Enhancing Polymer Sustainability: Eco-Conscious Strategies
Abstract
:1. Introduction
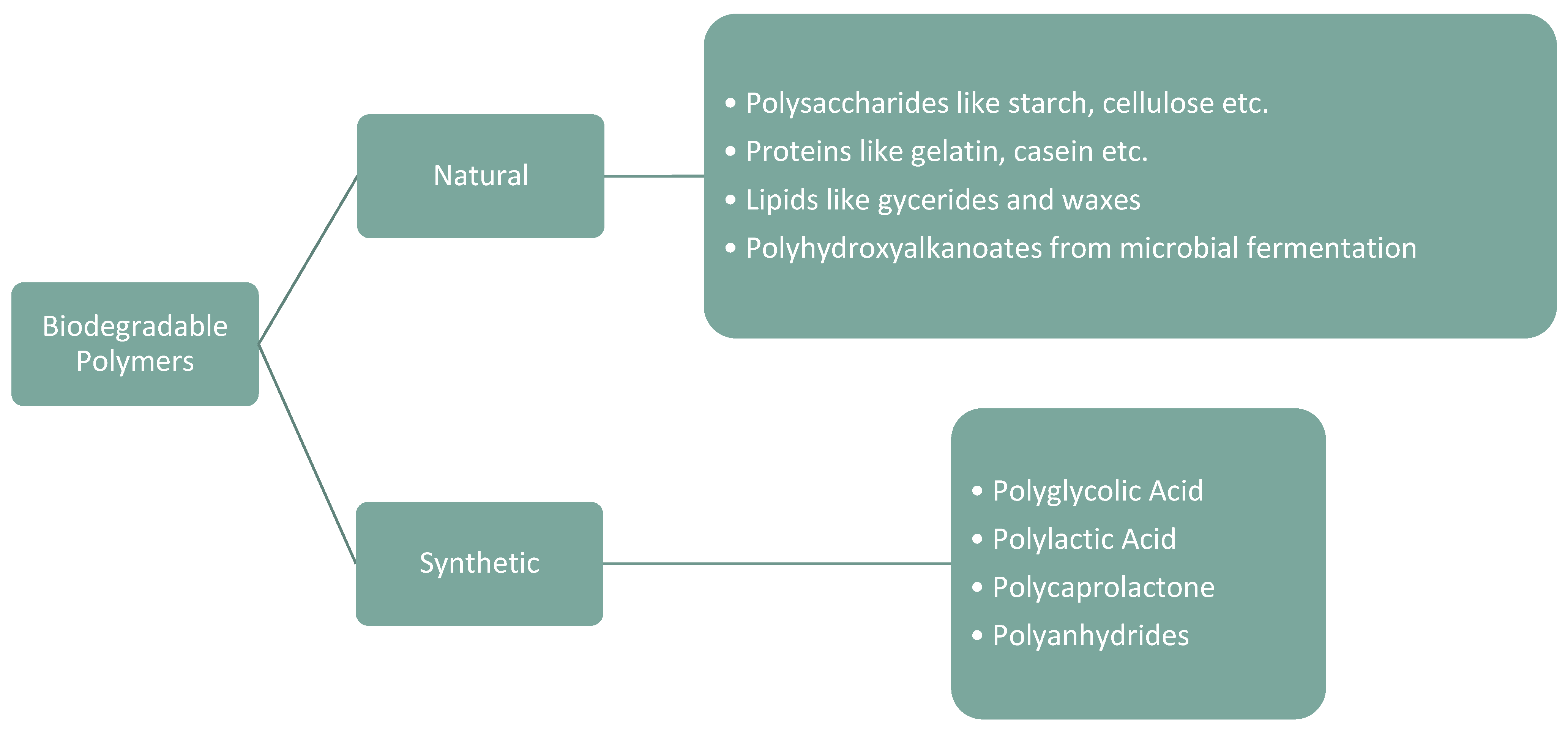
2. Biodegradable Polymers
2.1. Natural Biodegradable Polymers
2.2. Various Synthesis Methods
2.2.1. Chemical Modification
2.2.2. Enzymatic Reactions
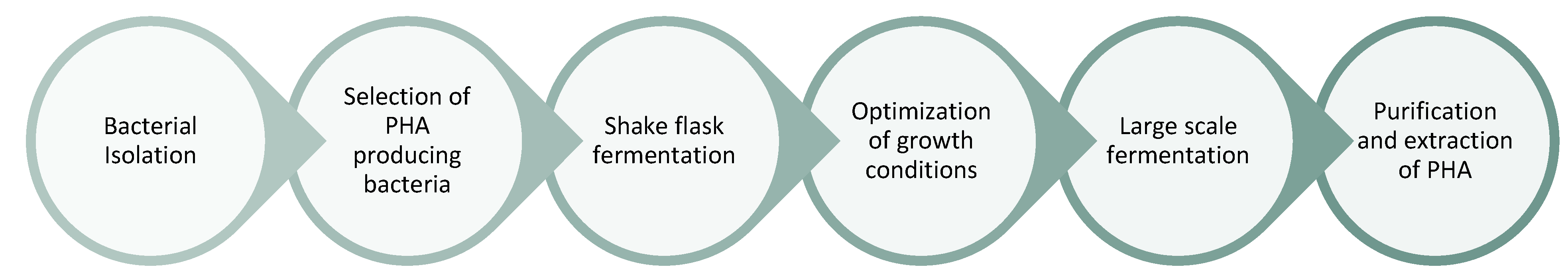
2.2.3. Microbial Fermentation Processes
3. Synthetic Biodegradable Polymers
3.1. Synthesis Methods
3.1.1. Ring-Opening Polymerization (ROP) [76,77]
3.1.2. Polycondensation [78,79]
3.1.3. Bacterial Fermentation [80,81]
3.1.4. Reactive Extrusion [82,83]
4. Green Polymer Synthesis Methods
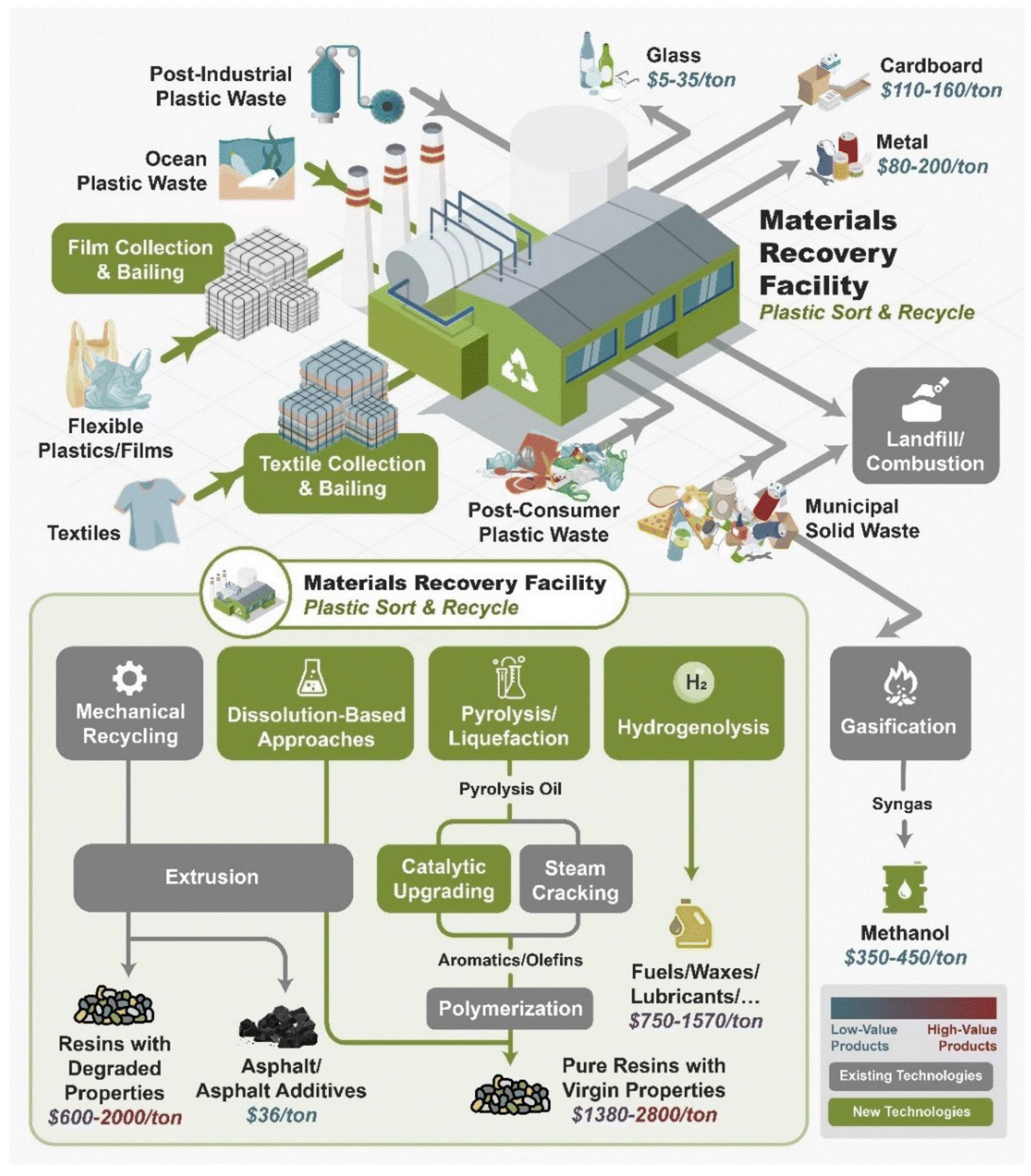
5. Sustainable Polymer Recycling Technologies
5.1. Mechanical Recycling
5.2. Chemical Recycling
5.3. Enzymatic Recycling
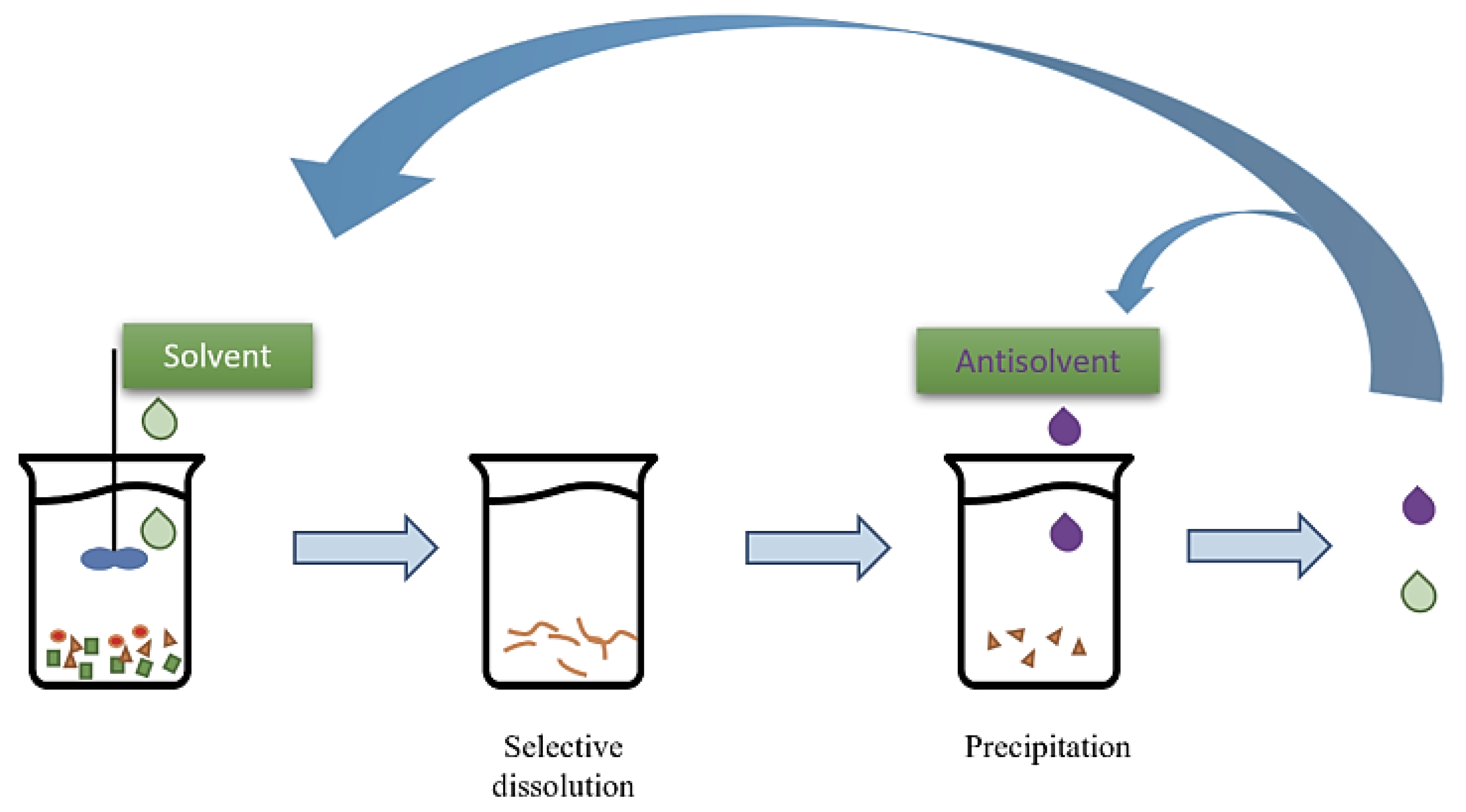
5.4. Dissolution-Based Recycling
5.5. Hybrid Recycling
6. Challenges and Future Prospects of Eco-Friendly Polymer Sustainability
7. Conclusions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Cowger, W.; Willis, K.A.; Bullock, S.; Conlon, K.; Emmanuel, J.; Erdle, L.M.; Eriksen, M.; Farrelly, T.A.; Hardesty, B.D.; Kerge, K. Global producer responsibility for plastic pollution. Sci. Adv. 2024, 10, eadj8275. [Google Scholar] [CrossRef]
- Filho, W.L.; Salvia, A.L.; Bonoli, A.; Saari, U.A.; Voronova, V.; Klõga, M.; Kumbhar, S.S.; Olszewski, K.; De Quevedo, D.M.; Barbir, J. An assessment of attitudes towards plastics and bioplastics in Europe. Sci. Total Environ. 2021, 755, 142732. [Google Scholar] [CrossRef] [PubMed]
- Europe, P. Circular Economy for Plastics—A European Overview. 2023. Available online: https://plasticseurope.org/de/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 1 June 2024).
- Real, L.E.P. Real, Plastics Statistics: Production, Recycling, and Market Data BT—Recycled Materials for Construction Applications: Plastic Products and Composites; Real, L.E.P., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 103–113. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Shenoy, S.R.; Wagdarikar, M.J.; Desai, N.D. Polymers in medical devices and pharmaceutical packaging. In Polymers for Pharmaceutical and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 333–382. [Google Scholar]
