The Synthesis of Narrowly Dispersed Poly(ε-caprolactone) Microspheres by Dispersion Polymerization Using a Homopolymer Poly(dodecyl acrylate) as the Stabilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RAFT Polymerization of PDA as Stabilizer
2.3. Synthesis of BCY
2.4. Synthesis of PCL Microspheres
2.5. Characterization
3. Results and Discussion
3.1. Synthesis of PDA
3.2. Synthesis of BCY
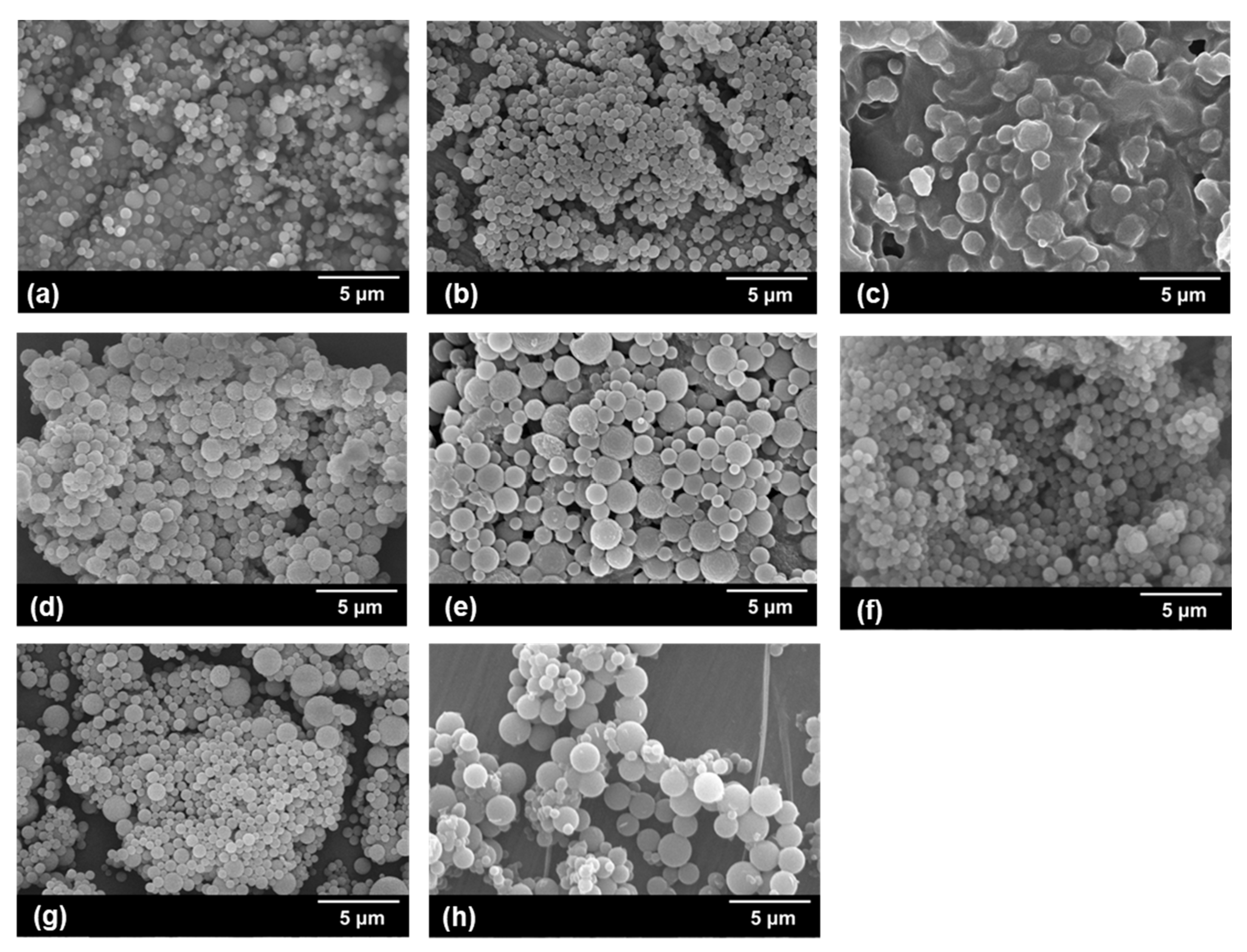
3.3. Synthesis of PCL Microspheres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, G.; Yue, H. Advances in Uniform Polymer Microspheres and Microcapsules: Preparation and Biomedical Applications. Chin. J. Chem. 2020, 38, 911–923. [Google Scholar] [CrossRef]
- Kim, T.; Mays, J.; Chung, I. Porous poly(ε-caprolactone) microspheres via UV photodegradation of block copolymers prepared by RAFT polymerization. Polymer 2018, 158, 198–203. [Google Scholar] [CrossRef]
- Li, Z.; Wei, X.; Ngai, T. Controlled production of polymer microspheres from microgel-stabilized high internal phase emulsions. Chem. Commun. 2011, 47, 331–333. [Google Scholar] [CrossRef]
