Synergistic Construction of Sub-Nanometer Channel Membranes through MOF–Polymer Composites: Strategies and Nanofiltration Applications
Abstract
:1. Introduction
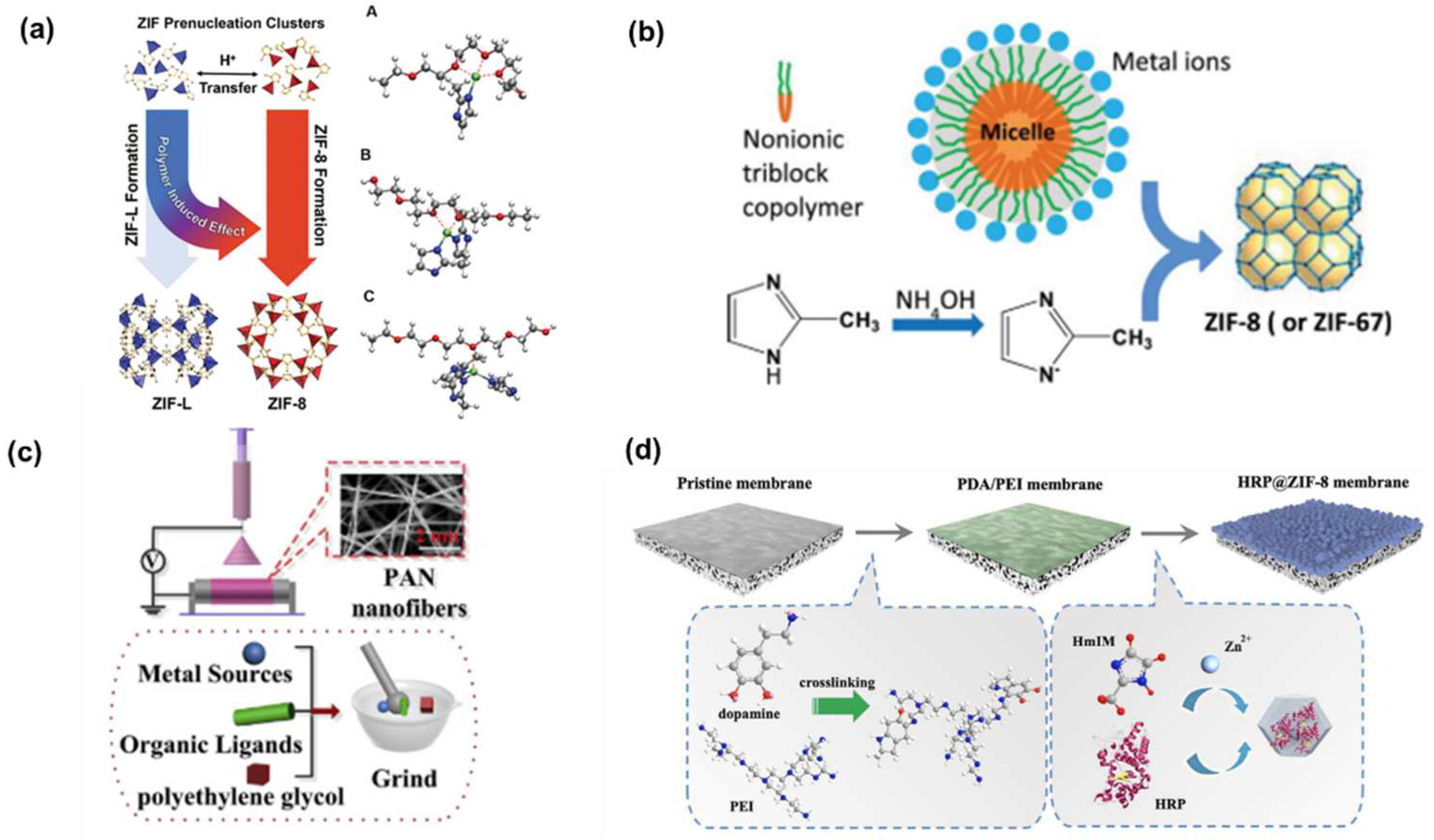
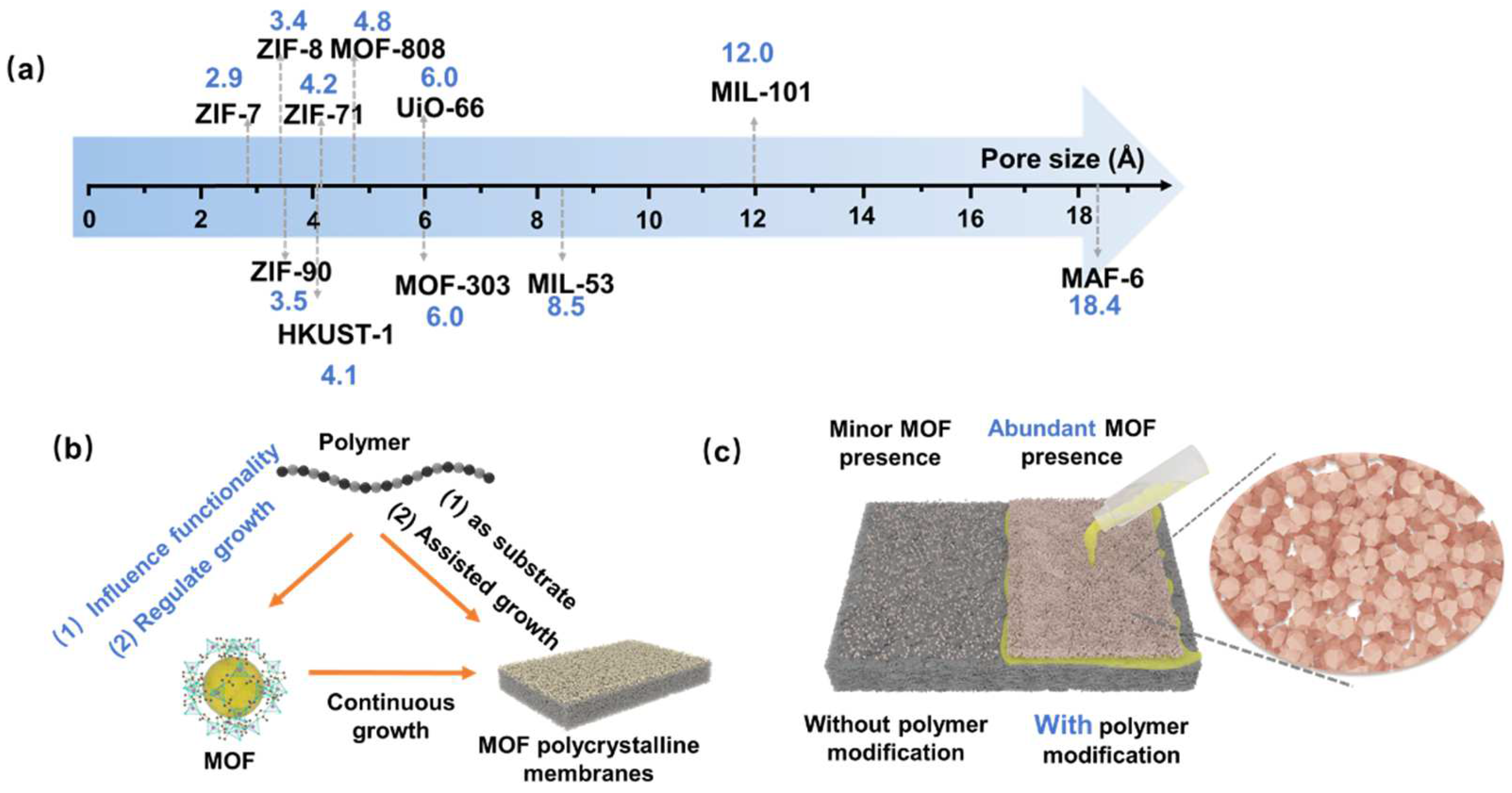
2. Polymer Regulation of MOF Functionality and Growth
2.1. Regulation of MOF Functionality by Polymers
2.2. Regulation of MOF Growth by Polymers
3. Strategies for the Preparation of MOF–Polymer Membranes
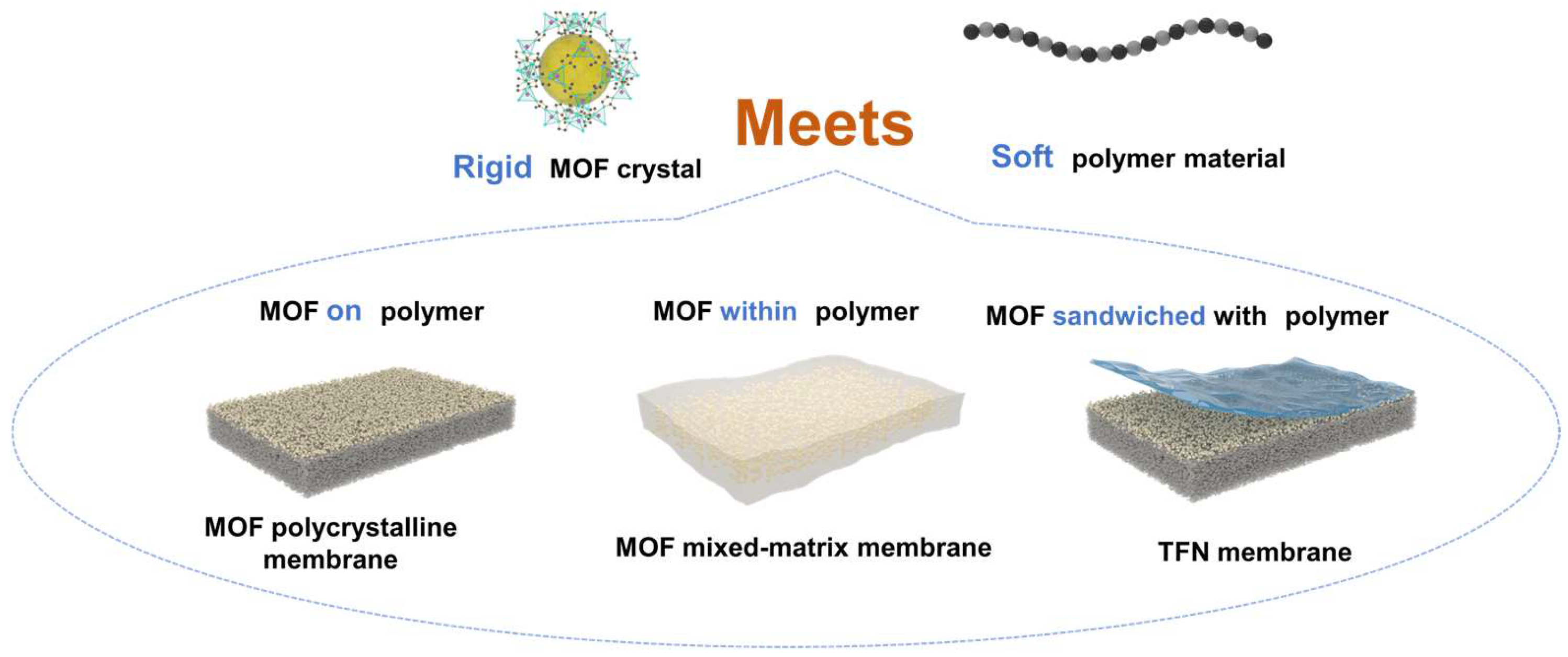
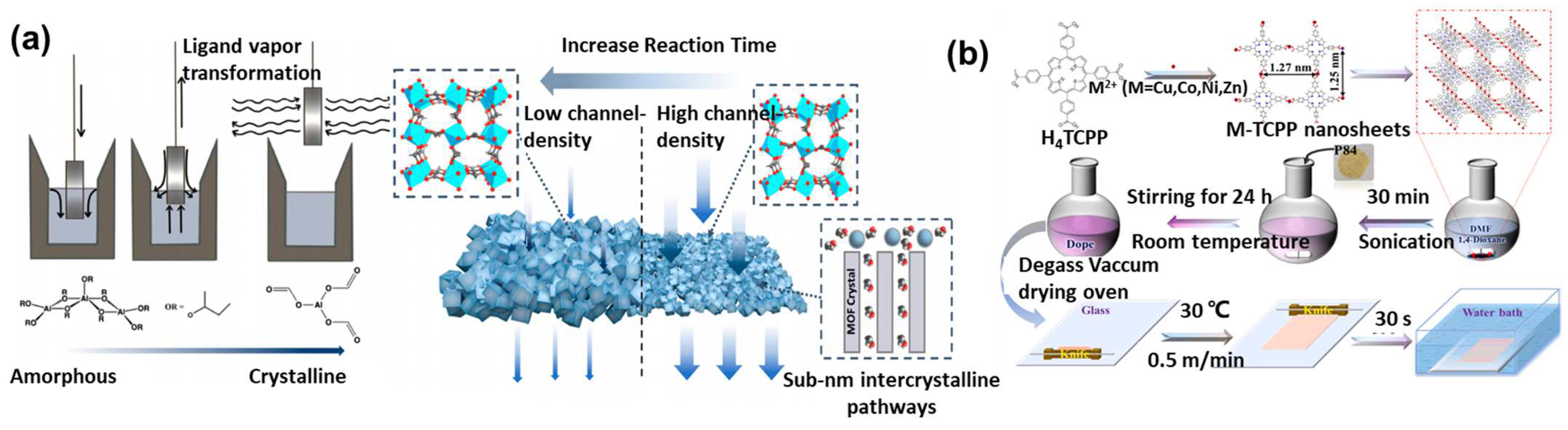
3.1. MOF Polycrystalline Membranes
3.1.1. Growth of MOFs on the Inorganic Carriers Modified with Polymers
3.1.2. Growth of MOFs on the Polymer Substrates
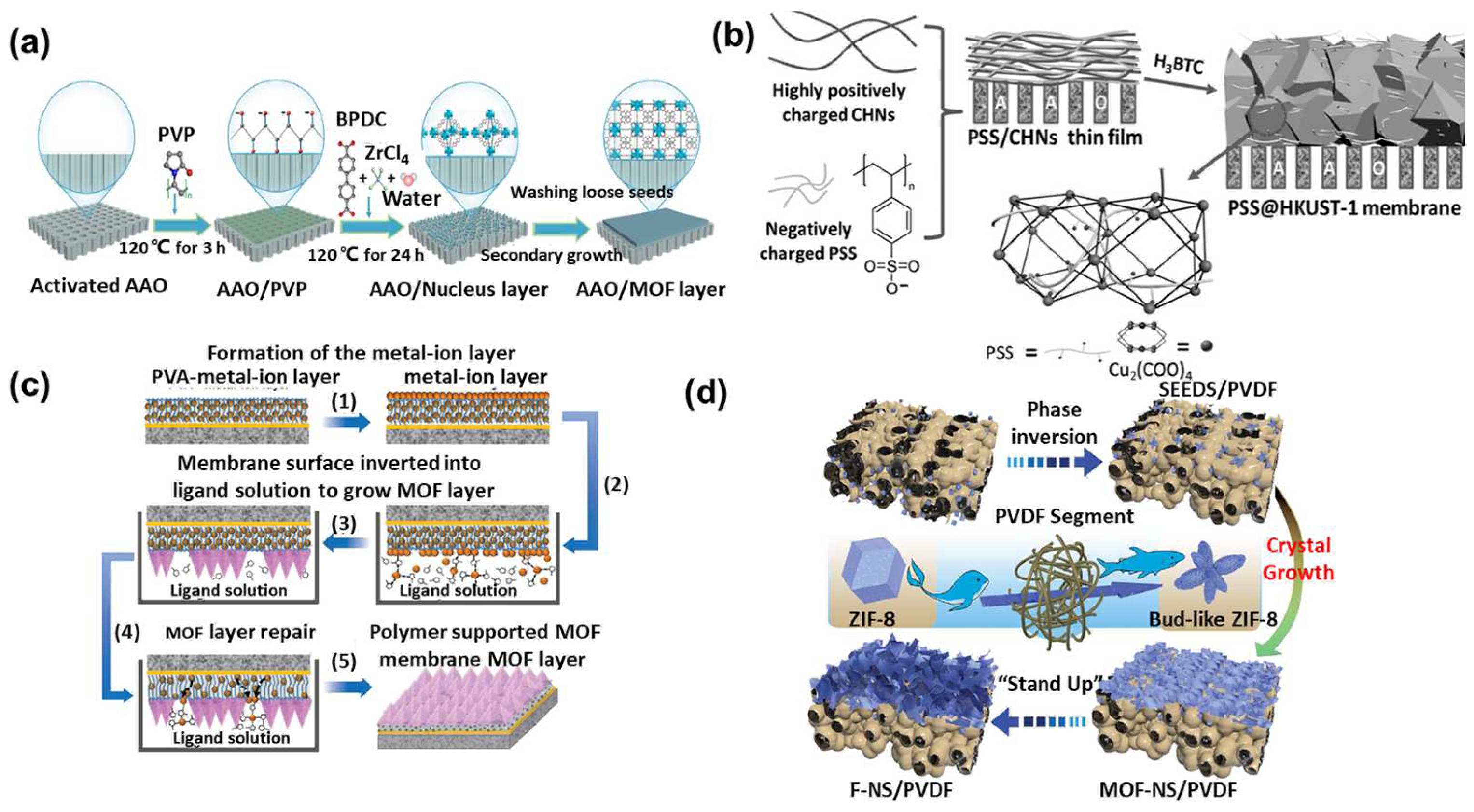
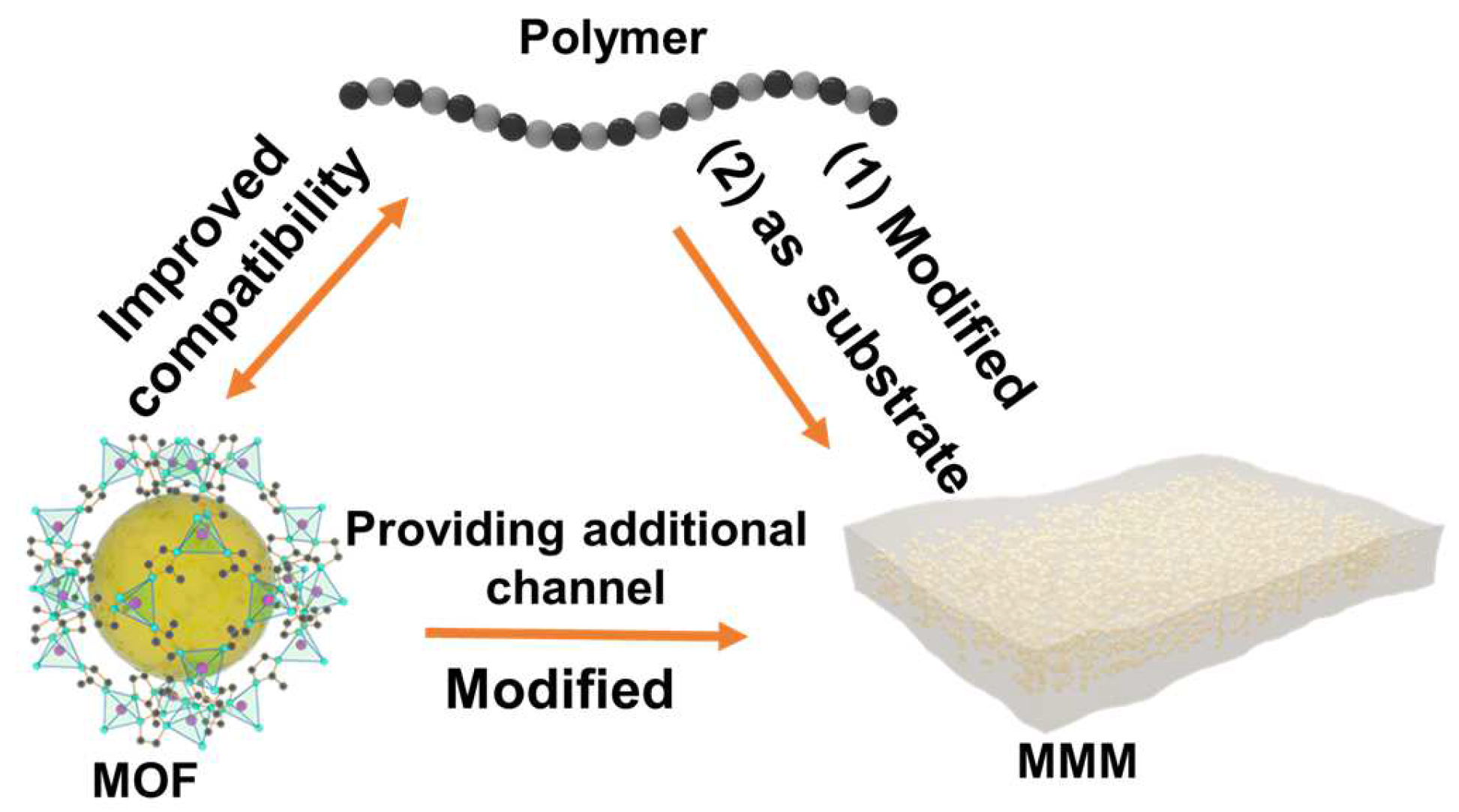
3.2. Growth of MOFs within Polymers: MOF MMMs
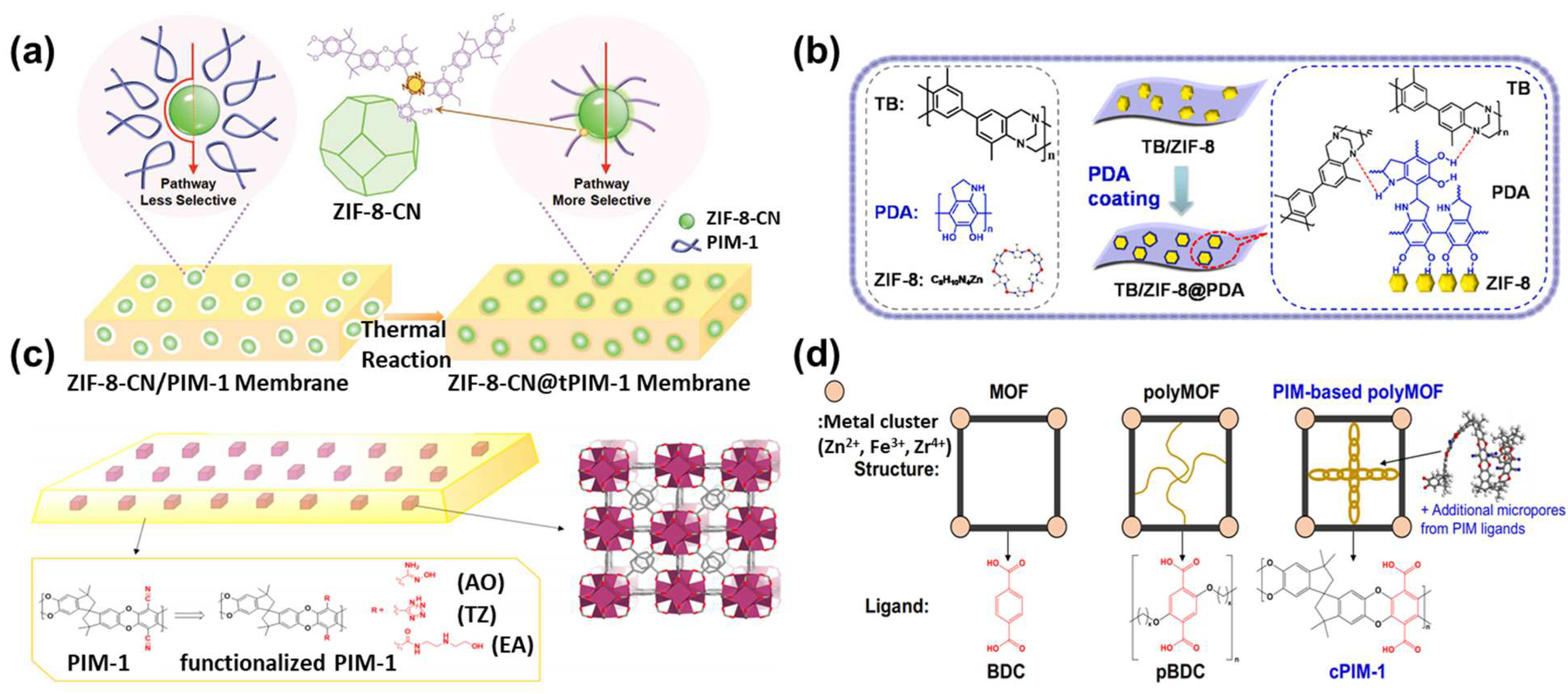
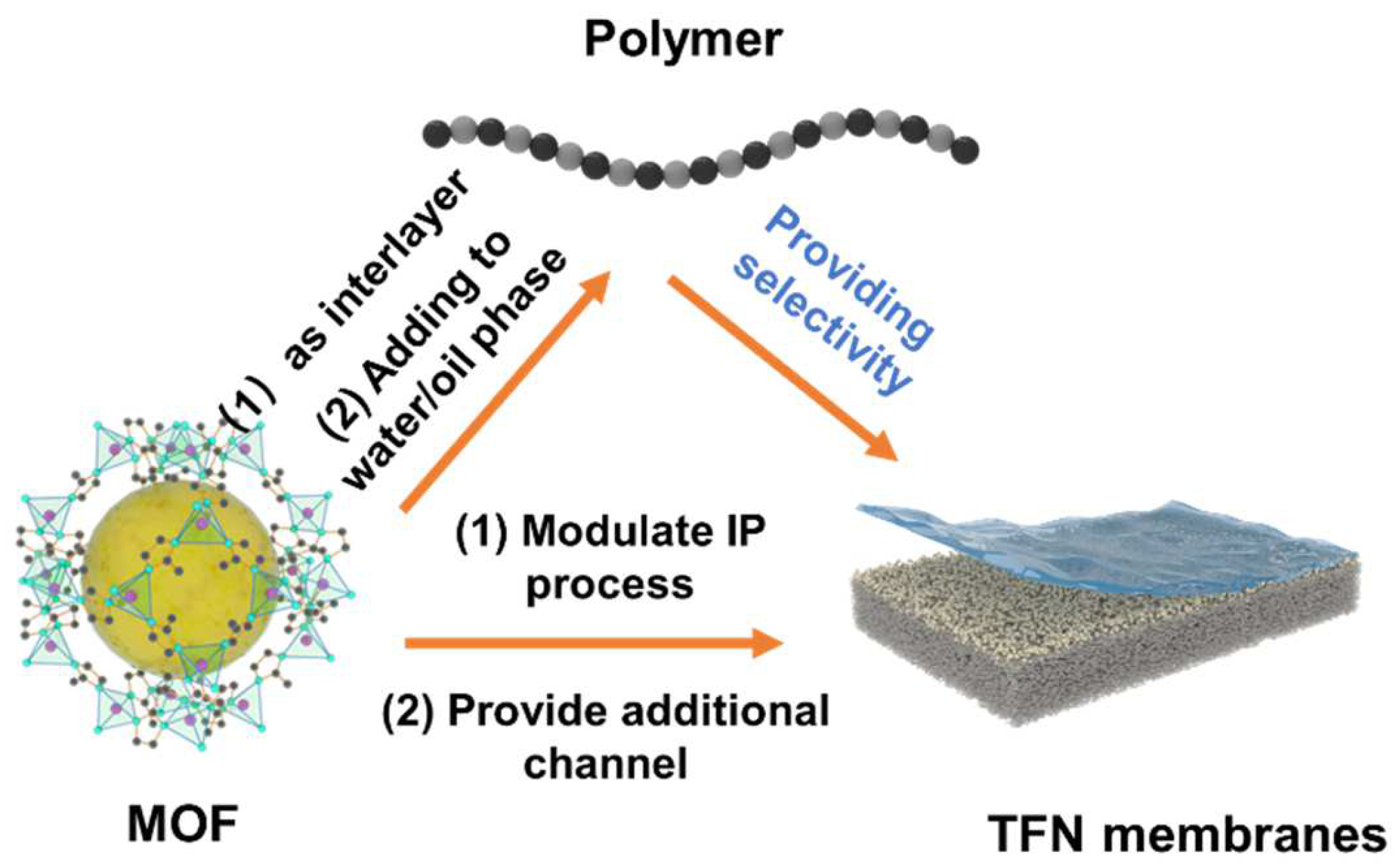
3.3. MOF Sandwiched by Polymers: TFN Membranes
3.3.1. MOF as an Interlayer for Interfacial Polymerization
3.3.2. Doping MOFs into the Aqueous/Organic Phase
3.3.3. In Situ Cogrowth of MOFs
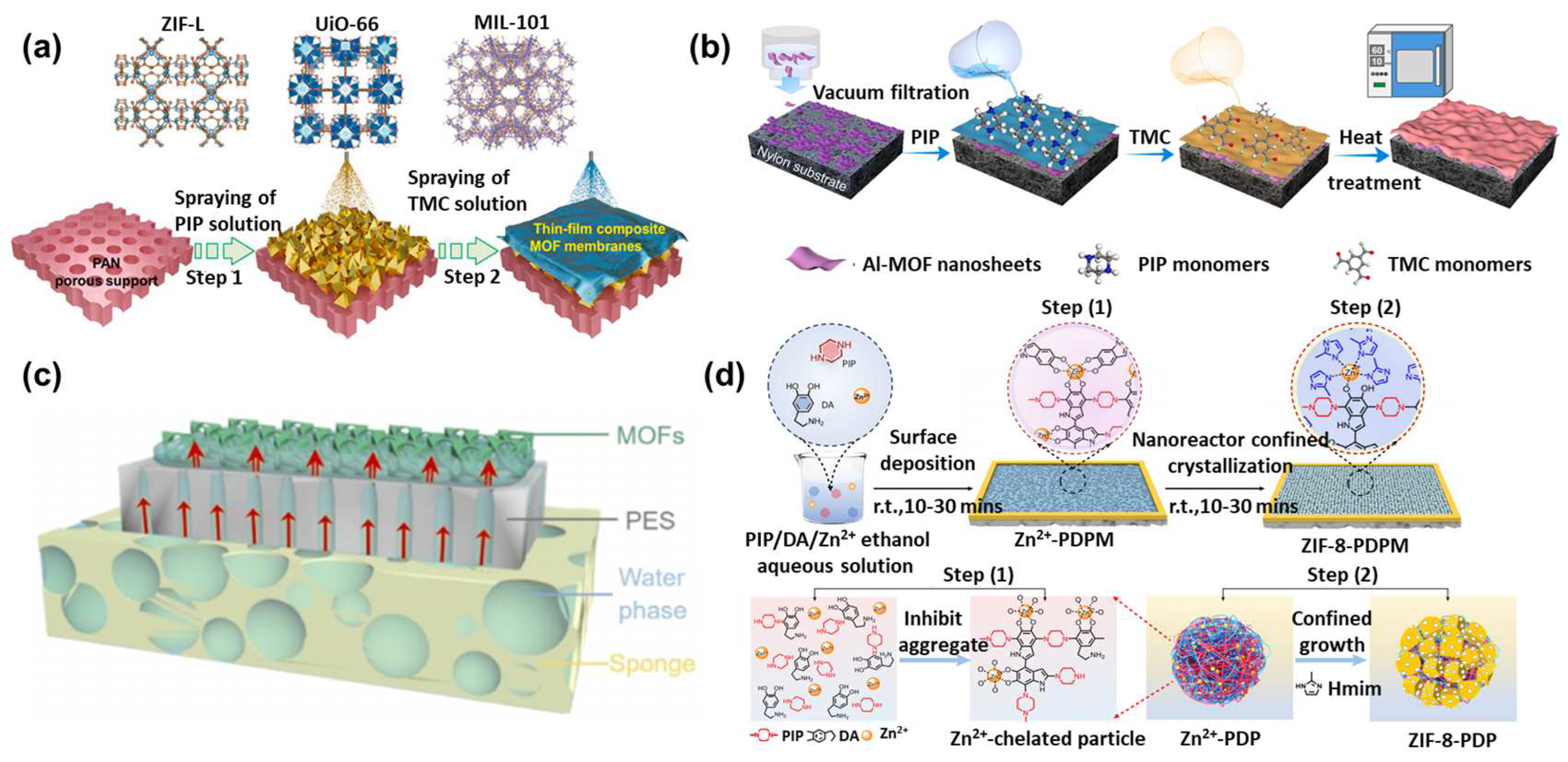
4. Nanofiltration Applications
4.1. Water Treatment
4.2. Organic Solvent Nanofiltration
5. Summary and Outlook
- (1)
- Interaction mechanisms between polymers and MOFs
- (2)
- Coordinated regulation of MOF crystallization and polymer membrane formation
- (3)
- Scaling up the fabrication of the MOF-based membrane for practical applications
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.; Dong, R.; Evans, A.M.; Biere, N.; Ebrahim, M.A.; Li, S.; Anselmetti, D.; Dichtel, W.R.; Livingston, A.G. Aligned macrocycle pores in ultrathin films for accurate molecular sieving. Nature 2022, 609, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-L.; Zhang, C.-X.; Tang, M.-J.; Sun, S.-P.; Xing, W.; Lee, Y.M. Evolution of functional nanochannel membranes. Prog. Mater. Sci. 2023, 139, 101162. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, X.; Zhang, J.; Zhang, F.; Fang, W.; Jin, J. Polymer membranes for organic solvent nanofiltration: Recent progress, challenges and perspectives. Adv. Membr. 2023, 3, 100063. [Google Scholar] [CrossRef]
- Cheng, Y.; Datta, S.J.; Zhou, S.; Jia, J.; Shekhah, O.; Eddaoudi, M. Advances in metal–organic framework-based membranes. Chem. Soc. Rev. 2022, 51, 8300–8350. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Xu, M.; Wang, B.; Zhang, Z.; Wen, G.; Zheng, Y.; Luo, D.; Zhao, L.; Yu, A.; Zhang, L.; et al. Microporous framework membranes for precise molecule/ion separations. Chem. Soc. Rev. 2021, 50, 986–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Q.; Cheng, J.; He, D.; Zhang, L.; Lu, P.; Jin, H.; Choi, J.; Li, Y. Crystallization-controlled defect minimization of a ZIF-67 membrane for the robust separation of propylene and propane. J. Membr. Sci. 2022, 664, 121072. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, Q.; Tong, T.; Sun, K.; Lin, L.-C.; Tang, C.Y.; Yang, F.; Guiver, M.D.; Quan, X.; Dong, Y. Robust ultrathin nanoporous MOF membrane with intra-crystalline defects for fast water transport. Nat. Commun. 2022, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, H.; Yao, J. Synthesis of 2D nanoporous zeolitic imidazolate framework nanosheets for diverse applications. Coord. Chem. Rev. 2021, 431, 213677. [Google Scholar] [CrossRef]