- Benson, R.; He, W. Polymeric Biomaterials. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2024; pp. 167–187. [Google Scholar]
- Lee, S.; Huang, M.; Lee, J.; Choi, H.; Jo, I.; Na, H.; Lee, Y.; Youk, J.H.; Yoon, M.; Shim, B.S. Eco-Friendly Materials for a Zero E-Waste Society: Challenges and Opportunities in Engineering Plastics. Adv. Sustain. Syst. 2024, 2300428. [Google Scholar] [CrossRef]
- Maraveas, C. Environmental sustainability of plastic in agriculture. Agriculture 2020, 10, 310. [Google Scholar] [CrossRef]
- Hofmann, T.; Ghoshal, S.; Tufenkji, N.; Adamowski, J.F.; Bayen, S.; Chen, Q.; Demokritou, P.; Flury, M.; Hüffer, T.; Ivleva, N.P. Plastics can be used more sustainably in agriculture. Commun. Earth Environ. 2023, 4, 332. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed, T.N.; Wang, B.; Zhou, R. Plastic pollution in agriculture as a threat to food security, the ecosystem, and the environment: An overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Cressey, D. The plastic ocean. Nature 2016, 536, 263–265. [Google Scholar] [CrossRef]
- Wayman, C.; Niemann, H. The fate of plastic in the ocean environment–a minireview. Environ. Sci. Process. Impacts. 2021, 23, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Astrup, T.; Fruergaard, T.; Christensen, T.H. Recycling of plastic: Accounting of greenhouse gases and global warming contributions. Waste Manag. Res. 2009, 27, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Benavides, P.T.; Kneifel, J.D.; Beers, K.L.; Hawkins, T.R. Cross-database comparisons on the greenhouse gas emissions, water consumption, and fossil-fuel use of plastic resin production and their post-use phase impacts. Resour. Conserv. Recycl. 2023, 198, 107168. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; Van Franeker, J.A.; Thompson, R.C.; Amaral-Zettler, L.A. The ecological impacts of marine debris: Unraveling the demonstrated evidence from what is perceived. Ecology 2016, 97, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Gao, D.; Lv, J.; Lee, P.S. Natural polymer in soft electronics: Opportunities, challenges, and future prospects. Adv. Mater. 2022, 34, 2105020. [Google Scholar] [CrossRef] [PubMed]
