.M.; Gandhe, B.R.; Sikder, A.K. Studies on thermal decomposition mechanism of CL-20 by pyrolysis gas chromatography–mass spectrometry (Py-GC/MS). J. Hazard. Mater. 2008, 159, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Banerjee, S.; Khan, M.A.S.; Sikder, N.; Sikder, A.K. Understanding metastable phase transformation during crystallization of RDX, HMX and CL-20: Experimental and DFT studies. Phys. Chem. Chem. Phys. 2016, 18, 23554–23571. [Google Scholar] [CrossRef] [PubMed]
- Gump, J.C.; Peiris, S.M. Phase transitions and isothermal equations of state of epsilon hexanitrohexaazaisowurtzitane (CL-20). J. Appl. Phys. 2008, 104, 083509. [Google Scholar] [CrossRef]
- Smith, N.; Guthrie, S.; Dreger, Z.; Giri, G. Selective morphological and polymorphic control of CL-20 thin films using meniscus-guided coating. Cryst. Growth Des. 2021, 22, 1164–1171. [Google Scholar] [CrossRef]
- Li, Y.; Li, B. Biomass materials self-assembled core-shell CL-20@Biomass materials-TiO2 energetic composites with reduced impact sensitivity and enhanced thermal stability. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133160. [Google Scholar] [CrossRef]
- Utkin, A.; Mochalova, V.; Zubareva, A.; Rykova, V.; Sosikov, V.; Yakushev, V. Shock initiation and detonation parameters of hexanitrohexaazaisowurtzitane (CL-20). Propellants Explos. Pyrotech. 2022, 47, e202200051. [Google Scholar] [CrossRef]
- Kumari, A.; Chaudhary, A.K.; Rajasekhar, K. Study of charge transfer mechanism of PEDOT polymer for detection of solid TEX and CL-20 explosives using pulsed photoacoustic technique. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118597. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.; Huang, B.; Liu, S.; Huang, Z.; Nie, F. Preparation and properties study of core-shell CL-20/TATB composites. Propellants Explos. Pyrotech. 2014, 39, 51–58. [Google Scholar] [CrossRef]
- Hemmatpour, H.; De Luca, O.; Crestani, D.; Stuart, M.C.; Lasorsa, A.; van der Wel, P.C.; Loos, K.; Giousis, T.; Haddadi-Asl, V.; Rudolf, P. New insights in polydopamine formation via surface adsorption. Nat. Commun. 2023, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- Mulyati, S.; Muchtar, S.; Arahman, N.; Syamsuddin, Y.; Mat Nawi, N.I.; Yub Harun, N.; Bilad, M.R.; Firdaus, Y.; Takagi, R.; Matsuyama, H. Two-step dopamine-to-polydopamine modification of polyethersulfone ultrafiltration membrane for enhancing anti-fouling and ultraviolet resistant properties. Polymers 2020, 12, 2051. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Weil, T.; Ng, D.Y.W.; Ball, V. Polydopamine at biological interfaces. Adv. Colloid Interface Sci. 2022, 305, 102689. [Google Scholar] [CrossRef] [PubMed]
- Saraf, M.; Prateek, R.R.; Balasubramaniam, B.; Thakur, V.K.; Gupta, R.K. Polydopamine-enabled biomimetic surface engineering of materials: New insights and promising applications. Adv. Mater. Interfaces 2024, 11, 2300670. [Google Scholar] [CrossRef]
- Kim, H.W.; McCloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.J.; Freeman, B.D.; Park, H.B. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Interfaces 2013, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Liu, S.; Bei, Y.; Ding, Y.; Liu, J.; He, W. Bio-inspired synthesis of energetic microcapsules core-shell structured with improved thermal stability and reduced sensitivity via in situ polymerization for application potential in propellants. Adv. Mater. Interfaces 2021, 8, 2101248. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, Q.; Zhao, W.; Guo, X.; Li, D.; Sun, X. Enhanced crystal stabilities of ε-CL-20 via core-shell structured energetic composites. Appl. Sci. 2020, 10, 2663. [Google Scholar] [CrossRef]