- Uemura, T.; Uchida, N.; Asano, A.; Saeki, A.; Seki, S.; Tsujimoto, M.; Isoda, S.; Kitagawa, S. Highly Photoconducting π-Stacked Polymer Accommodated in Coordination Nanochannels. J. Am. Chem. Soc. 2012, 134, 8360–8363. [Google Scholar] [CrossRef]
- Yang, S.L.; Karve, V.V.; Justin, A.; Kochetygov, I.; Espín, J.; Asgari, M.; Trukhina, O.; Sun, D.T.; Peng, L.; Queen, W.L. Enhancing MOF performance through the introduction of polymer guests. Coord. Chem. Rev. 2021, 427, 213525. [Google Scholar] [CrossRef]
- Peng, L.; Yang, S.L.; Sun, D.T.; Asgari, M.; Queen, W.L. MOF/polymer composite synthesized using a double solvent method offers enhanced water and CO adsorption properties. Chem. Commun. 2018, 54, 10602–10605. [Google Scholar] [CrossRef]
- Huang, G.; Yang, Q.; Xu, Q.; Yu, S.H.; Jiang, H.L. Polydimethylsiloxane Coating for a Palladium/MOF Composite: Highly Improved Catalytic Performance by Surface Hydrophobization. Angew. Chem. Int. Ed. 2016, 55, 7379–7383. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Sun, K.; Liu, X.; Yang, W.; Li, L.; Jiang, H.L. Electronic State and Microenvironment Modulation of Metal Nanoparticles Stabilized by MOFs for Boosting Electrocatalytic Nitrogen Reduction. Adv. Mater. 2023, 35, 202210669. [Google Scholar] [CrossRef] [PubMed]
- Koutsianos, A.; Pallach, R.; Frentzel-Beyme, L.; Das, C.; Paulus, M.; Sternemann, C.; Henke, S. Breathing porous liquids based on responsive metal-organic framework particles. Nat. Commun. 2023, 14, 4200. [Google Scholar] [CrossRef]
- He, S.; Chen, L.; Cui, J.; Yuan, B.; Wang, H.; Wang, F.; Yu, Y.; Lee, Y.; Li, T. General Way To Construct Micro- and Mesoporous Metal–Organic Framework-Based Porous Liquids. J. Am. Chem. Soc. 2019, 141, 19708–19714. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.; Sun, K.; Jiao, L.; Xie, C.; Ding, C.; Jiang, H.L. Interfacial Microenvironment Modulation Boosting Electron Transfer between Metal Nanoparticles and MOFs for Enhanced Photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 16372–16376. [Google Scholar] [CrossRef]
- Zuo, Q.; Liu, T.; Chen, C.; Ji, Y.; Gong, X.; Mai, Y.; Zhou, Y. Ultrathin Metal–Organic Framework Nanosheets with Ultrahigh Loading of Single Pt Atoms for Efficient Visible-Light-Driven Photocatalytic H2 Evolution. Angew. Chem. Int. Ed. 2019, 58, 10198–10203. [Google Scholar] [CrossRef] [PubMed]
- Zimpel, A.; Al Danaf, N.; Steinborn, B.; Kuhn, J.; Hohn, M.; Bauer, T.; Hirschle, P.; Schrimpf, W.; Engelke, H.; Wagner, E.; et al. Coordinative Binding of Polymers to Metal-Organic Framework Nanoparticles for Control of Interactions at the Biointerface. ACS Nano 2019, 13, 3884–3895. [Google Scholar] [CrossRef]
- Lyu, F.J.; Zhang, Y.F.; Zare, R.N.; Ge, J.; Liu, Z. One-Pot Synthesis of Protein-Embedded Metal-Organic Frameworks with Enhanced Biological Activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Albayati, N.; Kadhom, M. Preparation of functionalised UiO-66 metal–organic frameworks (MOFs) nanoparticles using deep eutectic solvents as a benign medium. Micro Nano Lett. 2020, 15, 1075–1078. [Google Scholar] [CrossRef]
- Wang, C.Z.; Tadepalli, S.; Luan, J.Y.; Liu, K.K.; Morrissey, J.J.; Kharasch, E.D.; Naik, R.R.; Singamaneni, S. Metal-Organic Framework as a Protective Coating for Biodiagnostic Chips. Adv. Mater. 2017, 29, 1604433. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cheng, F.F.; Xiong, W.W.; Zhang, Q.C. New synthetic strategies to prepare metal-organic frameworks. Inorg. Chem. Front. 2018, 5, 2693–2708. [Google Scholar] [CrossRef]