- Liu, D.; Cito, S.; Zhang, Y.; Wang, C.-F.; Sikanen, T.M.; Santos, H.A. A Versatile and Robust Microfluidic Platform Toward High Throughput Synthesis of Homogeneous Nanoparticles with Tunable Properties. Adv. Mater. 2015, 27, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Yang, X.; Huang, W. Synthesis of Narrow or Monodisperse Poly(divinylbenzene) Microspheres by Distillation−Precipitation Polymerization. Macromolecules 2004, 37, 9746–9752. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Li, Z. Preparation of fluorinated PCL porous microspheres and a super-hydrophobic coating on fabrics via electrospraying. Nanoscale 2018, 10, 18857–18868. [Google Scholar] [CrossRef] [PubMed]
- Omi, S.; Katami, K.i.; Yamamoto, A.; Iso, M. Synthesis of polymeric microspheres employing SPG emulsification technique. J. Appl. Polym. Sci. 1994, 51, 1–11. [Google Scholar] [CrossRef]
- Thiangpak, P.; Rodchanarowan, A. The synthesis of polycaprolactone (PCL) microspheres containing cerium (III) nitrate (Ce(NO3)3) self-healing agent via double emulsion evaporation method. Mater. Today Commun. 2020, 25, 101668. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Slomkowski, S. 4.25—Ring-Opening Dispersion Polymerization. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 645–660. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. 3.12—Copper-Mediated Atom Transfer Radical Polymerization. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 377–428. [Google Scholar] [CrossRef]