- Lin, C.; Yang, X.; He, G.; Wen, Y.; Qian, W.; Liu, R.; Liu, S.; Gong, F.; Zhang, J.; Zeng, C.; et al. Mussel-inspired interfacial reinforcement of thermoplastic polyurethane based energetic composites. Compos. Sci. Technol. 2023, 232, 109875. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Lin, M.F.; Ma, J.; Lu, X.; Lee, P.S. Polydopamine spheres as active templates for convenient synthesis of various nanostructures. Small 2013, 9, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.B.; Chen, Y.; Narain, R. Bioinspired dopamine and zwitterionic polymers for non-fouling surface engineering. Chem. Soc. Rev. 2021, 50, 11668–11683. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Zhang, J.; Ding, L.; Yang, Z.; Liu, X. Mussel-inspired coating of energetic crystals: A compact core–shell structure with highly enhanced thermal stability. Chem. Eng. J. 2017, 309, 140–150. [Google Scholar] [CrossRef]
- Zmerli, I.; Michel, J.P.; Makky, A. Bioinspired polydopamine nanoparticles: Synthesis, nanomechanical properties, and efficient PEGylation strategy. J. Mater. Chem. B 2020, 8, 4489–4504. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, D.; Gao, B.; Wang, J.; Luo, B.; Yang, G.; Nie, F. Solid–solid phase transition study of ε-CL-20/binder composites. RSC Adv. 2016, 6, 859–865. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Thakur, A.; Soni, P.K.; Kumar, M. Recrystallization of CL-20 to ε-polymorphic form. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1033, 012056. [Google Scholar] [CrossRef]
- Attia, A.A.M.; Abas, K.M.; Ahmed Nada, A.A.; Shouman, M.A.H.; Šišková, A.O.; Mosnáček, J. Fabrication, modification, and characterization of lignin-based electrospun fibers derived from distinctive biomass sources. Polymers 2021, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.H.; Xu, R.; Wang, Z.; Yu, M.; Zhao, X.; Yan, Q.L. Interfacial self-assembling of nano-TATB@ PDA embedded football-like CL-20 co-particles with reduced sensitivity. Chem. Eng. J. 2024, 488, 151010. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Chernyi, A.N.; Egorshev, V.Y.; Dashko, D.V.; Tel’man, K.G.; Shishov, N.I. Combustion of CL-20 cocrystals. Combust. Flame 2019, 207, 51–62. [Google Scholar] [CrossRef]
- Walley, S.M.; Field, J.E.; Greenaway, M.W. Crystal sensitivities of energetic materials. Mater. Sci. Technol. 2006, 22, 402–413. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Z.; Ye, L.; Yuan, W.; Xiao, L.; Qu, W.; Cui, C.; Chen, X.; Ren, H.; Cai, J.; et al. Direct mass spectrometric observation and reaction mechanism of gas-phase initial intermediates during CL-20 decomposition. Combust. Flame 2022, 241, 112095. [Google Scholar] [CrossRef]
- Lal, S.; Gao, H.; Jean’ne, M.S. Design and computational insight into two novel CL-20 analogues, BNMTNIW and BNIMTNIW: High performance energetic materials. New J. Chem. 2022, 46, 16693–16701. [Google Scholar] [CrossRef]
- Sultan, M.; Wu, J.; Haq, I.U.; Mudassar, M.; Yang, L.; Wu, J.; Lu, J.; Chen, L. A complete thermal decomposition mechanism study of an energetic-energetic CL-20/DNT cocrystal at different extreme temperatures by using ReaxFF reactive molecular dynamics simulations. J. Mol. Struct. 2022, 1269, 133691. [Google Scholar] [CrossRef]
- Duan, B.; Lu, X.; Mo, H.; Tan, B.; Wang, B.; Liu, N. Fabrication of CL-20/HMX cocrystal@ melamine–formaldehyde resin core–shell composites featuring enhanced thermal and safety performance via in situ polymerization. Int. J. Mol. Sci. 2022, 23, 6710. [Google Scholar] [CrossRef] [PubMed]
