Development of Cellulose Microfibers from Mixed Solutions of PAN-Cellulose in N-Methylmorpholine-N-Oxide
Abstract
1. Introduction
- -
- How does deformation affect the fibrillar morphology of mixed solutions;
- -
- Is it possible to obtain continuous cellulose microfibers using the dry-jet wet spinning method;
- -
- What are the mechanical properties of cellulose fibrils separated from the matrix polymer;
- -
- What role does the cellulose fibrillar phase play in composite fibers?
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rojas, O.J. Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Cham, Switzerland, 2016; 333p. [Google Scholar]
- Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Kichigina, G.A.; Kushch, P.P.; Kiryukhin, D.P.; Krasnikova, S.S.; Badamshina, E.R.; Vasil’ev, S.G.; Soldatenkov, T.A.; et al. Cellulose from Annual Plants and Its Use for the Production of the Films Hydrophobized with Tetrafluoroethylene Telomers. Molecules 2022, 27, 6002. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Rocha, T.M.; Philippini, R.R.; Martiniano, S.E.; Prado, C.A.; Mier-Alba, E.; Hernandez-Perez, A.F.; Jofre, F.M.; Abdeshahian, P.; Ribeaux, D.R.; et al. The Potential of Vegetal Biomass for Biomolecules Production. In Comprehensive Renewable Energy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–164. [Google Scholar]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Egorov, Y.E.; Kulichikhin, V.G.; Mikhailov, Y.M. New Hydrated Cellulose Fiber Based on Flax Cellulose. Russ. J. Gen. Chem. 2021, 91, 1807–1815. [Google Scholar] [CrossRef]
- Moryganov, A.P.; Zakharov, A.G.; Zhivetin, V.V. Promising polymer materials for chemical and textile production. Russ. Chem. J. 2002, 46, 58–65. [Google Scholar]
- Mboowa, D. A review of the traditional pulping methods and the recent improvements in the pulping processes. Biomass Convers. Biorefinery 2024, 14, 1–12. [Google Scholar] [CrossRef]