- Pal, J.; Kankariya, N.; Sanwaria, S.; Nandan, B.; Srivastava, R.K. Control on molecular weight reduction of poly(ε-caprolactone) during melt spinning—A way to produce high strength biodegradable fibers. Mater. Sci. Eng. C 2013, 33, 4213–4220. [Google Scholar] [CrossRef]
- Zhu, S.; Hamielec, A. 4.32—Polymerization Kinetic Modeling and Macromolecular Reaction Engineering. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 779–831. [Google Scholar] [CrossRef]
- Muranaka, M.; Ono, T. Preparation of Monodisperse Polylactide Microspheres by Dispersion Polymerization Using a Polymeric Stabilizer with Hydroxy Groups. Macromol. Rapid Commun. 2009, 30, 152–156. [Google Scholar] [CrossRef]
- Huda, M.K.; Das, P.P.; Saikia, P.J.; Baruah, S.D. Synthesis of poly(n-octadecyl methacrylate-co-2-hydroxyethyl methacrylate) copolymer and their utilization as polymeric stabilizer in the preparation of PCL microspheres. Polym. Bull. 2017, 74, 1661–1676. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Thurecht, K.J.; Howdle, S.M.; Irvine, D.J. Facile one-spot synthesis of highly branched polycaprolactone. Polym. Chem. 2014, 5, 2997–3008. [Google Scholar] [CrossRef]