- Gao, J.K.; He, M.; Lee, Z.Y.; Cao, W.F.; Xiong, W.W.; Li, Y.X.; Ganguly, R.; Wu, T.; Zhang, Q.C. A surfactant-thermal method to prepare four new three-dimensional heterometal-organic frameworks. Dalton Trans. 2013, 42, 11367–11370. [Google Scholar] [CrossRef]
- Westendorff, K.S.; Paolucci, C.; Giri, G. Polymer-induced polymorphism in a Zn-based metal organic framework. Chem. Commun. 2021, 57, 887–890. [Google Scholar] [CrossRef]
- Yao, J.; He, M.; Wang, K.; Chen, R.; Zhong, Z.; Wang, H. High-yield synthesis of zeolitic imidazolate frameworks from stoichiometric metal and ligand precursor aqueous solutions at room temperature. CrystEngComm 2013, 15, 3601–3606. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.; Sun, Y.; Xing, Y.; Li, C. Heavy metal elimination based on metal organic framework highly loaded on flexible nanofibers. Environ. Res. 2020, 188, 109742. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liang, J.; Mohammad, M.I.B.; Lv, D.; Cao, Y.; Qi, J.; Liang, K.; Ma, J. Biocatalytic metal–organic framework membrane towards efficient aquatic micropollutants removal. Chem. Eng. J. 2021, 426, 131861. [Google Scholar] [CrossRef]
- Uemura, T.; Hoshino, Y.; Kitagawa, S.; Yoshida, K.; Isoda, S. Effect of organic polymer additive on crystallization of porous coordination polymer. Chem. Mater. 2006, 18, 992–995. [Google Scholar] [CrossRef]
- Jiang, D.; Mallat, T.; Krumeich, F.; Baiker, A. Polymer-assisted synthesis of nanocrystalline copper-based metal–organic framework for amine oxidation. Catal. Commun. 2011, 12, 602–605. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Wang, Y.; Wu, J.; Kirlikovali, K.O.; Li, P.; Zhou, Y.; Farha, O.K. A General Strategy for the Synthesis of Hierarchically Ordered Metal–Organic Frameworks with Tunable Macro-, Meso-, and Micro-Pores. Small 2022, 19, 202206116. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, C.; Xiao, L.; Meng, Q.; Zhang, G. Metal-organic frameworks-based mixed matrix pervaporation membranes for recovery of organics. Adv. Membr. 2024, 4, 100092. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Li, T.; Ren, Y.; Wang, H.; Song, Z.; Li, J.; Zhao, Q.; Li, J.; Li, L. Balancing the Crystallinity and Film Formation of Metal-Organic Framework Membranes through In Situ Modulation for Efficient Gas Separation. Angew. Chem. Int. Ed. Engl. 2023, 62, e202309095. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Liang, F.Y.; Bux, H.; Feldhoff, A.; Yang, W.S.; Caro, J. Molecular sieve membrane: Supported metal-organic framework with high hydrogen selectivity. Angew. Chem. Int. Ed. Engl. 2010, 49, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; Dou, W.; Caro, J. Steam-Stable Zeolitic Imidazolate Framework ZIF-90 Membrane with Hydrogen Selectivity through Covalent Functionalization. J. Am. Chem. Soc. 2010, 132, 15562–15564. [Google Scholar] [CrossRef]
- Wu, M.; Sun, Y.; Ji, T.; Yu, K.; Liu, L.; He, Y.; Yan, J.; Meng, S.; Hu, W.; Fan, X.; et al. Fabrication of water-stable MOF-808 membrane for efficient salt/dye separation. J. Membr. Sci. 2023, 686, 122023. [Google Scholar] [CrossRef]