- Korchagina, A.A.; Gismatulina, Y.A.; Budaeva, V.V.; Zolotukhin, V.N.; Bychin, N.V.; Sakovich, G.V. Miscanthus × Giganteus var. KAMIS as a new feedstock for cellulose nitrates. J. Sib. Fed. Univ. Chem. 2020, 13, 565–577. [Google Scholar] [CrossRef]
- Thygesen, A.; Madsen, B.; Bjerre, A.B.; Lilholt, H. Cellulosic Fibers: Effect of Processing on Fiber Bundle Strength. J. Nat. Fibers 2011, 8, 161–175. [Google Scholar] [CrossRef]
- Kudela, J.; Kurjatko, S. Wood Structure and Properties ’02; Arbora Publishers: Zvolen, Slovakia, 2002. [Google Scholar]
- Lavrenteva, E.P.; Sanina, O.K.; Belousov, R.O. The deep processing of bast fibers as the way to the revival of the national traditions of Russia. Text. Technol. Int. 2022, 3, 130–139. [Google Scholar]
- Smyslov, A.G. Method for Producing Cellulose. RF Patent 2779000:C1, 9 September 2022. [Google Scholar]
- Sevastyanova, J.V.; Makarov, I.S.; Potashev, A.V.; Medvedev, V.V.; Vinogradov, M.I.; Legkov, S.A.; Palchikova, E.E. Modern Technology for the Production of Hydrated Cellulose Fibers. Fibers Polym. 2024, 25, 913–921. [Google Scholar] [CrossRef]
- Yadav, R.D.; Chaudhry, S.; Dhiman, S.S. Biopulping and its potential to reduce effluent loads from bleaching of hardwood kraft pulp. BioResources 2010, 5, 159–171. [Google Scholar] [CrossRef]
- Quintana, E.; Valls, C.; Vidal, T.; Roncero, M.B. An enzymecatalysed bleaching treatment to meet dissolving pulp characteristics for cellulose derivatives applications. Bioresour. Technol. 2013, 148, 1–8. [Google Scholar] [CrossRef]
- Schneider, T.; Behn, C.; Windeisen-Holzhauser, E.; Roffael, E. Influence of thermo-mechanical and chemo-thermo-mechanical pulping on the properties of oak fibres. Eur. J. Wood Prod. 2019, 77, 229–234. [Google Scholar] [CrossRef]
- Novikov, A.O.; Temruk, V.I.; Solovyova, T.V.; Gorzhanov, V.V.; Dubovik, A.A. Study of the grinding ability and paper-forming properties of cotton pulp. Proceedings of BSTU. Chem. Technol. Wood Proc. 2012, 4, 157–161. [Google Scholar]