- Pendhari, S.S.; Kant, T.; Desai, Y.M. Application of polymer composites in civil construction: A general review. Compos. Struct. 2008, 84, 114–124. [Google Scholar] [CrossRef]
- Koniuszewska, A.G.; Kaczmar, J.W. Application of polymer based composite materials in transportation. Prog. Rubber Plast. Recycl. Technol. 2016, 32, 1–24. [Google Scholar] [CrossRef]
- Millican, J.M.; Agarwal, S. Plastic pollution: A material problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Singh, S. Plastic waste: Environmental hazards, its biodegradation, and challenges. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Springer: Singapore, 2020; pp. 99–133. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic waste: Challenges and opportunities to mitigate pollution and effective management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef]
- Skoczinski, P.; Carus, M.; Tweddle, G.; Ruiz, P.; Zhang, H.; Guzman, N.D.; De, A.; Ravenstijn, J.; Käb, H.; Raschka, A. Bio-based Building Blocks and Polymers—Global Capacities, Production and Trends. Ind. Biotechnol. 2023, 19, 185–194. [Google Scholar] [CrossRef]
- Das, O.; Babu, K.; Shanmugam, V.; Sykam, K.; Tebyetekerwa, M.; Neisiany, R.E.; Försth, M.; Sas, G.; Gonzalez-Libreros, J.; Capezza, A.J. Natural and industrial wastes for sustainable and renewable polymer composites, Renew. Sustain. Energy Rev. 2022, 158, 112054. [Google Scholar] [CrossRef]
- Samanta, S.; Kim, S.; Saito, T.; Sokolov, A.P. Polymers with dynamic bonds: Adaptive functional materials for a sustainable future. J. Phys. Chem. B 2021, 125, 9389–9401. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Addressing the Sustainability Conundrums and Challenges within the Polymer Value Chain. Sustainability 2023, 15, 15758. [Google Scholar] [CrossRef]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent developments in smart food packaging focused on biobased and biodegradable polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Helanto, K.; Matikainen, L.; Talja, R.; Rojas, O.J. Bio-based polymers for sustainable packaging and biobarriers: A critical review. BioResources 2019, 14, 4902–4951. [Google Scholar] [CrossRef]