- Xu, R.; Kang, Y.; Zhang, W.; Zhang, X.; Pan, B. Oriented UiO-67 Metal–Organic Framework Membrane with Fast and Selective Lithium-Ion Transport. Angew. Chem. Int. Ed. 2021, 61, 202115443. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wang, Y.; Liu, Y.T.; Wu, H.; Zhao, M.G.; Ren, Y.X.; Pu, Y.C.A.; Li, W.P.; Wang, S.Y.; Song, S.Q.; et al. Ultrathin ZIF-8 Membrane through Inhibited Ostwald Ripening for High-Flux CH/CH Separation. Adv. Funct. Mater. 2022, 32, 202208064. [Google Scholar] [CrossRef]
- Guo, Y.; Ying, Y.; Mao, Y.; Peng, X.; Chen, B. Polystyrene Sulfonate Threaded through a Metal–Organic Framework Membrane for Fast and Selective Lithium-Ion Separation. Angew. Chem. Int. Ed. 2016, 55, 15120–15124. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.-F.; Yao, J.; Wang, H. Contra-diffusion synthesis of metal-organic framework separation membranes: A review. Sep. Purif. Technol. 2022, 300, 121837. [Google Scholar] [CrossRef]
- Yao, J.F.; Dong, D.H.; Li, D.; He, L.; Xu, G.S.; Wang, H.T. Contra-diffusion synthesis of ZIF-8 films on a polymer substrate. Chem. Commun. 2011, 47, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Wu, H.; Zhang, R.; Su, Y.; Cao, L.; Yu, Q.; Yuan, J.; Xiao, K.; He, M.; Jiang, Z. Metal-coordinated sub-10 nm membranes for water purification. Nat. Commun. 2019, 10, 4160. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; You, X.; Wu, H.; Li, R.; Xiao, K.; Ren, Y.; Wang, H.; Song, S.; Wang, Y.; Pu, Y.; et al. A facile metal ion pre-anchored strategy for fabrication of defect-free MOF membranes on polymeric substrates. J. Membr. Sci. 2022, 650, 120419. [Google Scholar] [CrossRef]
- Yu, C.; Cen, X.; Zhang, Z.; Sun, Y.; Xue, W.; Qiao, Z.; Guiver, M.D.; Zhong, C. Step-Nucleation In Situ Self-Repair to Prepare Rollable Large-Area Ultrathin MOF Membranes. Adv. Mater. 2023, 35, 202307013. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Jia, Y.; Fang, K.; Qin, Y.; Deng, N.; Liang, Y. Preparation hierarchical porous MOF membranes with island-like structure for efficient gas separation. J. Membr. Sci. 2022, 663, 121036. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Chen, C.; Chen, Y.; Li, W.; Wang, J. Secondary-assembled defect-free MOF membrane via triple-needle electrostatic atomization for highly stable and selective organics permeation. J. Membr. Sci. 2022, 648, 120382. [Google Scholar] [CrossRef]
- Xu, L.H.; Li, S.H.; Mao, H.; Li, Y.; Zhang, A.S.; Wang, S.; Liu, W.M.; Lv, J.; Wang, T.; Cai, W.W.; et al. Highly flexible and superhydrophobic MOF nanosheet membrane for ultrafast alcohol-water separation. Science 2022, 378, abo5680. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Mahdi, E.M.; Peng, Y.; Qian, Y.; Zhang, B.; Yan, N.; Yuan, D.; Tan, J.-C.; Zhao, D. Kinetically controlled synthesis of two-dimensional Zr/Hf metal–organic framework nanosheets via a modulated hydrothermal approach. J. Mater. Chem. A 2017, 5, 8954–8963. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, G.; Qiao, Z.; Li, N.; Buenconsejo, P.J.S.; Xi, S.; Karmakar, A.; Li, M.; Cai, H.; Pennycook, S.J.; et al. Solution-Processable Metal–Organic Framework Nanosheets with Variable Functionalities. Adv. Mater. 2021, 33, 202101257. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Li, K.R.; Shi, D.C.; Yang, H.; Yu, X.; Fan, W.D.; Buenconsejo, P.J.S.; Zhao, D. Large-Area Fabrication of Ultrathin Metal-Organic Framework Membranes. Adv. Mater. 2023, 35, 202211859. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zeng, T.; Ma, D.; Zhang, P.; Zhao, S.; Yang, L.; Zou, X.; Zhu, G. Covalent-Linking-Enabled Superior Compatibility of ZIF-8 Hybrid Membrane for Efficient Propylene Separation. Adv. Mater. 2021, 34, 202104606. [Google Scholar] [CrossRef] [PubMed]