- Kholmova, M.A.; Komarov, V.I.; Guryev, A.V. High yield cellulose. Methods of obtaining. Properties (overview). Chem. Plant Raw Mater. 2007, 2, 5–12. [Google Scholar]
- Bochek, A.M.; Shevchuk, I.L.; Lavrent’ev, V.N. Fabrication of Microcrystalline and Powdered Cellulose from Short Flax Fiber and Flax Straw. Russ. J. Appl. Chem. 2003, 76, 1679–1682. [Google Scholar] [CrossRef]
- Ward, K. Crystallinity of Cellulose and Its Significance for the Fiber Properties. Text. Res. J. 1950, 20, 363–372. [Google Scholar] [CrossRef]
- Meyer, K.H.; Lotmar, W. Sur l’élasticité de la cellulose. (Sur la constitution de la partie cristallisée de la cellulose IV). Helv. Chim. Acta 1936, 19, 68–86. [Google Scholar] [CrossRef]
- Diddens, I.; Murphy, B.; Krisch, M.; Muller, M. Anisotropic elastic properties of cellulose measured using inelastic X-ray scattering. Macromolecules 2008, 41, 9755–9759. [Google Scholar] [CrossRef]
- Shrinivasan, A.V.; Haritos, G.H.; Hedberg, F.L. Biomimetics: Advancing man-made materials through guidance from nature. Appl. Mech. Rev. 1991, 44, 463–481. [Google Scholar] [CrossRef]
- Lu, N.; Swan, R.H., Jr.; Ferguson, I. Composition, structure, and mechanical properties of hemp fiber reinforced composite with recycled high-density polyethylene matrix. J. Compos. Mater. 2012, 46, 1915–1924. [Google Scholar] [CrossRef]
- Marrot, L.; Lefeuvre, A.; Pontoire, B.; Bourmaud, A.; Baley, C. Analysis of the hemp fibre mechanical properties and their scattering (Fedora 17). Ind. Crops Prod. 2013, 51, 317–327. [Google Scholar] [CrossRef]
- Zugenmaier, P. Crystalline Cellulose and Derivatives, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Charlet, K.; Jernot, J.P.; Gomina, M. Mechanical properties of flax fibers and of the derived unidirectional composites. J. Compos. Mater. 2010, 44, 2887–2896. [Google Scholar] [CrossRef]