- Ramesh, M.; Rajeshkumar, L.; Sasikala, G.; Balaji, D.; Saravanakumar, A.; Bhuvaneswari, V.; Bhoopathi, R. A critical review on wood-based polymer composites: Processing, properties, and prospects. Polymers 2022, 14, 589. [Google Scholar] [CrossRef] [PubMed]
- Klöpffer, W. The critical review of life cycle assessment studies according to ISO 14040 and 14044: Origin, purpose and practical performance. Int. J. Life Cycle Assess. 2012, 17, 1087–1093. [Google Scholar] [CrossRef]
- Greene, J. Biodegradation of compostable plastics in green yard-waste compost environment. J. Polym. Environ. 2007, 15, 269–273. [Google Scholar] [CrossRef]
- Wojtowicz, A. Biodegradability and compostability of biopolymers. In Thermoplast. Starch A Green Material for Various Industries; Wiley Online Library: Hoboken, NJ, USA, 2009; pp. 55–76. [Google Scholar] [CrossRef]
- Bailey, C. USDA programs to support development and use of biobased industrial products. In Testing and Use of Environmentally Acceptable Lubricants; ASTM Internationa: West Conshehoken, PA, USA, 2012. [Google Scholar]
- Higashida, K.; Jinji, N. Strategic use of recycled content standards under international duopoly. J. Environ. Econ. Manag. 2006, 51, 242–257. [Google Scholar] [CrossRef]
- Cakmak, O.K. Biodegradable polymers—A review on properties, processing, and degradation mechanism. Circ. Econ. Sustain. 2024, 4, 339–362. [Google Scholar] [CrossRef]
- Dhanvijay, P.U.; Shertukde, V.V. Crystallization of biodegradable polymers. Polym. Plast. Technol. Eng. 2011, 50, 1289–1304. [Google Scholar] [CrossRef]
- Belboom, S.; Léonard, A. Does biobased polymer achieve better environmental impacts than fossil polymer? Comparison of fossil HDPE and biobased HDPE produced from sugar beet and wheat. Biomass Bioenergy 2016, 85, 159–167. [Google Scholar] [CrossRef]
- Harding, K.G.; Dennis, J.S.; von Blottnitz, H.; Harrison, S.T.L. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bai, Y.; Yang, W.; Bu, K.; Tanveer, S.K.; Hai, J. Global trends in natural biopolymers in the 21st century: A scientometric review. Front. Chem. 2022, 10, 915648. [Google Scholar] [CrossRef] [PubMed]
- Top 9 Biopolymer Trends & Innovations in 2024, StartUS Insights. 2022. Available online: https://www.startus-insights.com/innovators-guide/biopolymer-trends-innovation/ (accessed on 1 June 2024).