- Erdal, N.B.; Lando, G.A.; Yadav, A.; Srivastava, R.K.; Hakkarainen, M. Hydrolytic Degradation of Porous Crosslinked Poly(ε-Caprolactone) Synthesized by High Internal Phase Emulsion Templating. Polymers 2020, 12, 1849. [Google Scholar] [CrossRef]
- Doskocilova, D.; Schneider, B. NMR studies of swollen crosslinked polymer gels. Pure Appl. Chem. 1982, 54, 575–584. [Google Scholar] [CrossRef]
- Sosnowski, S.; Gadzinowski, M.; Slomkowski, S.; Penczek, S. Synthesis of Bioerodible Poly(ε-caprolactone) Latexes and Poly(D, L-lactide) Microspheres by Ring-Opening Polymerization. J. Bioact. Compat. Polym. 1994, 9, 345–366. [Google Scholar] [CrossRef]
- Itoh, T.; Okuno, M.; Moriya, Y.; Shimomoto, H.; Ihara, E. Poly(acrylic acid) block copolymers as stabilizers for dispersion polymerization. Polymer 2022, 256, 125265. [Google Scholar] [CrossRef]
- Chen, R.; Ren, N.; Jin, X.; Zhu, X. Stabilization capacity of PNIPAM microgels as particulate stabilizer in dispersion polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 789–794. [Google Scholar] [CrossRef]
- Watanabe, S.; Kobayashi, T.; Sumitomo, H.; Murata, M.; Masuda, Y. Preparation of monodisperse PMMA particles by dispersion polymerization of MMA using poly(styrene-co-methacrylic acid) copolymer as a steric stabilizer. Polym. Bull. 2010, 65, 543–550. [Google Scholar] [CrossRef]