- Cseri, L.; Hardian, R.; Anan, S.; Vovusha, H.; Schwingenschlögl, U.; Budd, P.M.; Sada, K.; Kokado, K.; Szekely, G. Bridging the interfacial gap in mixed-matrix membranes by nature-inspired design: Precise molecular sieving with polymer-grafted metal–organic frameworks. J. Mater. Chem. A 2021, 9, 23793–23801. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Li, N.; Fan, Y. Sealing Tröger base/ZIF-8 mixed matrix membranes defects for improved gas separation performance. J. Membr. Sci. 2021, 636, 119582. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Wang, H.; Fan, Y.; Zhang, S.; He, S.; Wang, F.; Tao, Y.; Zhao, X.; Zhang, Y.-B.; et al. Enhancing the Gas Separation Selectivity of Mixed-Matrix Membranes Using a Dual-Interfacial Engineering Approach. J. Am. Chem. Soc. 2020, 142, 18503–18512. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, D.; Shan, M.; Feng, X.; Gascon, J.; Wang, Y.; Zhang, Y. Uniformly Distributed Mixed Matrix Membranes via a Solution Processable Strategy for Propylene/Propane Separation. Angew. Chem. Int. Ed. 2024, 63, 202316093. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ni, L.; Zhang, H.; Li, L.; Guo, X.; Qi, J.; Zhou, Y.; Zhu, Z.; Sun, X.; Li, J. Phenolic resin regulated interface of ZIF-8 based mixed matrix membrane for enhanced gas separation. J. Membr. Sci. 2023, 666, 121117. [Google Scholar] [CrossRef]
- Carja, I.-D.; Tavares, S.R.; Shekhah, O.; Ozcan, A.; Semino, R.; Kale, V.S.; Eddaoudi, M.; Maurin, G. Insights into the Enhancement of MOF/Polymer Adhesion in Mixed-Matrix Membranes via Polymer Functionalization. ACS Appl. Mater. Interfaces 2021, 13, 29041–29047. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, B.K.; Yoo, S.Y.; Lee, H.; Wu, W.-N.; Smith, Z.P.; Park, H.B. PolyMOF nanoparticles constructed from intrinsically microporous polymer ligand towards scalable composite membranes for CO2 separation. Nat. Commun. 2023, 14, 8330. [Google Scholar] [CrossRef]
- Seoane, B.; Sebastián, V.; Téllez, C.; Coronas, J. Crystallization in THF: The possibility of one-pot synthesis of mixed matrix membranes containing MOF MIL-68(Al). CrystEngComm 2013, 15, c3ce40847g. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.J.; Wang, Z.X.; Jin, C.G.; Yin, M.J.; An, Q.F. Rapid Fabrication of High-Permeability Mixed Matrix Membranes at Mild Condition for CO2 Capture. Small 2023, 19, 202208177. [Google Scholar] [CrossRef]
- Chen, G.; Chen, C.; Guo, Y.; Chu, Z.; Pan, Y.; Liu, G.; Liu, G.; Han, Y.; Jin, W.; Xu, N. Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation. Science 2023, 381, 1350–1356. [Google Scholar] [CrossRef]
- He, S.; Zhu, B.; Jiang, X.; Han, G.; Li, S.; Lau, C.H.; Wu, Y.; Zhang, Y.; Shao, L. Symbiosis-inspired de novo synthesis of ultrahigh MOF growth mixed matrix membranes for sustainable carbon capture. Proc. Natl. Acad. Sci. USA 2022, 119, 2114964119. [Google Scholar] [CrossRef]
- Morgan, P.W.; Kwolek, S.L. Interfacial polycondensation. II. Fundamentals of polymer formation at liquid interfaces. J. Polym. Sci. 2003, 40, 299–327. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Synthesis of high-performance thin film composite (TFC) membranes by controlling the preparation conditions: Technical notes. J. Water Process Eng. 2019, 30, 100542. [Google Scholar] [CrossRef]