- Quagliano Amado, J.C.; Ross, P.G.; Mattos Silva Murakami, L.; Narciso Dutra, J.C. Properties of hydroxyl-terminal polybutadiene (HTPB) and its use as a liner and binder for composite propellants: A review of recent advances. Propellants Explos. Pyrotech. 2022, 47, e202100283. [Google Scholar] [CrossRef]
- Lysien, K.; Stolarczyk, A.; Jarosz, T. Energetic polyoxetanes as high-performance binders for energetic composites: A critical review. Polymers 2022, 14, 4651. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Prakash, C.; Tomar, V.; Harr, M.; Gunduz, I.E.; Oskay, C. Experimentally-validated mesoscale modeling of the coupled mechanical-thermal response of AP-HTPB energetic material under dynamic loading. Int. J. Fract. 2017, 203, 277–298. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Wang, Z.; Zhang, Y.; Ge, Z.; Luo, Y. Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions. Polym. Bull. 2015, 72, 1835–1847. [Google Scholar] [CrossRef]
- Razaviamri, S.; Wang, K.; Liu, B.; Lee, B.P. Catechol-based antimicrobial polymers. Molecules 2021, 26, 559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, Z.; Wu, Y.; Zhao, W. Designing strong interfacial adhesion between carbon fiber and epoxy resin via dopamine towards excellent protection ability under high hydrostatic pressure and severe erosion corrosion condition. Compos. Sci. Technol. 2022, 217, 109090. [Google Scholar] [CrossRef]






| Sample | Contents of Component (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| HTPB a | TDI b | DOS c | HNIW | HNIW@PDA | AP d | Al | T313 e | |
| SP-0 | 10.70 | 0.60 | 3.70 | 15.00 | 0 | 52.00 | 18.00 | 0.18 |
| SP-25% | 11.25 | 3.75 | ||||||
| SP-50% | 7.50 | 7.50 | ||||||
| SP-75% | 3.75 | 11.25 | ||||||
| SP-100% | 0 | 15 | ||||||
| Sample | C 1s (%) | N 1s (%) | O 1s (%) | N/C |
|---|---|---|---|---|
| HNIW | 32.98 | 33.65 | 33.37 | 1.02 |
| PDA | 70.55 | 8.92 | 20.54 | 0.13 |
| HNIW@PDA | 43.24 | 26.98 | 29.78 | 0.62 |
| Sample | TT (°C) | TP (°C) |
|---|---|---|
| HNIW | 163.9 | 242.9 |
| HNIW@PDA-Air-3 h | 162.4 | 243.1 |
| HNIW@PDA-Air-6 h | 174.8 | 243.7 |
| HNIW@PDA-Air-9 h | 161.0 | 244.6 |
| HNIW@PDA-O2-3 h | 168.1 | 244.6 |
| HNIW@PDA-O2-6 h | 184.7 | 239.9 |
| HNIW@PDA-O2-9 h | 171.5 | 247.2 |
| Sample | Impact Energy (J) | Reduction Rate of IS (%) |
|---|---|---|
| HNIW | 5.5 | - |
| HNIW@PDA-Air-3 h | 8.0 | 45.45 |
| HNIW@PDA-Air-6 h | 10.0 | 81.82 |
| HNIW@PDA-Air-9 h | 8.5 | 54.55 |
| HNIW@PDA-O2-3 h | 9.0 | 63.64 |
| HNIW@PDA-O2-6 h | 13.5 | 145.45 |
| HNIW@PDA-O2-9 h | 12.5 | 127.27 |
| Sample | Mass Ratio of C (%) |
|---|---|
| HNIW | 16.76 |
| PDA | 53.52 |
| HNIW@PDA | 17.19 |
| Sample | QV (kJ/kg) |
|---|---|
| SP-0 | 6297 |
| SP-25% | 6289 |
| SP-50% | 6281 |
| SP-75% | 6292 |
| SP-100% | 6287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Liu, C.; Yang, D.; Li, G. Preparation of Polydopamine Functionalized HNIW Crystals and Application in Solid Propellants. Polymers 2024, 16, 1566. https://doi.org/10.3390/polym16111566
Zhu F, Liu C, Yang D, Li G. Preparation of Polydopamine Functionalized HNIW Crystals and Application in Solid Propellants. Polymers. 2024; 16(11):1566. https://doi.org/10.3390/polym16111566
Chicago/Turabian StyleZhu, Fengdan, Chang Liu, Desheng Yang, and Guoping Li. 2024. "Preparation of Polydopamine Functionalized HNIW Crystals and Application in Solid Propellants" Polymers 16, no. 11: 1566. https://doi.org/10.3390/polym16111566
APA StyleZhu, F., Liu, C., Yang, D., & Li, G. (2024). Preparation of Polydopamine Functionalized HNIW Crystals and Application in Solid Propellants. Polymers, 16(11), 1566. https://doi.org/10.3390/polym16111566