- Bentz, K.C.; Ejaz, M.; Arencibia, S.; Sultan, N.; Grayson, S.M.; Savin, D.A. Hollow amphiphilic crosslinked nanocapsules from sacrificial silica nanoparticle templates and their application as dispersants for oil spill remediation. Polym. Chem. 2017, 8, 5129–5138. [Google Scholar] [CrossRef]
- Song, J.-S.; Tronc, F.; Winnik, M.A. Two-Stage Dispersion Polymerization toward Monodisperse, Controlled Micrometer-Sized Copolymer Particles. J. Am. Chem. Soc. 2004, 126, 6562–6563. [Google Scholar] [CrossRef]
- Lok, K.P.; Ober, C.K. Particle size control in dispersion polymerization of polystyrene. Can. J. Chem. 1985, 63, 209–216. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Choi, J.H.; Matsumoto, Y. Preparation of micron-size monodisperse poly(methyl methacrylate) particles using poly(2-oxazoline) macromonomer. Polym. Int. 1993, 30, 265–270. [Google Scholar] [CrossRef]
- Paine, A.J. Dispersion polymerization of styrene in polar solvents. 7. A simple mechanistic model to predict particle size. Macromolecules 1990, 23, 3109–3117. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Ito, K. Dispersion Polymerization. In Polymer Particles; Okubo, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 299–328. [Google Scholar] [CrossRef]