- Shu, L.; Peng, Y.; Song, H.; Zhu, C.; Yang, W. Modular Customization and Regulation of Metal-Organic Frameworks for Efficient Membrane Separations. Angew. Chem. Int. Ed. Engl. 2023, 62, e202315057. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.W.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Zhao, Z.; Shehzad, M.A.; Wu, B.; Wang, X.; Yasmin, A.; Zhu, Y.; Wang, X.; He, Y.; Ge, L.; Li, X.; et al. Spray-deposited thin-film composite MOFs membranes for dyes removal. J. Membr. Sci. 2021, 635, 119475. [Google Scholar] [CrossRef]
- Hussain, S.; Bahadar, S.; Wang, G.; Zhu, L.; Ye, Z.; Peng, X. Photothermal-driven interfacial-polymerized ultrathin polyamide selective layer for nanofiltration. Chem. Eng. J. 2022, 440, 136012. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, Y.; Wang, X.; Fan, K.; Li, P.; Xia, S. Regulating interfacial polymerization via constructed 2D metal-organic framework interlayers for fabricating nanofiltration membranes with enhanced performance. Desalination 2022, 544, 116134. [Google Scholar] [CrossRef]
- Jin, X.-G.; Liang, X.-K.; Liu, J.-H.; Mo, J.-W.; Ren, T.-X.; Ma, X.-H.; Xu, Z.-L. Development of high permeability nanofiltration membranes through porous 2D MOF nanosheets. Chem. Eng. J. 2023, 471, 144566. [Google Scholar] [CrossRef]
- Wen, Y.; Dai, R.B.; Li, X.S.; Zhang, X.R.; Cao, X.Z.; Wu, Z.C.; Lin, S.H.; Tang, C.Y.; Wang, Z.W. Metal-organic framework enables ultraselective polyamide membrane for desalination and water reuse. Sci. Adv. 2022, 8, abm4149. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, X.; Kim, J.; Tong, T.; Huang, C.-H.; Chen, Y. Capillary-Assisted Fabrication of Thin-Film Nanocomposite Membranes for Improved Solute–Solute Separation. Environ. Sci. Technol. 2022, 56, 5849–5859. [Google Scholar] [CrossRef]
- Gong, Y.; Gao, S.; Tian, Y.; Zhu, Y.; Fang, W.; Wang, Z.; Jin, J. Thin-film nanocomposite nanofiltration membrane with an ultrathin polyamide/UIO-66-NH2 active layer for high-performance desalination. J. Membr. Sci. 2020, 600, 117874. [Google Scholar] [CrossRef]
- Fang, S.-Y.; Gong, J.-L.; Tang, L.; Li, J.; Qin, M.; Zhou, H.-Y.; Tang, L.-X.; Zhao, J. Thin-Film Nanocomposite Membranes with Nature-Inspired MOFs Incorporated for Removing Fluoroquinolone Antibiotics. ACS Appl. Mater. Interfaces 2023, 15, 25633–25649. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jiang, X.; Urban, J.J.; Lau, C.H.; Liu, S.; Shao, L. Robust natural nanocomposites realizing unprecedented ultrafast precise molecular separations. Mater. Today 2020, 36, 40–47. [Google Scholar] [CrossRef]
- Jiao, C.; Song, X.; Zhang, X.; Sun, L.; Jiang, H. MOF-Mediated Interfacial Polymerization to Fabricate Polyamide Membranes with a Homogeneous Nanoscale Striped Turing Structure for CO2/CH4 Separation. ACS Appl. Mater. Interfaces 2021, 13, 18380–18388. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.F.; Peng, X.S.; Zhang, L.; Gao, C.J. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef]