- Lemoigne, M. Études sur l’autolyse microbienne origine de l’acide β-oxybutyrique formé par autolyse. Ann. Inst. Pasteur. 1927, 41, 148. [Google Scholar]
- Steinbüchel, A.; Füchtenbusch, B. Bacterial and other biological systems for polyester production. Trends Biotechnol. 1998, 16, 419–427. [Google Scholar] [CrossRef]
- Panchal, S.S.; Vasava, D.V. Biodegradable polymeric materials: Synthetic approach. ACS Omega 2020, 5, 4370–4379. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Domb, A.J.; Nudelman, R. Biodegradable polymers derived from natural fatty acids. J. Polym. Sci. Part. A Polym. Chem. 1995, 33, 717–725. [Google Scholar] [CrossRef]
- Rivard, C.; Moens, L.; Roberts, K.; Brigham, J.; Kelley, S. Starch esters as biodegradable plastics: Effects of ester group chain length and degree of substitution on anaerobic biodegradation. Enzyme Microb. Technol. 1995, 17, 848–852. [Google Scholar] [CrossRef]
- Kaith, B.S.; Mittal, H.; Jindal, R.; Maiti, M.; Kalia, S. Environment benevolent biodegradable polymers: Synthesis, biodegradability, and applications. In Cellulose Fibers: Bio-and Nano-Polymer Composites: Green Chemistry and Technology; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Sarkar, M.; Upadhyay, A.; Pandey, D.; Sarkar, C.; Saha, S. Cellulose-Based Biodegradable Polymers: Synthesis, Properties, and Their Applications. In Biodegradable Polymers and Their Emerging Applications; Springer: Singapore, 2023; pp. 89–114. [Google Scholar]
- Xu, J.; McCarthy, S.P.; Gross, R.A.; Kaplan, D.L. Chitosan film acylation and effects on biodegradability. Macromolecules 1996, 29, 3436–3440. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Hadjichristidis, N. Poly (amino ester) s as an emerging synthetic biodegradable polymer platform: Recent developments and future trends. Prog. Polym. Sci. 2023, 136, 101634. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Rapid synthesis of graft copolymers from natural cellulose fibers. Carbohydr. Polym. 2013, 98, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Cheng, S.; Zhuo, R. Synthesis and characterization of novel biodegradable amphiphilic graft polymers based on aliphatic polycarbonate. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1356–1361. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Tran, R.T.; Thevenot, P.; Gyawali, D.; Chiao, J.-C.; Tang, L.; Yang, J. Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism. Soft Matter. 2010, 6, 2449–2461. [Google Scholar] [CrossRef]
- Iffland, K.; Sherwood, J.; Carus, M.; Raschka, A.; Farmer, T.; Clark, J. Definition, Calculation and Comparison of the “Biomass Utilization Efficiency (BUE)” of Various Bio-based Chemicals, Polymers and Fuels, Hürth 2015-11. Available online: https://renewable-carbon.eu/news/nova-paper-8-published/ (accessed on 1 June 2024).
- Jambunathan, P.; Zhang, K. Engineered biosynthesis of biodegradable polymers. J. Ind. Microbiol. Biotechnol. 2016, 43, 1037–1058. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Singh, P.; Alsanie, W.F.; Grammatikos, S.A.; Thakur, V.K. Synthesis of Bio-based monomers and polymers using microbes for a sustainable bioeconomy. Bioresour. Technol. 2022, 344, 126156. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. PLA Synthesis. From the Monomer to the Polymer; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly (lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, properties, and applications. Encycl. Polym. Sci. Technol. 2002, 1–36. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A.; Steinbüchel, A. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int. J. Biol. Macromol. 1999, 25, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V.A. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Lee, J.A.; Ahn, J.H.; Kim, I.; Li, S.; Lee, S.Y. Synthesis, characterization, and application of fully biobased and biodegradable nylon-4, 4 and-5, 4. ACS Sustain. Chem. Eng. 2020, 8, 5604–5614. [Google Scholar]
- Fukuda, Y.; Sasanuma, Y. Computational characterization of nylon 4, a biobased and biodegradable polyamide superior to nylon 6. ACS Omega 2018, 3, 9544–9555. [Google Scholar] [CrossRef]
- Lee, Y.; Porter, R.S. Effects of thermal history on crystallization of poly (ether ether ketone)(PEEK). Macromolecules 1988, 21, 2770–2776. [Google Scholar] [CrossRef]
- Matyszczak, G.; Wrzecionek, M.; Gadomska-Gajadhur, A.; Rus, P. Kinetics of polycondensation of sebacic acid with glycerol. Org. Process Res. Dev. 2020, 24, 1104–1111. [Google Scholar] [CrossRef]
- Radojčić, D.; Ionescu, M.; Petrović, Z.S. Novel potentially biodegradable polyurethanes from bio-based polyols. Contemp. Mater. 2013, 4, 9–21. [Google Scholar] [CrossRef]
- Guelcher, S.A. Biodegradable polyurethanes: Synthesis and applications in regenerative medicine. Tissue Eng. Part B Rev. 2008, 14, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, R.; Patel, J. Polyvinyl alcohol: A comprehensive study. Acta Sci. Pharm. Sci. 2019, 3, 34–44. [Google Scholar]
- Dubois, P.; Coulembier, O.; Raquez, J.M. Handbook of Ring-Opening Polymerization; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Duda, A.; Kowalski, A. Thermodynamics and kinetics of ring-opening polymerization. In Handbook of Ring-Opening Polymerization; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 1–51. [Google Scholar]