- Shen, S.; Sudol, E.D.; El-Aasser, M.S. Control of particle size in dispersion polymerization of methyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1393–1402. [Google Scholar] [CrossRef]
- Zuo, Q.; Shi, T.; Zhou, X.; Zhang, Y.; He, J. Study on dispersion polymerization of polyvinyl pyrrolidone assisted with mixed-nonpolar solvent. Appl. Chem. Ind. 2011, 40, 1758. [Google Scholar]
- Downey, J.S.; Frank, R.S.; Li, W.-H.; Stöver, H.D.H. Growth Mechanism of Poly(divinylbenzene) Microspheres in Precipitation Polymerization. Macromolecules 1999, 32, 2838–2844. [Google Scholar] [CrossRef]
- Jinhua, L.; Guangyuan, Z. Polystyrene Microbeads by Dispersion Polymerization: Effect of Solvent on Particle Morphology. Int. J. Polym. Sci. 2014, 2014, 703205. [Google Scholar] [CrossRef]
- Koleske, J.V.; Lundberg, R.D. Lactone polymers. I. Glass transition temperature of poly-ε-caprolactone by means of compatible polymer mixtures. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 795–807. [Google Scholar] [CrossRef]
- Martínez, J.; Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Alonso-Moreno, C.; Sánchez-Barba, L.F.; Fernandez-Baeza, J.; Rodríguez, A.M.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Otero, A.; et al. Versatile organoaluminium catalysts based on heteroscorpionate ligands for the preparation of polyesters. Dalton Trans. 2018, 47, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