- Tang, M.J.; Liu, M.L.; Wang, D.A.; Shao, D.D.; Wang, H.J.; Cui, Z.L.; Cao, X.L.; Sun, S.P. Precisely Patterned Nanostrand Surface of Cucurbituril [6]-Based Nanofiltration Membranes for Effective Alcohol-Water Condensation. Nano Lett. 2020, 20, 2717–2723. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kang, D.Y.; Copeland, J.R.; Brunelli, N.A.; Didas, S.A.; Bollini, P.; Sievers, C.; Kamegawa, T.; Yamashita, H.; Jones, C.W. Dramatic Enhancement of CO Uptake by Poly(ethyleneimine) Using Zirconosilicate Supports. J. Am. Chem. Soc. 2012, 134, 10757–10760. [Google Scholar] [CrossRef]
- Han, G.; Studer, R.M.; Lee, M.; Rodriguez, K.M.; Teesdale, J.J.; Smith, Z.P. Post-synthetic modification of MOFs to enhance interfacial compatibility and selectivity of thin-film nanocomposite (TFN) membranes for water purification. J. Membr. Sci. 2023, 666, 121133. [Google Scholar] [CrossRef]
- Xu, T.; Sheng, F.; Wu, B.; Shehzad, M.A.; Yasmin, A.; Wang, X.; He, Y.; Ge, L.; Zheng, X.; Xu, T. Ti-exchanged UiO-66-NH2–containing polyamide membranes with remarkable cation permselectivity. J. Membr. Sci. 2020, 615, 118608. [Google Scholar] [CrossRef]
- Zhang, B.; Dai, X.; Wei, N.; Cui, X.; Fan, F.; Zhang, J.; Zhang, D.; Meng, F.; Qi, W.; Fu, Y. Fabrication of Oriented MOF-Based Mixed Matrix Membrane via Ion-Induced Synchronous Synthesis. Small 2023, 20, 202305688. [Google Scholar] [CrossRef]
- Ji, Y.-L.; Gu, B.-X.; Huo, H.-Q.; Xie, S.-J.; Peng, H.; Zhang, W.-H.; Yin, M.-J.; Xiong, B.; Lu, H.; Villalobos, L.F.; et al. Roll-to-roll fabrication of large-area metal–organic framework-based membranes for high-performance aqueous separations. Nat. Water 2024, 2, 183–192. [Google Scholar] [CrossRef]
- Ji, Y.L.; Gu, B.X.; Xie, S.J.; Yin, M.J.; Qian, W.J.; Zhao, Q.; Hung, W.S.; Lee, K.R.; Zhou, Y.; An, Q.F.; et al. Superfast Water Transport Zwitterionic Polymeric Nanofluidic Membrane Reinforced by Metal–Organic Frameworks. Adv. Mater. 2021, 33, 202102292. [Google Scholar] [CrossRef]
- Jia, M.-M.; Feng, J.-H.; Shao, W.; Chen, Z.; Yu, J.-R.; Sun, J.-J.; Wu, Q.-Y.; Li, Y.; Xue, M.; Chen, X.-M. In-situ interfacial synthesis of metal-organic framework/polyamide thin-film nanocomposite membranes with elevated nanofiltration performances. J. Membr. Sci. 2024, 694, 122418. [Google Scholar] [CrossRef]
- Meng, Q.-W.; Cheng, L.; Ge, Q. Recent advances and future challenges of polyamide-based chlorine-resistant membrane. Adv. Membr. 2023, 3, 100075. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lau, S.K.; Yong, W.F. Recent advances of thin film composite nanofiltration membranes for Mg2+/Li+ separation. Adv. Membr. 2024, 4, 100093. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Wang, J.; Wang, Z.; Kapteijn, F.; Liu, X. Highly Water-Permeable Metal–Organic Framework MOF-303 Membranes for Desalination. J. Am. Chem. Soc. 2021, 143, 20055–20058. [Google Scholar] [CrossRef]
- Jian, M.P.; Qiu, R.S.; Xia, Y.; Lu, J.; Chen, Y.; Gu, Q.F.; Liu, R.P.; Hu, C.Z.; Qu, J.H.; Wang, H.T.; et al. Ultrathin water-stable metal-organic framework membranes for ion separation. Sci. Adv. 2020, 6, aay3998. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, L.; Wang, Y.; Li, R.; Gu, X.; Yuan, Y.D.; Qian, Y.; Hu, Z.; Zhao, D. Improving Water-Treatment Performance of Zirconium Metal-Organic Framework Membranes by Postsynthetic Defect Healing. ACS Appl. Mater. Interfaces 2017, 9, 37848–37855. [Google Scholar] [CrossRef]
- Zhang, C.; Mu, Y.; Zhang, W.; Zhao, S.; Wang, Y. PVC-based hybrid membranes containing metal-organic frameworks for Li+/Mg2+ separation. J. Membr. Sci. 2020, 596, 117724. [Google Scholar] [CrossRef]