- Makarov, I.S.; Smyslov, A.G.; Palchikova, E.E.; Vinogradov, M.I.; Shandryuk, G.A.; Levin, I.S.; Arkharova, N.A.; Kulichikhin, V.G. Nonwoven materials based on natural and artificial fibers. Cellulose 2024, 31, 1927–1940. [Google Scholar] [CrossRef]
- Golova, L.K.; Kulichikhin, V.G.; Papkov, S.P. The mechanism of cellulose dissolution in non-aqueous solvent systems. Polym. Sci. Ser. A 1986, 27, 1795–1809. [Google Scholar]
- Liu, S.; Zhang, L. Effects of polymer concentration and coagulation temperature on the properties of regenerated cellulose films prepared from LiOH/urea solution. Cellulose 2009, 16, 189–198. [Google Scholar] [CrossRef]
- Yudianti, R.; Syampurwadi, A.; Onggo, H.; Karina, M.; Uyama, H.; Azuma, J. Properties of bacterial cellulose transparent film regenerated from dimethylacetamide–LiCl solution. Polym. Adv. Technol. 2016, 27, 1102–1107. [Google Scholar] [CrossRef]
- Iovleva, M.M. About new fibers obtained from cellulose systems—An aqueous solution of sodium hydroxide. Fibre Chem. 1996, 1, 11–14. [Google Scholar]
- Sen, S.; Losey, B.P.; Gordon, E.E.; Argyropoulos, D.S.; Martin, J.D. Ionic Liquid Character of Zinc Chloride Hydrates Define Solvent Characteristics that Afford the Solubility of Cellulose. J. Phys. Chem. B 2016, 120, 1134–1141. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Aizenshtein, E.M. Global and Domestic Production and Consumption of Nonwovens. Fibre Chem. 2018, 49, 372–381. [Google Scholar] [CrossRef]
- Higashi, T.; Toyama, T.; Sakurai, H.; Nakaza, M.; Omae, K.; Nakadate, T.; Yamaguchi, N. Cross-sectional Study of Respiratory Symptoms and Pulmonary Functions in Rayon Textile Workers with Special Reference to H2S Exposure. Ind. Health 1983, 21, 281–292. [Google Scholar] [CrossRef]
- Golova, L.K. Processing of cellulose via highly concentrated “solid solutions”. Fibre Chem. 1996, 28, 5–16. [Google Scholar] [CrossRef]