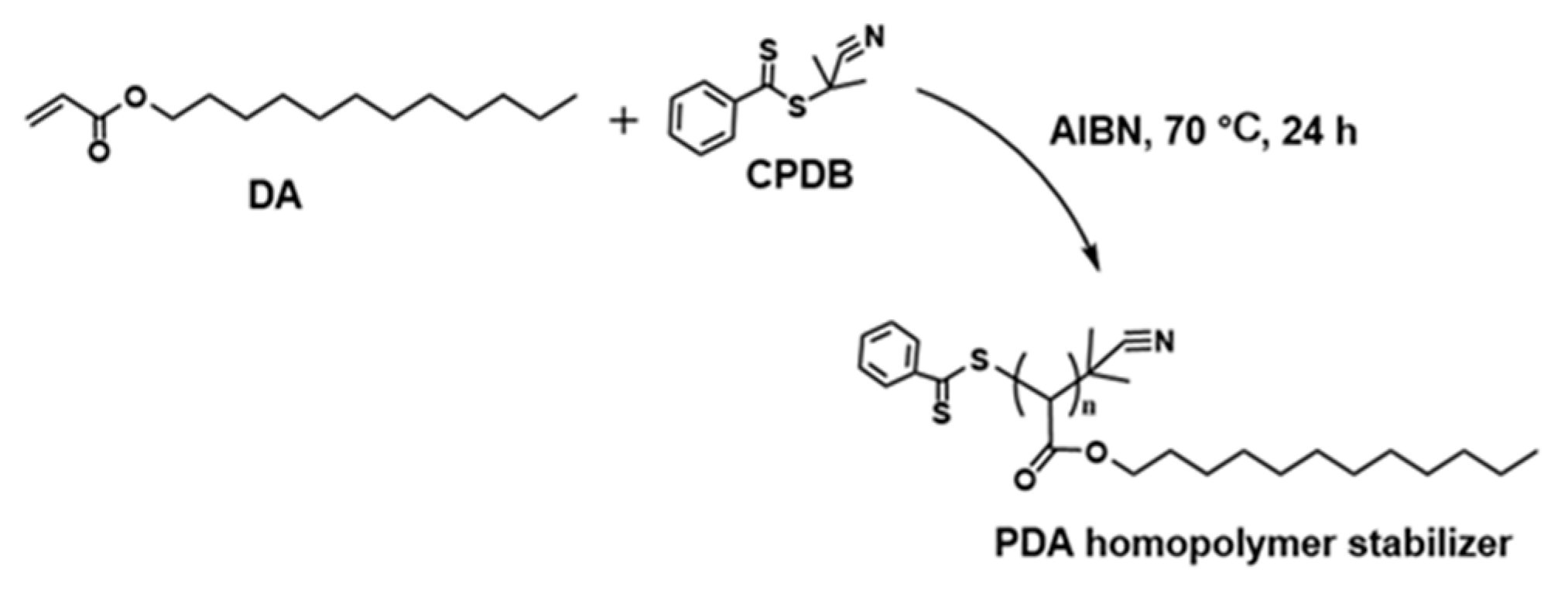
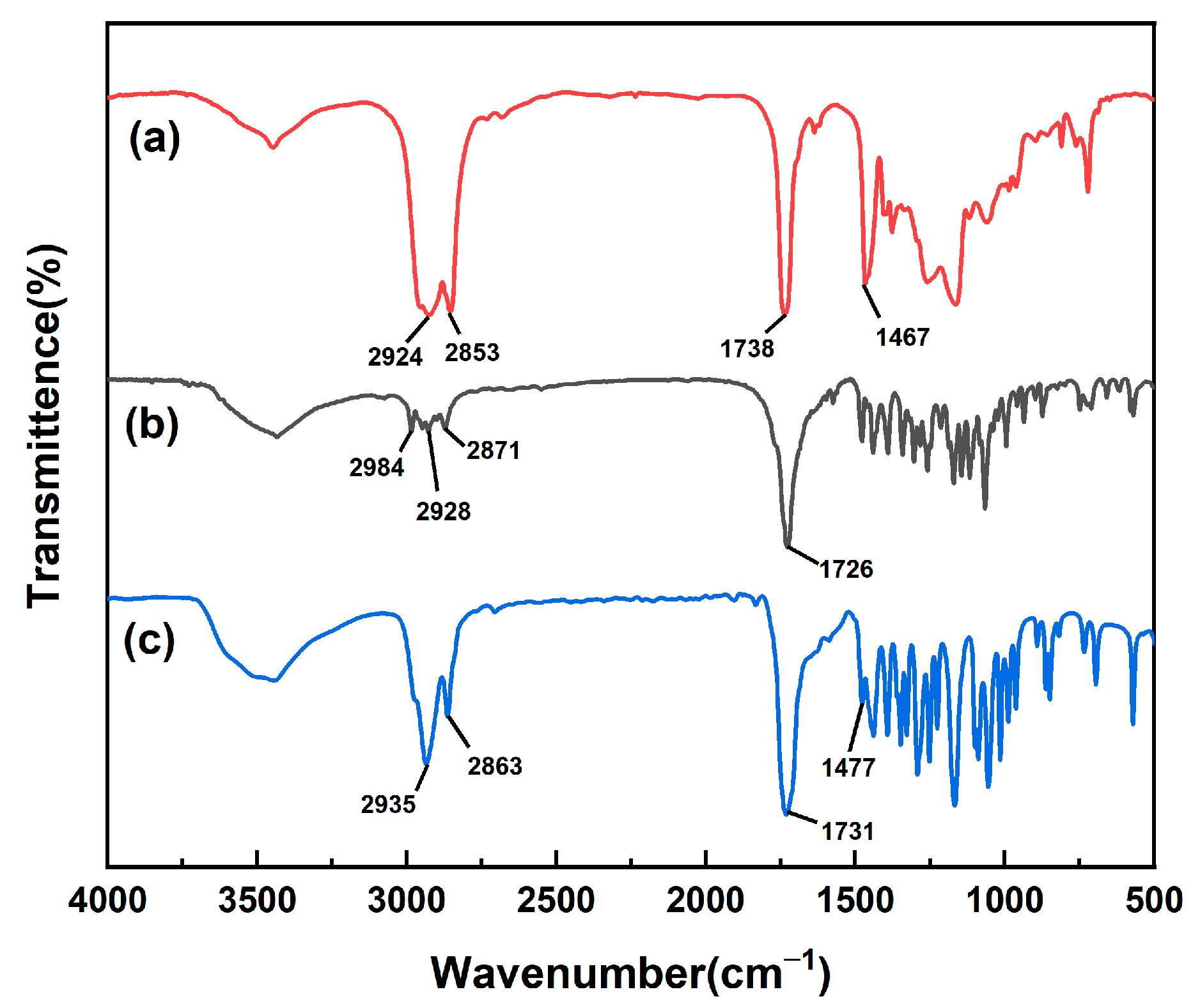
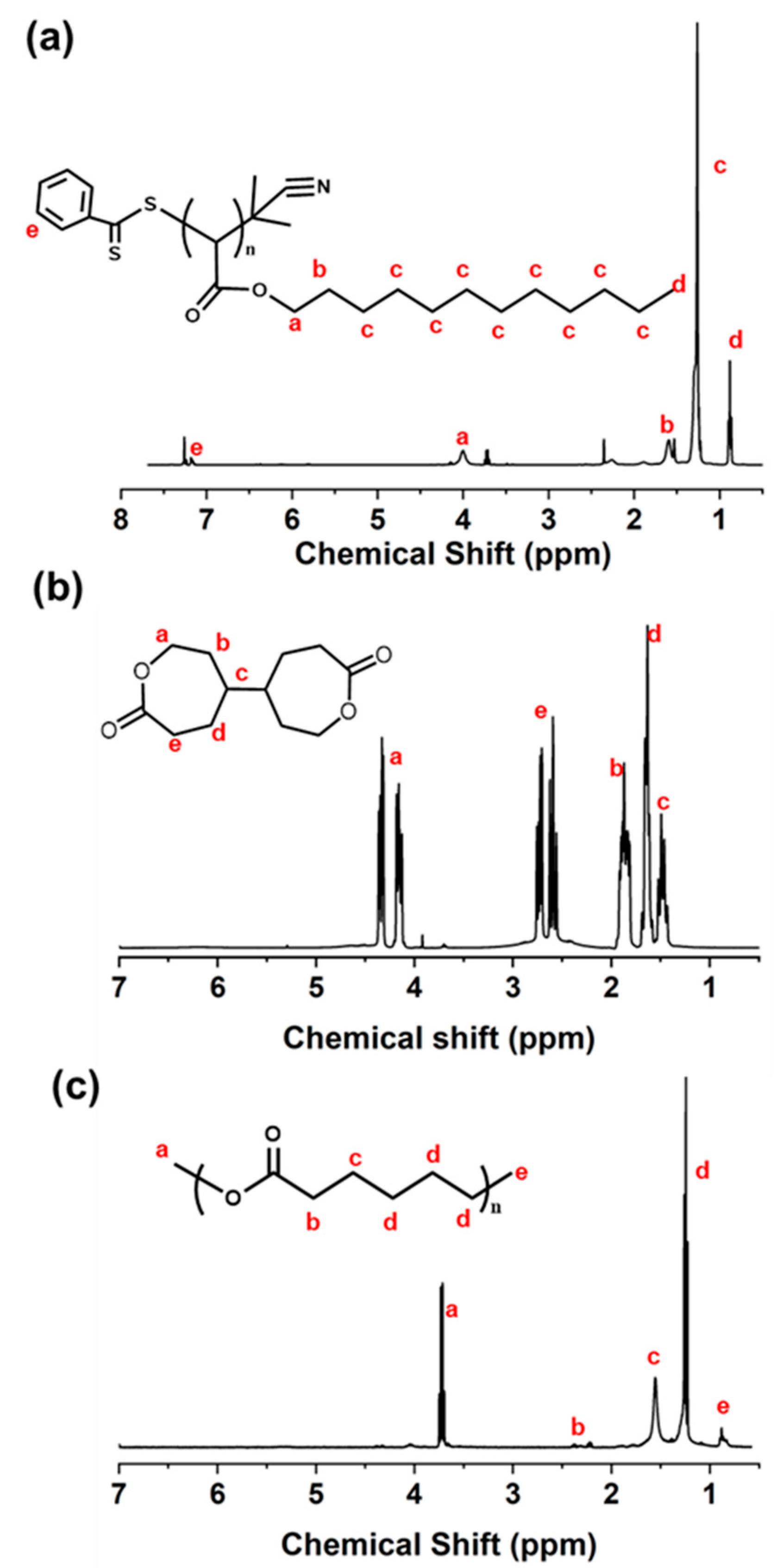
| Sample | DA (mmol) | CPDB (mmol) | DA/CPDB (Molar Ratio) | Mn in Theory | Mn Determined by GPC | Mw Determined by GPC | Mw/Mn |
|---|---|---|---|---|---|---|---|
| PDA-1 | 42 | 0.3 | 140:1 | 33,800 | 39,200 | 45,900 | 1.17 |
| PDA-2 | 42 | 0.8 | 53:1 | 13,000 | 14,300 | 17,000 | 1.18 |
| PDA-3 | 42 | 2 | 21:1 | 5200 | 4600 | 5200 | 1.12 |
| PDA-4 | 42 | 0 | - | 17,000 | 15,600 | 41,000 | 2.62 |
| Sample | Stabilizer | Mn of PDA | Mw/Mn of PDA | Dw/Dn of PCL Microspheres |
|---|---|---|---|---|
| S1 | PDA-1 | 39,200 | 1.17 | 1.18 |
| S2 | PDA-2 | 14,300 | 1.18 | 1.09 |
| S8 | PDA-4 | 15,600 | 2.62 | 1.40 |
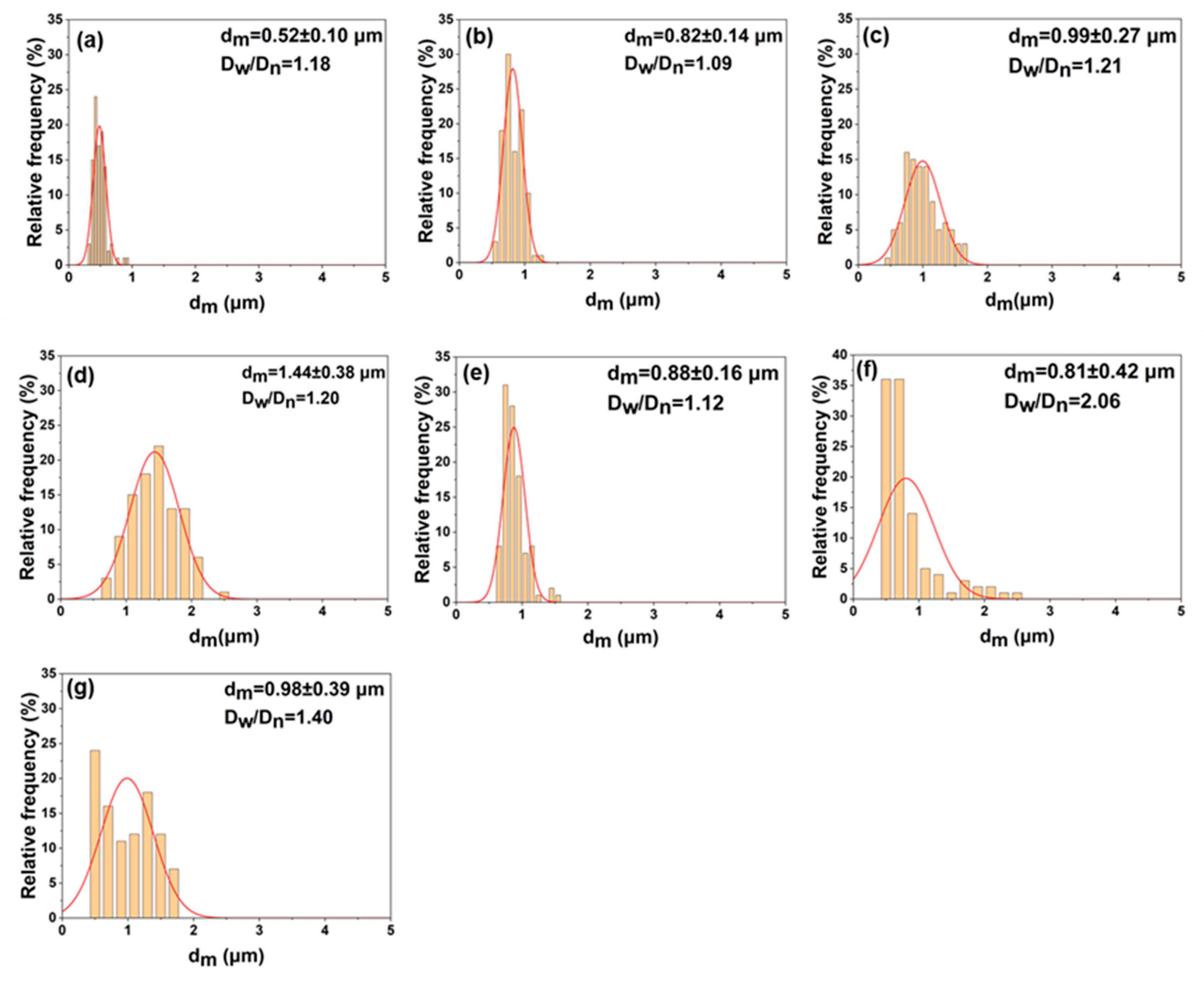
| Sample | Stabilizer | 1,4-Dioxane/Heptane (v/v) | Ta (°C) | PCL Morphology | Dnb (μm) | Dw/Dnc |
|---|---|---|---|---|---|---|
| S1 | PDA-1 | 1/8 | 5 | Spherical | 0.52 | 1.18 |
| S2 | PDA-2 | 1/8 | 5 | Spherical | 0.82 | 1.09 |
| S3 | PDA-3 | 1/8 | 5 | Agglomerated * | - | - |
| S4 | PDA-2 | 1.2/7.8 | 5 | Spherical | 0.99 | 1.21 |
| S5 | PDA-2 | 1.5/7.5 | 5 | Spherical | 1.44 | 1.20 |
| S6 | PDA-2 | 1/8 | 0 | Spherical | 0.88 | 1.12 |