- Nakabayashi, K. Direct arylation polycondensation as conjugated polymer synthesis methodology. Polym. J. 2018, 50, 475–483. [Google Scholar] [CrossRef]
- Zhang, J.; Krishnamachari, P.; Lou, J.; Shahbazi, A. Synthesis of poly (L (+) lactic acid) by polycondensation method in solution. In Proceedings of the 2007 National Conference on Environmental Science and Technology, Greensboro, NC, USA, 12–14 September 2007; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–8. [Google Scholar]
- Lee, S.Y.; Choi, J.; Wong, H.H. Recent advances in polyhydroxyalkanoate production by bacterial fermentation: Mini-review. Int. J. Biol. Macromol. 1999, 25, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, J.; Lee, S.H. Production of polyhydroxyalkanoates by fermentation of bacteria. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2000; pp. 259–266. [Google Scholar]
- Li, T.-T.; Feng, L.-F.; Gu, X.-P.; Zhang, C.-L.; Wang, P.; Hu, G.-H. Intensification of polymerization processes by reactive extrusion. Ind. Eng. Chem. Res. 2021, 60, 2791–2806. [Google Scholar] [CrossRef]
- Raquez, J.; Narayan, R.; Dubois, P. Recent advances in reactive extrusion processing of biodegradable polymer-based compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Liboiron, M. Redefining pollution and action: The matter of plastics. J. Mater. Cult. 2016, 21, 87–110. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Rudnik, E. Preparation. In Handbook of Biopolymers and Biodegradable Plastics; William Andrew Publishing: Boston, MA, USA, 2013; pp. 189–211. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids. 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Kobayashi, S. Enzymatic polymerization: A new method of polymer synthesis. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3041–3056. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef] [PubMed]
- Oster, G.; Yang, N.-L. Photopolymerization of vinyl monomers. Chem. Rev. 1968, 68, 125–151. [Google Scholar] [CrossRef]
- Kaur, M.; Srivastava, A.K. Photopolymerization: A review. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 481–512. [Google Scholar] [CrossRef]
- Kamps, J.H.; Hoeks, T.; Kung, E.; Lens, J.P.; McCloskey, P.J.; Noordover, B.A.J.; Heuts, J.P.A. Activated carbonates: Enabling the synthesis of differentiated polycarbonate resins via melt transcarbonation. Polym. Chem. 2016, 7, 5294–5303. [Google Scholar] [CrossRef]
- Nair, K.P.; Gray, S.D. Melt Polymerization of Low Melt Viscosity Liquid Crystalline Polymers. U.S. Patent No. 9,057,016, 16 June 2015. [Google Scholar]
- Papaspyrides, C.D.; Vouyiouka, S.N. Solid State Polymerization; Wiley Online Library: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nishii, M.; Hayashi, K. Solid-state polymerization. Annu. Rev. Mater. Sci. 1975, 5, 135–149. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Arrighi, V. Polymers: Chemistry and Physics of Modern Materials; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green. Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Michaudel, Q.; Kottisch, V.; Fors, B.P. Cationic polymerization: From photoinitiation to photocontrol, Angew. Chem. Int. Ed. 2017, 56, 9670–9679. [Google Scholar] [CrossRef] [PubMed]
- Crivello, J.V.; Lam, J.H.W. The Photoinitiated Cationic Polymerization of Epoxy Resins; ACS Publications: Washington, DC, USA, 1979. [Google Scholar]
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3D printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Jousset, S.; Qiu, J.; Matyjaszewski, K.; Granel, C. Atom transfer radical polymerization of methyl methacrylate in water-borne system. Macromolecules 2001, 34, 6641–6648. [Google Scholar] [CrossRef]
- Mueller, L.; Jakubowski, W.; Matyjaszewski, K.; Pietrasik, J.; Kwiatkowski, P.; Chaladaj, W.; Jurczak, J. Synthesis of high molecular weight polystyrene using AGET ATRP under high pressure. Eur. Polym. J. 2011, 47, 730–734. [Google Scholar] [CrossRef]
- Tanaka, K.; Matyjaszewski, K. Copolymerization of (meth) acrylates with olefins using activators regenerated by electron transfer for atom transfer radical polymerization (ARGET ATRP). In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 1–9. [Google Scholar]
- Ning, W.; Shang, P.; Wu, J.; Shi, X.; Liu, S. Novel amphiphilic, biodegradable, biocompatible, thermo-responsive ABA triblock copolymers based on PCL and PEG analogues via a combination of ROP and RAFT: Synthesis, characterization, and sustained drug release from self-assembled micelles. Polymers 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Glaied, O.; Delaite, C.; Riess, G. Synthesis of PCL-b-PVAc block copolymers by combination of click chemistry, ROP, and RAFT polymerizations. Polym. Bull. 2012, 68, 607–621. [Google Scholar] [CrossRef]
- Meldal, M.; Diness, F. Recent fascinating aspects of the CuAAC click reaction. Trends Chem. 2020, 2, 569–584. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper (I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ameta, C.; Ameta, R.; Punjabi, P.B. Click chemistry: A tool for green chemical organic synthesis. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–48. [Google Scholar]
- Tremblay-Parrado, K.-K.; García-Astrain, C.; Avérous, L. Click chemistry for the synthesis of biobased polymers and networks derived from vegetable oils. Green Chem. 2021, 23, 4296–4327. [Google Scholar] [CrossRef]