- Golova, L.K.; Borodina, O.E.; Kuznetsova, L.K.; Lyubova, T.A.; Krylova, T.B. The Solid-Phase MMO Process. Fibre Chem. 2000, 32, 243–251. [Google Scholar] [CrossRef]
- Fink, H.-P.; Weigel, P.; Purz, H.; Ganster, J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Golova, L.K.; Vasilyeva, N.V.; Borodina, O.E.; Krylova, T.B.; Kuznetsova, L.K.; Rogovina, S.Z.; Zelenetsky, S.N. Method for Producing Cellulose Solution for the Manufacture of Molded Products. PR Patent 94004914 A1, 20 August 1996. [Google Scholar]
- Palchikova, E.E.; Makarov, I.S.; Vinogradov, M.I.; Golova, L.K.; Shambilova, G.K.; Kulichikhin, V.G. Kinetics of Dissolution of Polyacrylonitrile in N-Methylmorpholine-N-oxide in the Absence and Presence of Cellulose. Polym. Sci. Ser. B 2021, 63, 833–842. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated cellulose by the Lyocell process, a brief review of the process and properties. BioResources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- Vinogradov, M.I.; Golova, L.K.; Makarov, I.S.; Bondarenko, G.N.; Levin, I.S.; Arkharova, N.A.; Kulichikhin, V.G. Transformation of Specific Dispersion Interactions between Cellulose and Polyacrylonitrile in Solutions into Covalent Interactions in Fibers. Materials 2023, 16, 5843. [Google Scholar] [CrossRef]
- Purkait, M.K.; Haldar, D. Value-added products derived from lignocellulosic biomass. In Lignocellulosic Biomass to Value-Added Products; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 9; pp. 125–140. [Google Scholar]
- Hachaichi, A.; Kouini, B.; Kian, L.K.; Asim, M.; Fouad, H.; Jawaid, M.; Sain, M. Nanocrystalline Cellulose from Microcrystalline Cellulose of Date Palm Fibers as a Promising Candidate for Bio-Nanocomposites: Isolation and Characterization. Materials 2021, 14, 5313. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. The Effect of Morphology and Chemical Characteristics of Cellulose Reinforcements on the Crystallinity of Polylactic Acid. J. Appl. Polym. Sci. 2006, 101, 300–310. [Google Scholar] [CrossRef]