| S7 | PDA-2 | 1/8 | 10 | Spherical | 0.81 | 2.06 |
| S8 | PDA-4 | 1/8 | 5 | Spherical | 0.98 | 1.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, C.; Liu, Q.; Guo, C.; Zhang, S. The Synthesis of Narrowly Dispersed Poly(ε-caprolactone) Microspheres by Dispersion Polymerization Using a Homopolymer Poly(dodecyl acrylate) as the Stabilizer. Polymers 2024, 16, 1911. https://doi.org/10.3390/polym16131911
Wang Y, Xu C, Liu Q, Guo C, Zhang S. The Synthesis of Narrowly Dispersed Poly(ε-caprolactone) Microspheres by Dispersion Polymerization Using a Homopolymer Poly(dodecyl acrylate) as the Stabilizer. Polymers. 2024; 16(13):1911. https://doi.org/10.3390/polym16131911
Chicago/Turabian StyleWang, Yiling, Chuangbang Xu, Qi Liu, Cuicui Guo, and Shengmiao Zhang. 2024. "The Synthesis of Narrowly Dispersed Poly(ε-caprolactone) Microspheres by Dispersion Polymerization Using a Homopolymer Poly(dodecyl acrylate) as the Stabilizer" Polymers 16, no. 13: 1911. https://doi.org/10.3390/polym16131911
APA StyleWang, Y., Xu, C., Liu, Q., Guo, C., & Zhang, S. (2024). The Synthesis of Narrowly Dispersed Poly(ε-caprolactone) Microspheres by Dispersion Polymerization Using a Homopolymer Poly(dodecyl acrylate) as the Stabilizer. Polymers, 16(13), 1911. https://doi.org/10.3390/polym16131911