- Liu, X.L.; Demir, N.K.; Wu, Z.T.; Li, K. Highly Water-Stable Zirconium Metal Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef]
- Shi, D.; Li, H.; Yu, X.; Zhang, Z.; Yuan, Y.D.; Fan, W.; Yuan, H.; Ying, Y.; Yang, H.; Shang, C.; et al. Intercrystalline Channels at Subnanometer Scale for Precise Molecular Nanofiltration. J. Am. Chem. Soc. 2023, 145, 15848–15858. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Hua, D.; Zhao, F.; Zheng, D.; Pan, J.; Hong, Y.; Liu, Y.; Rao, X.; Zhou, S.; Zhan, G. Integration of P84 and porphyrin–based 2D MOFs (M−TCPP, M = Zn, Cu, Co, Ni) for mixed matrix membranes towards enhanced performance in organic solvent nanofiltration. Sep. Purif. Technol. 2022, 282, 120022. [Google Scholar] [CrossRef]
- Ma, D.; Han, G.; Gao, Z.F.; Chen, S.B. Continuous UiO-66-Type Metal–Organic Framework Thin Film on Polymeric Support for Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 45290–45300. [Google Scholar] [CrossRef]

| Membrane | Type | Permeance (L m−2 h−1 bar−1) | Rejection or Selectivity | Ref |
|---|---|---|---|---|
| MOF-808 | PMOF | 4.37 | 287.3 (Salt/dye) | [36] |
| UiO-67 | PMOF | / | 159.4 (Li+/Mg2+) | [37] |
| HKUST-1 | PMOF | / | 10,296 (Li+/Mg2+) | [39] |
| NUS-8 | PMOF | 3 | 98%, MgCl2 | [49] |
| MOF-303 | PMOF | 3.0 | 96.0%, Na2SO4 | [88] |
| UiO-66 | PMOF | 0.14 | 98%, MgCl2 | [92] |
| UiO-66 | PMOF | 0.285 | 45%, NaCl | [90] |
| UiO-66-SO3H | MMM | / | 4 (Li+/Mg2+) | [91] |
| MIL-101-NH2 | TFN | 20.0 | 99.0% (Methyl blue, MW = 799.80) | [67] |
| Cu-TCPP | TFN | 32.7 | 271.7 (Cl−/SO42−) | [69] |
| Al-MOF | TFN | 42.4 | 97.0%, Na2SO4 | [70] |
| Cu-BDC | TFN | 4.0 | >90%, (Boron and NDMA) | [71] |
| UiO-66-NH2 | TFN | 46.0 | 97.1%, Na2SO4 | [73] |
| UiO-66-NH2 | TFN | 31.5 | 99.9%, Na2SO4 | [75] |
| Cu-BDC | TFN | / | 1221.95 (Li+/Mg2+) | [82] |
| ZIF-8 | TFN | 130.0 | 97% (>1 nm molecule) | [83] |
| ZIF-8 | TFN | 20.6 | 96.5%, Na2SO4 | [85] |
| Membrane | Type | Permeance (L m−2 h−1 bar−1) | Rejection or Selectivity | Ref |
|---|---|---|---|---|
| Al-MOF | PMOF | EtOH, 0.8-22 | 99% (300–650 Da molecule) | [93] |
| Cu-TCPP | MMM | EtOH, 2.82 | 95.7% (Brilliant Blue R, Mw = 825.97) | [94] |
| UiO-66−NH2 | MMM | EtOH, 0.88 | 96.33% (Rose Bengal, Mw = 1017.64) | [95] |
| Fe-TCPP | TFN | MeOH, 120 | 97.0% (Acid Fuchsin, Mw = 585.54) | [68] |
| UiO-66−NH2 | TFN | EtOH, 30.2 | 98%, (Rose Bengal, Mw = 1017.64) | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Tang, Y.; Ding, Y.-M.; Jiang, H.-Y.; Zhang, Z.-B.; Li, W.-X.; Liu, M.-L.; Sun, S.-P. Synergistic Construction of Sub-Nanometer Channel Membranes through MOF–Polymer Composites: Strategies and Nanofiltration Applications. Polymers 2024, 16, 1653. https://doi.org/10.3390/polym16121653
Chen Q, Tang Y, Ding Y-M, Jiang H-Y, Zhang Z-B, Li W-X, Liu M-L, Sun S-P. Synergistic Construction of Sub-Nanometer Channel Membranes through MOF–Polymer Composites: Strategies and Nanofiltration Applications. Polymers. 2024; 16(12):1653. https://doi.org/10.3390/polym16121653
Chicago/Turabian StyleChen, Qian, Ying Tang, Yang-Min Ding, Hong-Ya Jiang, Zi-Bo Zhang, Wei-Xing Li, Mei-Ling Liu, and Shi-Peng Sun. 2024. "Synergistic Construction of Sub-Nanometer Channel Membranes through MOF–Polymer Composites: Strategies and Nanofiltration Applications" Polymers 16, no. 12: 1653. https://doi.org/10.3390/polym16121653