- Petersson, L.; Oksman, K. Biopolymer Based Nanocomposites: Comparing Layered Silicates and Microcrystalline Cellulose as Nanoreinforcement. Compos. Sci. Technol. 2006, 66, 2187–2196. [Google Scholar] [CrossRef]
- Boran, S.; Kiziltas, A.; Kiziltas, E.E.; Gardner, D.J. Characterization of Ultrafine Cellulose-Filled High-Density Polyethylene Composites Prepared Using Different Compounding Methods. BioResources 2016, 11, 8178–8199. [Google Scholar] [CrossRef]
- Murr, L.E. Classification of Composite Materials and Structures. In Handbook of Materials Structures, Properties, Processing and Performance; Springer: Berlin/Heidelberg, Germany, 2015; pp. 405–418. [Google Scholar]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Shlyakhtin, A.V.; Nifantiev, I.E.; Kulichikhin, V.G. Method for Preparing a Solution of Acrylonitrile-Based Copolymer in n-Methylmorpholine-n-Oxide. RF Patent 2541473, 13 June 2013. [Google Scholar]
- Golova, L.K.; Romanov, V.V.; Lunina, O.B.; Platonov, V.A.; Papkov, S.P.; Khorozova, O.D.; Yakshin, V.V.; Belasheva, T.P.; Sokira, A.N. Method for Producing Solution for Spinning Fibers. RF Patent 1645308, 30 April 1991. [Google Scholar]
- TB 10099-2017; Code for Design of Railway Station and Terminal. China Railway Publishing House: Beijing, China, 2017.
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Pittenger, B.; Erina, N.; Su, C. Mechanical property mapping at the nanoscale using PeakForce QNM scanning probe technique. In Nanomechanical Analysis of High Performance Materials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 31–51. [Google Scholar]
- Flater, E.E.; Zacharakis-Jutz, G.E.; Dumba, B.G.; White, I.A.; Clifford, C.A. Towards easy and reliable AFM tip shape determination using blind tip reconstruction. Ultramicroscopy 2014, 146, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Tsebrenko, M.V.; Vinogradov, G.V.; Ablazova, T.I.; Yudin, A.V. On the mechanism of the phenomenon of specific fiber formation during the flow of melts of polymer mixtures. Colloid J. 1976, 38, 200. [Google Scholar]