- Shirame, S.P.; Bhosale, R.B. Green approach in click chemistry. In Green Chemistry; Books on Demand; InTech: Guindy, India, 2018. [Google Scholar]
- Kendall, J.L.; Canelas, D.A.; Young, J.L.; DeSimone, J.M. Polymerizations in supercritical carbon dioxide. Chem. Rev. 1999, 99, 543–564. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schubert, U.S. Microwave-assisted polymer synthesis: Recent developments in a rapidly expanding field of research. Macromol. Rapid Commun. 2007, 28, 368–386. [Google Scholar] [CrossRef]
- Kempe, K.; Becer, C.R.; Schubert, U.S. Microwave-Assisted Polymerizations: Recent Status and Future Perspectives. Macromolecules 2011, 44, 5825–5842. [Google Scholar] [CrossRef]
- Ehsani, A.; Shiri, H.M.; Kowsari, E.; Safari, R.; Torabian, J.; Hajghani, S. High performance electrochemical pseudocapacitors from ionic liquid assisted electrochemically synthesized p-type conductive polymer. J. Colloid. Interface Sci. 2017, 490, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Shamsuri, A.A.; Abdan, K.; Jamil, S.N.A.M. Preparations and properties of ionic liquid-assisted electrospun biodegradable polymer fibers. Polymers 2022, 14, 2308. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nagamani, C.; Moore, J.S. Polymer mechanochemistry: From destructive to productive. Acc. Chem. Res. 2015, 48, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef] [PubMed]
- Misono, M.; Ono, I.; Koyano, G.; Aoshima, A. Heteropolyacids. Versatile green catalysts usable in a variety of reaction media. Pure Appl. Chem. 2000, 72, 1305–1311. [Google Scholar] [CrossRef]
- Kobayashi, S. Green polymer chemistry: New methods of polymer synthesis using renewable starting materials. Struct. Chem. 2017, 28, 461–474. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding plastics recycling technologies: Chemical aspects, technology status and challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Kaminsky, W.; Schlesselmann, B.; Simon, C.M. Thermal degradation of mixed plastic waste to aromatics and gas. Polym. Degrad. Stab. 1996, 53, 189–197. [Google Scholar] [CrossRef]
- Kanerva, L.T. Methanolysis and Ethanolysis. Acta Chem. Scand. 1986, 40, 731–734. [Google Scholar] [CrossRef]
- Prifti, K.; Lechtenberg, F.; Manenti, F.; Espuña, A.; Graells, M. Comparing the climate impact of methanol production in Europe: Steam methane reforming vs. Plastic waste gasification processes. Resour. Conserv. Recycl. 2024, 208, 107653. [Google Scholar] [CrossRef]
- Afzal, S.; Singh, A.; Nicholson, S.R.; Uekert, T.; DesVeaux, J.S.; Tan, E.C.D.; Dutta, A.; Carpenter, A.C.; Baldwin, R.M.; Beckham, G.T. Techno-economic analysis and life cycle assessment of mixed plastic waste gasification for production of methanol and hydrogen. Green Chem. 2023, 25, 5068–5085. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Goodship, V. Plastic recycling. Sci. Prog. 2007, 90, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [PubMed]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical recycling of packaging plastics: A review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Delva, L.; Van Kets, K.; Kuzmanovic, M.; Demets, R.; Hubo, S.; Mys, N.; De Meester, S.; Ragaert, K. Mechanical recycling of polymers for dummies. In Capture-Plastics to Resource; Ghent University: Ghent, Belgium, 2019. [Google Scholar]
- Ghabezi, P.; Sam-Daliri, O.; Flanagan, T.; Walls, M.; Harrison, N.M. Circular economy innovation: A deep investigation on 3D printing of industrial waste polypropylene and carbon fibre composites. Resour. Conserv. Recycl. 2024, 206, 107667. [Google Scholar] [CrossRef]
- Shao, L.; Chang, Y.-C.; Hao, C.; Fei, M.; Zhao, B.; Bliss, B.J.; Zhang, J. A chemical approach for the future of PLA upcycling: From plastic wastes to new 3D printing materials. Green Chem. 2022, 24, 8716–8724. [Google Scholar] [CrossRef]
- Gomes, T.E.P.; Cadete, M.S.; Dias-de-Oliveira, J.; Neto, V. Controlling the properties of parts 3D printed from recycled thermoplastics: A review of current practices. Polym. Degrad. Stab. 2022, 196, 109850. [Google Scholar] [CrossRef]
- Achilias, D.S.; Antonakou, E.V.; Koutsokosta, E.; Lappas, A.A. Chemical recycling of polymers from waste electric and electronic equipment. J. Appl. Polym. Sci. 2009, 114, 212–221. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 46. [Google Scholar]
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020, 5, 501–516. [Google Scholar]
- Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. [Google Scholar] [CrossRef] [PubMed]
- Carniel, A.; de Abreu Waldow, V.; de Castro, A.M. A comprehensive and critical review on key elements to implement enzymatic PET depolymerization for recycling purposes. Biotechnol. Adv. 2021, 52, 107811. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, M.I.S.; Sousa, A.F.; Teixeira, G.; Tavares, A.P.M.; Ferreira, A.M.; Coutinho, J.A.P. Enhancing plastic waste recycling: Evaluating the impact of additives on the enzymatic polymer degradation. Catal. Today 2024, 429, 114492. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and enzymatic degradation of synthetic plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar]
- Martínez-Cutillas, A.; León, S.; Oh, S.; de Ilarduya, A.M. Enzymatic recycling of polymacrolactones. Polym. Chem. 2022, 13, 1586–1595. [Google Scholar] [CrossRef]