- Sysoev, A.V.; Belousov, S.I.; Godovsky, Y.K.; Chalykh, A.E. Features of the rheological behavior of some mixtures of thermoplastics with thermotropic liquid crystalline polyester. Polym. Sci. Ser. A 1996, 38, 1019–1024. [Google Scholar]
- Kulichikhin, V.G.; Plate, N.A. Blend compositions on the base of liquid crystalline thermoplastic. Polym. Sci. Ser. A 1991, 33, 3–38. [Google Scholar]
- Golubev, Y.V.; Makarov, I.S.; Vinogradov, M.I.; Golova, L.K.; Smyslov, A.G. Technical Fiber Based on Flax Cellulose as Tire Cord Precursor. Fibre Chem. 2023, 55, 223–231. [Google Scholar] [CrossRef]
- Chavan, R.; Patra, A. Development and processing of lyocell. IJFTR 2004, 29, 483. [Google Scholar]
- Skvortsov, I.Y.; Varfolomeeva, L.A.; Kulichikhin, V.G. The Effect of Tetraethoxysilane on the Phase State, Rheological Properties, and Coagulation Features of Polyacrylonitrile Solutions. Colloid J. 2019, 81, 165–175. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Skvortsov, I.Y.; Mironova, M.I.; Ozerin, A.N.; Kurkin, T.S.; Berkovich, A.K.; Frenkin, E.I.; Malkin, A.Y. From polyacrylonitrile, its solutions, and filaments to carbon fibers: I. Phase state and rheology of basic polymers and their solutions. Adv. Polym. Technol. 2018, 37, 1076–1084. [Google Scholar] [CrossRef]
- Pakshver, A.B. Karbotsepnye Volokna (Carbochain Fibers); Khimiya: Moscow, Russia, 1966. [Google Scholar]
- Palchikova, E.E.; Makarov, I.S.; Mironova, M.V.; Vinogradov, M.I.; Golova, L.K.; Kulichikhin, V.G. Phase Transformations in a PAN–N-Methylmorpholine-N-Oxide–Water System. Colloid J. 2022, 84, 730–740. [Google Scholar] [CrossRef]
- Kuleznev, V.N. (Ed.) Fundamentals of Physics and Chemistry of Polymers; Textbook for Universities; M. “Higher School”: Moscow, Russia, 1977. [Google Scholar]
- Golubev, Y.A.; Fokin, D.A.; Utkin, A.A.; Grishin, T. Mechanical properties of natural ultra-high-pressure high-temperature impact glasses at the nanoscale by PeakForce QNM. J. Non-Cryst. Solids 2022, 576, 121302. [Google Scholar] [CrossRef]
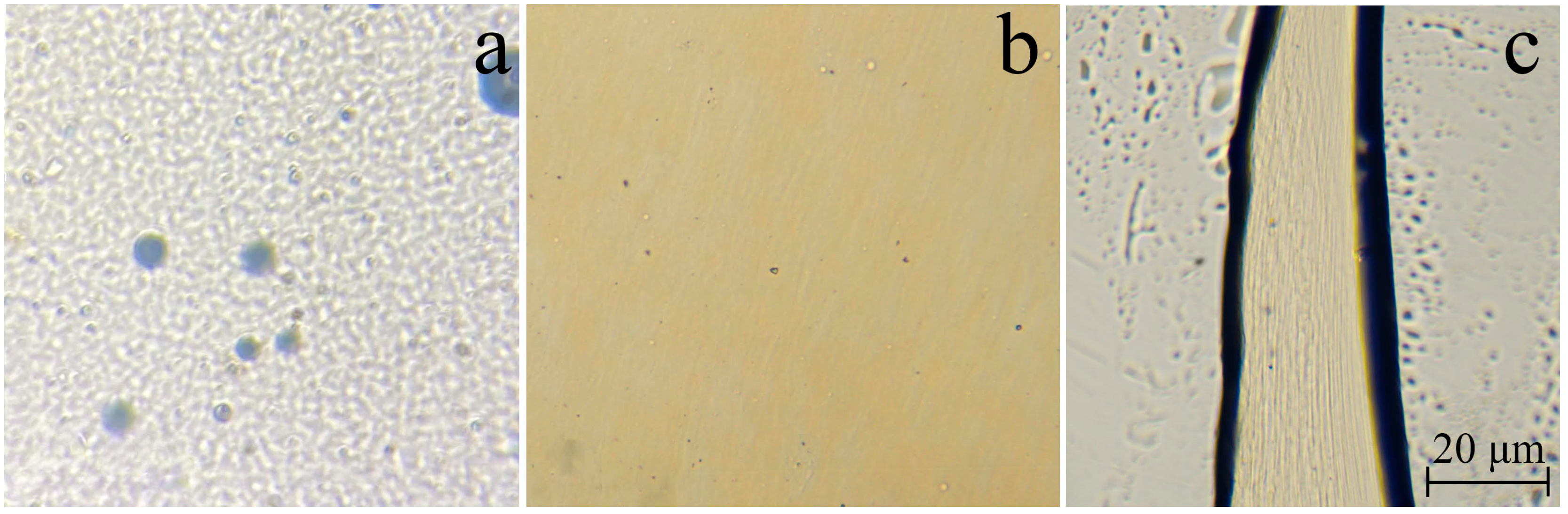
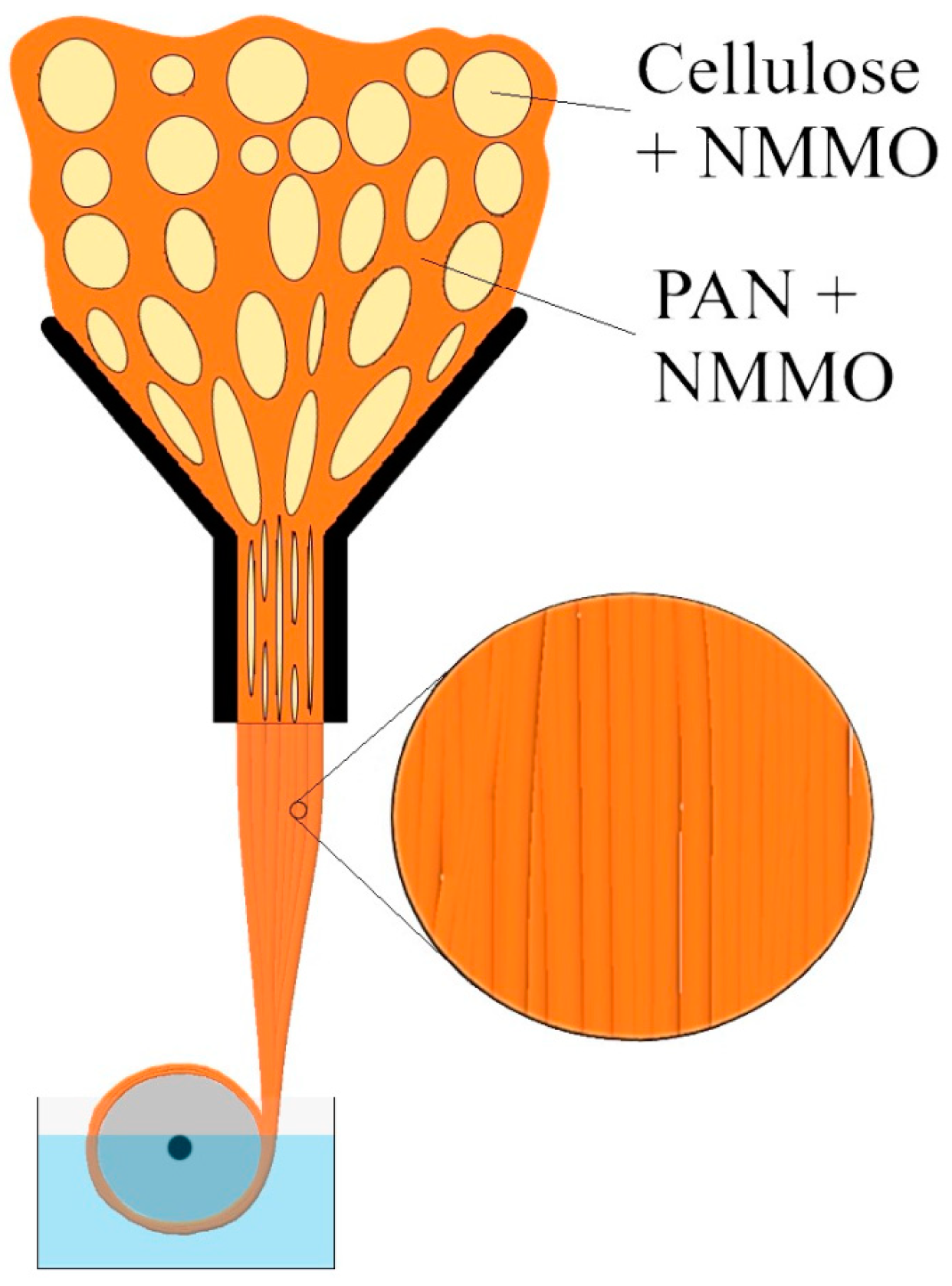
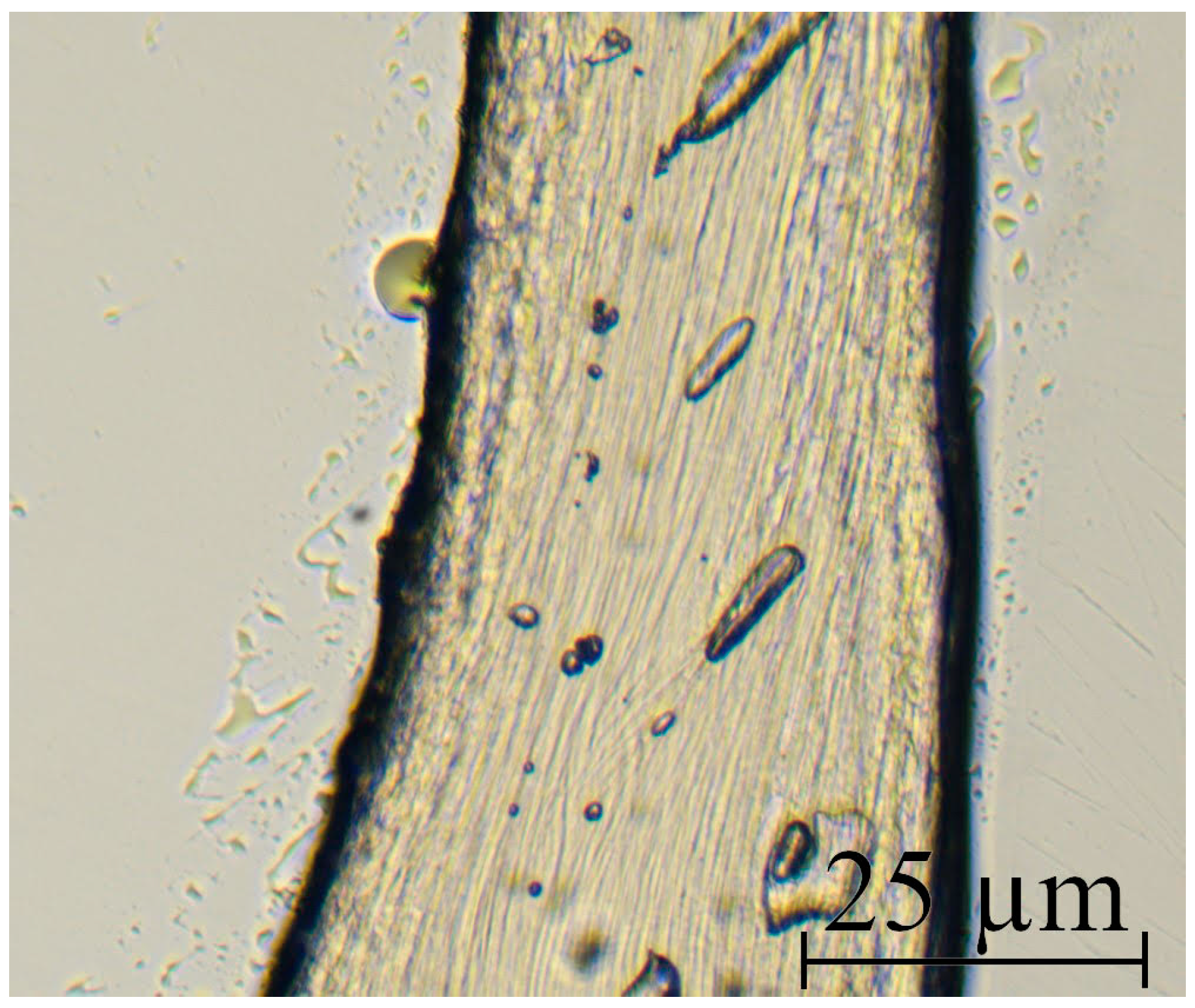

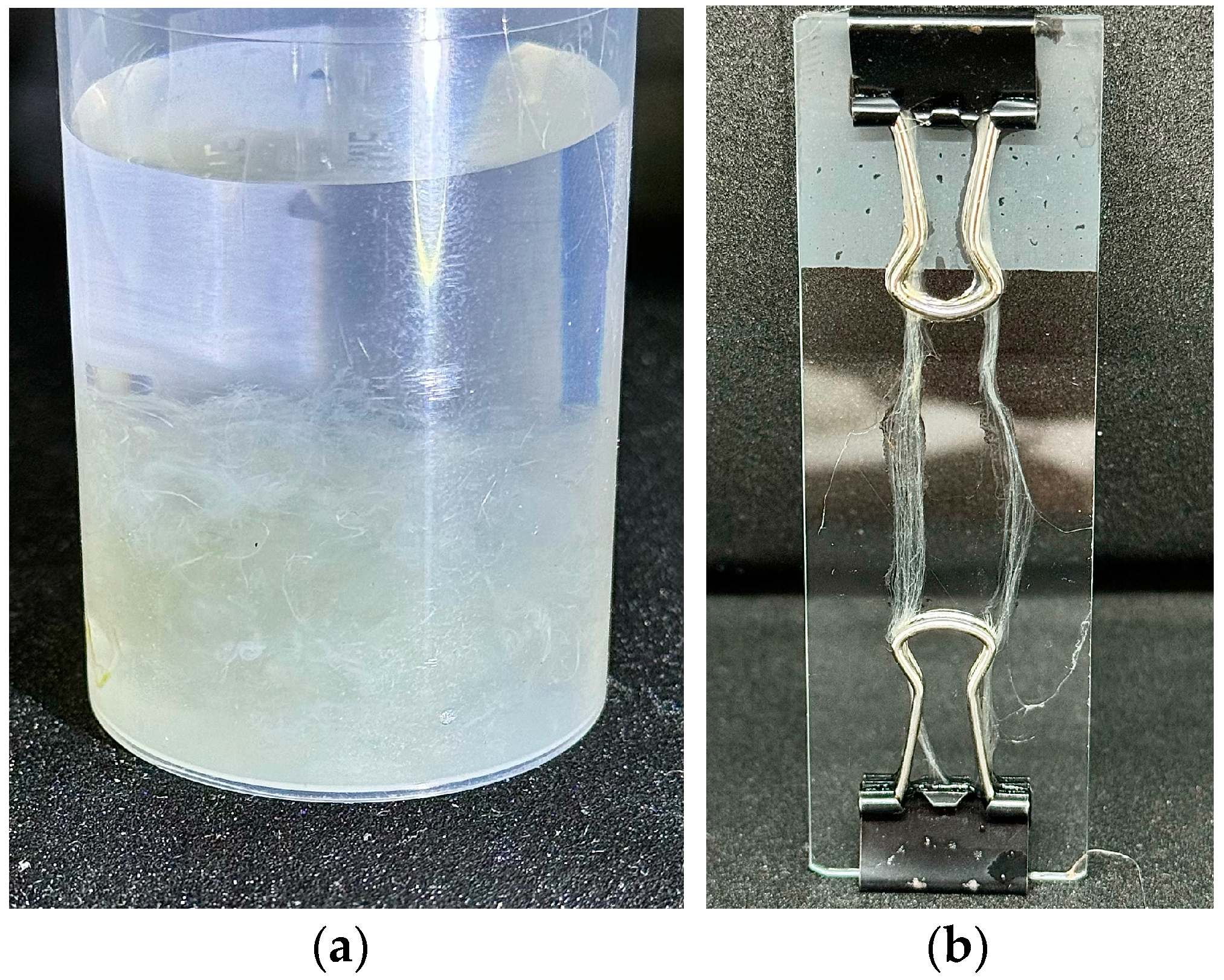
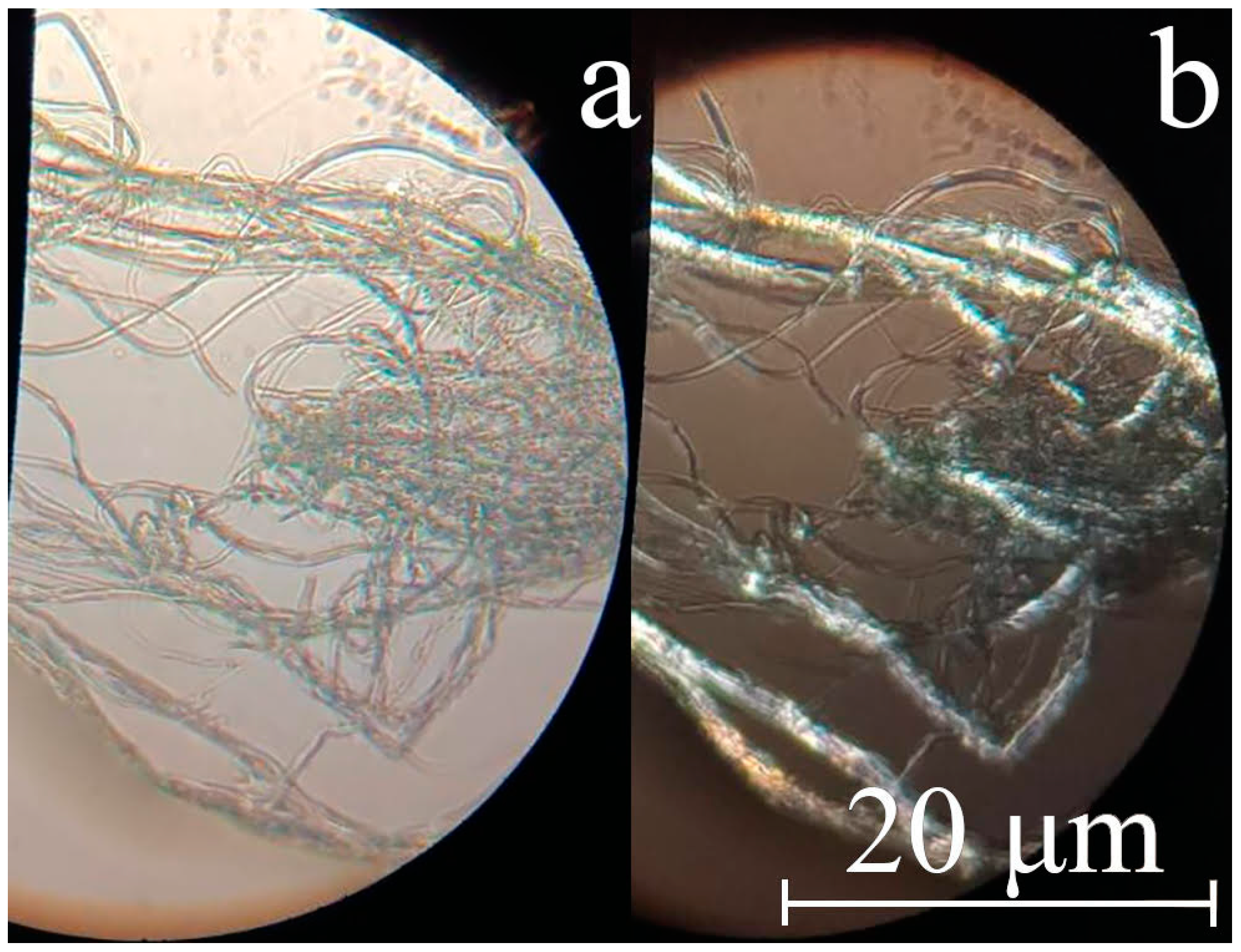


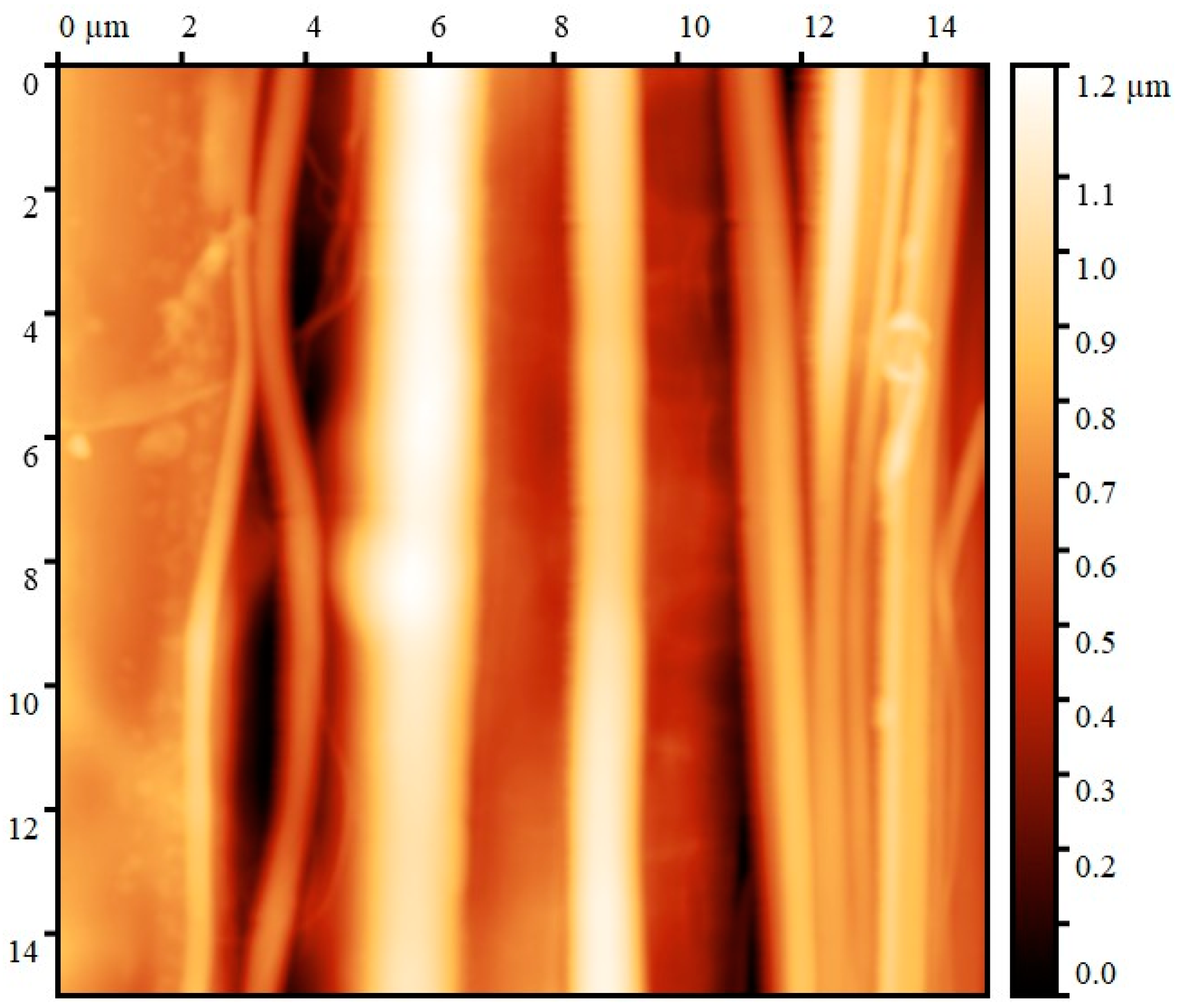

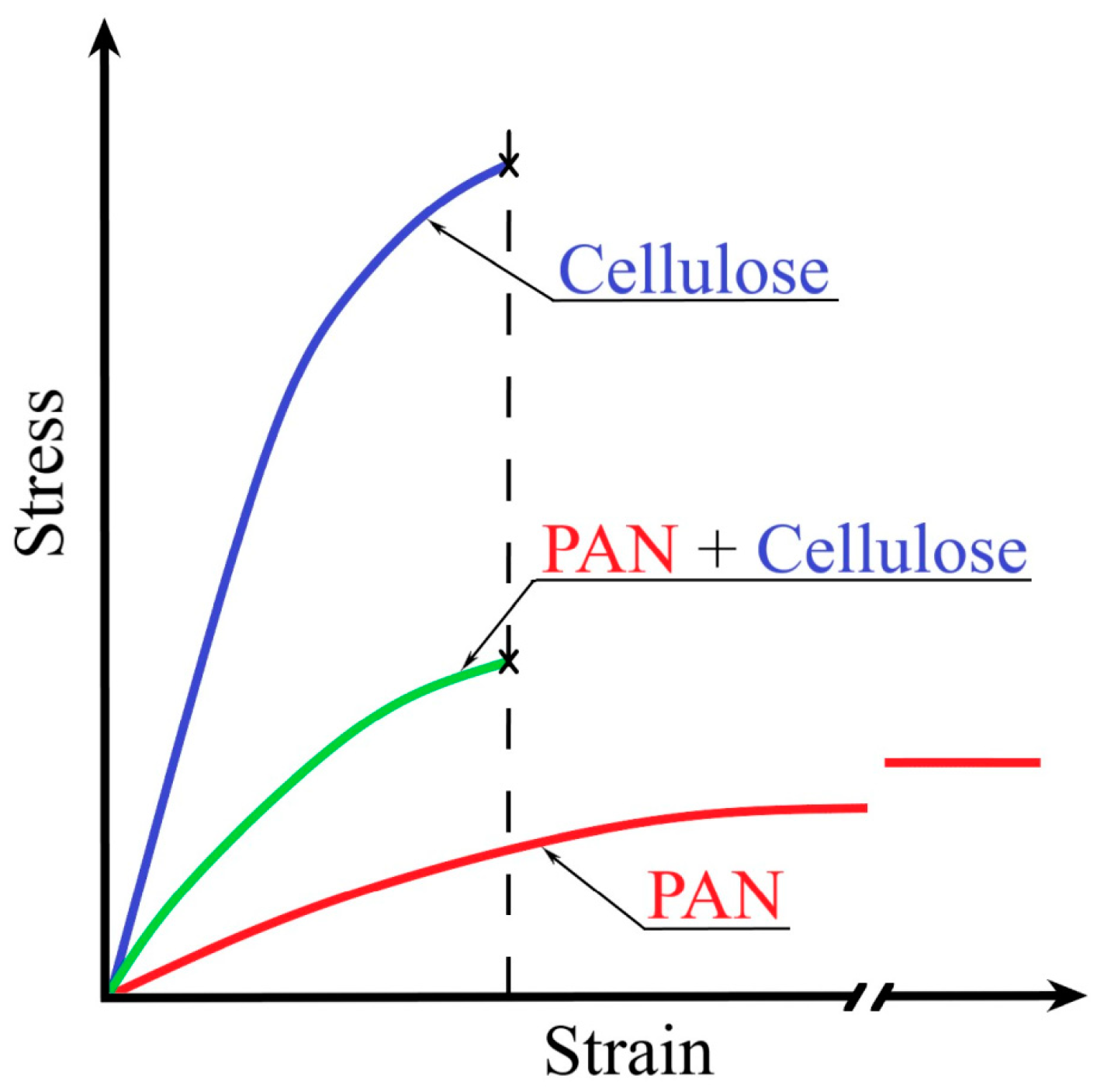
| Sample | Diameter, μm | Tensile Strength, MPa | Modulus of Elasticity, GPa | Elongation, % |
|---|---|---|---|---|
| 100% cellulose | 12.1–18.9 | 410–630 | 7.2–19.6 | 5–9 |
| 100% PAN | 12.2–19.3 | 96–200 | 2.1–4.1 | 60–130 |
| 70% PAN-30% cellulose | 10.8–13.8 | 96–250 | 2.4–5.4 | 4.5–8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, I.; Vinogradov, M.; Golubev, Y.; Palchikova, E.; Kulanchikov, Y.; Grishin, T. Development of Cellulose Microfibers from Mixed Solutions of PAN-Cellulose in N-Methylmorpholine-N-Oxide. Polymers 2024, 16, 1869. https://doi.org/10.3390/polym16131869
Makarov I, Vinogradov M, Golubev Y, Palchikova E, Kulanchikov Y, Grishin T. Development of Cellulose Microfibers from Mixed Solutions of PAN-Cellulose in N-Methylmorpholine-N-Oxide. Polymers. 2024; 16(13):1869. https://doi.org/10.3390/polym16131869
Chicago/Turabian StyleMakarov, Igor, Markel Vinogradov, Yaroslav Golubev, Ekaterina Palchikova, Yuriy Kulanchikov, and Timofey Grishin. 2024. "Development of Cellulose Microfibers from Mixed Solutions of PAN-Cellulose in N-Methylmorpholine-N-Oxide" Polymers 16, no. 13: 1869. https://doi.org/10.3390/polym16131869
APA StyleMakarov, I., Vinogradov, M., Golubev, Y., Palchikova, E., Kulanchikov, Y., & Grishin, T. (2024). Development of Cellulose Microfibers from Mixed Solutions of PAN-Cellulose in N-Methylmorpholine-N-Oxide. Polymers, 16(13), 1869. https://doi.org/10.3390/polym16131869