- Orlando, M.; Molla, G.; Castellani, P.; Pirillo, V.; Torretta, V.; Ferronato, N. Microbial Enzyme Biotechnology to Reach Plastic Waste Circularity: Current Status, Problems and Perspectives. Int. J. Mol. Sci. 2023, 24, 3877. [Google Scholar] [CrossRef]
- Arostegui, A.; Sarrionandia, M.; Aurrekoetxea, J.; Urrutibeascoa, I. Effect of dissolution-based recycling on the degradation and the mechanical properties of acrylonitrile–butadiene–styrene copolymer. Polym. Degrad. Stab. 2006, 91, 2768–2774. [Google Scholar] [CrossRef]
- Triebert, D.; Hanel, H.; Bundt, M.; Wohnig, K. Solvent-based recycling. In Circular Economy of Polymers: Topics in Recycling Technologies; American Chemical Society; ACS Publications: Washington, DC, USA, 2021; pp. 33–59. [Google Scholar]
- Lu, T. Advanced Recycling of Mixed Plastic Waste Using Safer Solvents and Supercritical Water. Ph.D. Thesis, University of Massachusetts Lowell, Lowell, MA, USA, 2023. [Google Scholar]
- Yan, N. Recycling plastic using a hybrid process. Science 2022, 378, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Choudhary, H.; Wang, Z.; Baral, N.R.; Mohan, M.; Aguilar, R.A.; Huang, S.; Holiday, A.; Banatao, D.R.; Singh, S.; et al. A hybrid chemical-biological approach can upcycle mixed plastic waste with reduced cost and carbon footprint. One Earth 2023, 6, 1576–1590. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Hossain, M.; Umer, R. Recycling of wind turbine blades through modern recycling technologies: A road to zero waste, Renew. Energy Focus. 2023, 44, 373–389. [Google Scholar] [CrossRef]
- European Commission, Circular Economy Action Plan. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 1 June 2024).
- OECD. Extended Producer Responsibility. In Updated Guidance for Efficient Waste Management; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Giacovelli, C. Single-Use Plastics: A Roadmap for Sustainability. 2018. Available online: https://www.unep.org/resources/report/single-use-plastics-roadmap-sustainability (accessed on 1 June 2024).
- Bucknall, D.G. Plastics as a materials system in a circular economy. Philos. Trans. R. Soc. A 2020, 378, 20190268. [Google Scholar] [CrossRef]




| Natural Biodegradable Polymers | Synthetic Biodegradable Polymers | |
|---|---|---|
| Source | Renewable biological sources (plants, animals, microorganisms) | Synthetic processes, renewable feedstocks, sometimes petroleum-based |
| Examples | Cellulose, chitosan, collagen, gelatin | PLA, PCL, PHAs, polyanhydrides, PVA |
| Properties | Biocompatible, nontoxic, lower mechanical strength, and thermal stability | Customizable properties, consistent quality, tailored mechanical, and thermal properties |
| Production Methods | Extraction, purification, chemical, or enzymatic modification | Polymerization (ring-opening, polycondensation), bacterial fermentation |
| Applications | Food packaging, medical applications, agriculture, textiles | Packaging, medical devices, drug delivery, agriculture, consumer goods |
| Advantages | High biocompatibility, nontoxicity, renewable sources, easier biodegradation | Customizable, consistent quality, wide range of applications |
| Disadvantages | Quality variability, limited property customization, potential allergenicity | Higher production costs, potential use of toxic intermediates, specific biodegradation conditions |
| Biodegradability | Often readily biodegradable due to natural enzyme activity | Biodegradability depends on environmental conditions |
| Environmental Impact | Generally lower carbon footprint, promotes sustainable use of resources | Can be designed to have minimal environmental impact, but dependent on production methods |
| Economic Considerations | Potential for lower production costs but variability in quality and supply | Higher production costs but more consistent performance and quality |
| Feature | Depolymerization | Pyrolysis | Gasification |
|---|---|---|---|
| Process | Breaks down polymers into monomers through chemical reactions. | Breaks down polymers into smaller molecules through high temperature and absence of oxygen. | Converts organic materials into syngas through high temperature and controlled oxygen. |
| Feedstock | Various plastic polymers. | Plastic waste, biomass, and organic materials. | Biomass, coal, and organic waste. |
| Products | Monomers suitable for polymer production. | Pyrolysis oil, gas, and char. | Syngas. |
| Circular Economy | Facilitates closed-loop recycling of plastics, reducing reliance on virgin materials. | Can process mixed or contaminated plastics that are difficult to recycle mechanically. | Offers potential for energy recovery from waste materials, contributing to waste-to-energy initiatives. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beena Unni, A.; Muringayil Joseph, T. Enhancing Polymer Sustainability: Eco-Conscious Strategies. Polymers 2024, 16, 1769. https://doi.org/10.3390/polym16131769
Beena Unni A, Muringayil Joseph T. Enhancing Polymer Sustainability: Eco-Conscious Strategies. Polymers. 2024; 16(13):1769. https://doi.org/10.3390/polym16131769
Chicago/Turabian StyleBeena Unni, Aparna, and Tomy Muringayil Joseph. 2024. "Enhancing Polymer Sustainability: Eco-Conscious Strategies" Polymers 16, no. 13: 1769. https://doi.org/10.3390/polym16131769
APA StyleBeena Unni, A., & Muringayil Joseph, T. (2024). Enhancing Polymer Sustainability: Eco-Conscious Strategies. Polymers, 16(13), 1769. https://doi.org/10.3390/polym16131769






