The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review
Abstract
:1. Introduction
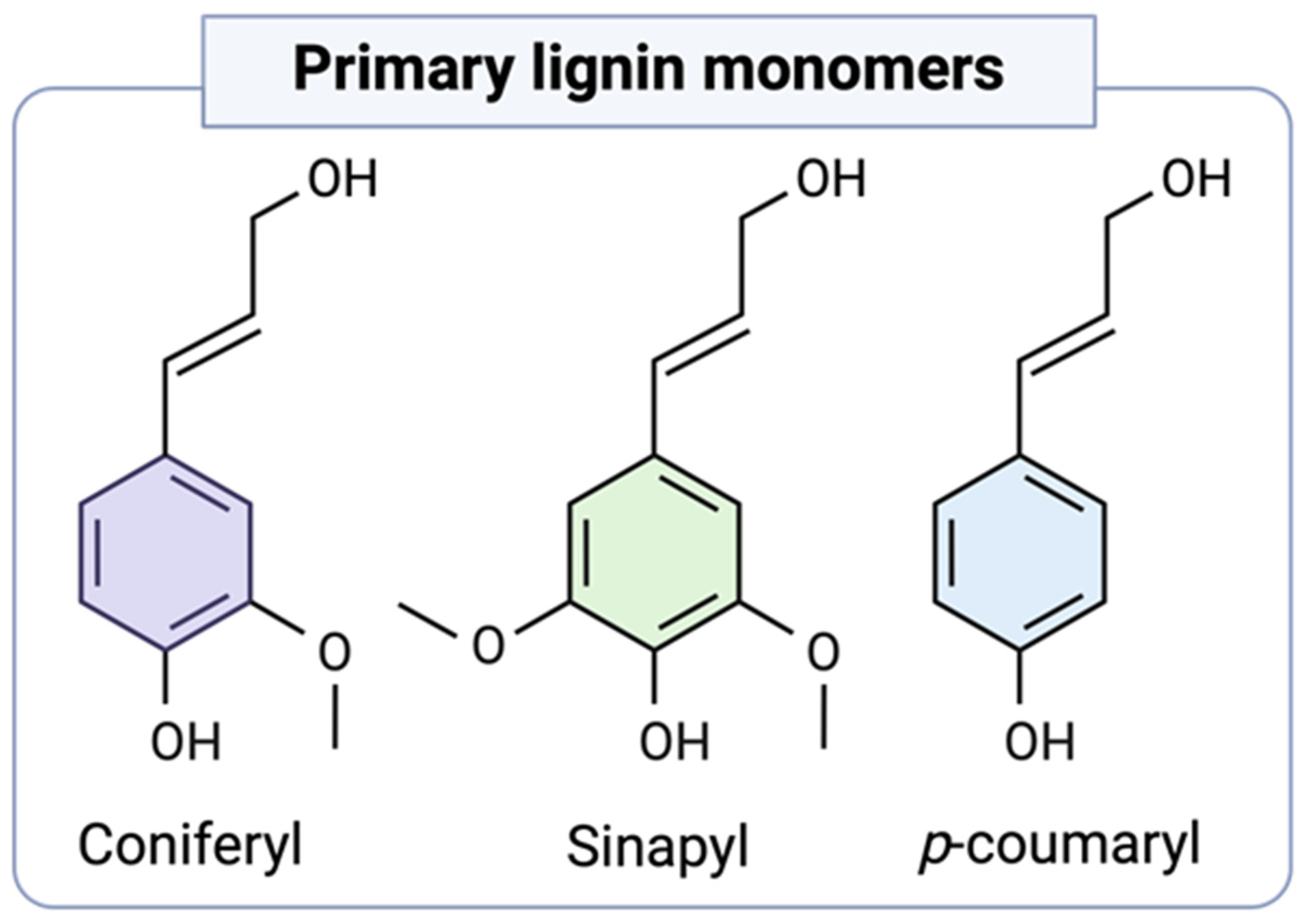
2. Native Lignin Chemical Properties
3. Technical Lignins and Their Antimicrobial Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cazacu, G.; Capraru, M.; Popa, V.I. Advances Concerning Lignin Utilization in New Materials. In Advances in Natural Polymers; Thomas, S., Visakh, P.M., Mathew, A.P., Eds.; Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2013; Volume 18, pp. 255–312. ISBN 978-3-642-20939-0. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; De Jong, E.; Guran, B.; Abächerli, A. Co-Ordination Network for Lignin—Standardisation, Production and Applications Adapted to Market Requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 978-0-444-59561-4. [Google Scholar]
- Lora, J. Industrial Commercial Lignins: Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 225–241. ISBN 978-0-08-045316-3. [Google Scholar]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and Antioxidant Activities of Lignin from Residue of Corn Stover to Ethanol Production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Jha, A.; Kumar, A. Deciphering the Role of Sodium Lignosulfonate against Candida spp. as Persuasive Anticandidal Agent. Int. J. Biol. Macromol. 2018, 107, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Férir, G.; D’huys, T.; Petrova, M.I.; Lebeer, S.; Snoeck, R.; Andrei, G.; Schols, D. The Low-Cost Compound Lignosulfonic Acid (LA) Exhibits Broad-Spectrum Anti-HIV and Anti-HSV Activity and Has Potential for Microbicidal Applications. PLoS ONE 2015, 10, e0131219. [Google Scholar] [CrossRef]
- Flickinger, E.A.; Campbell, J.M.; Schmitt, L.G.; Fahey, G.C. Selected Lignosulfonate Fractions Affect Growth Performance, Digestibility, and Cecal and Colonic Properties in Rats. J. Anim. Sci. 1998, 76, 1626. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fernandes, M.M.; Matamá, T.; Loureiro, A.; Gomes, A.C.; Cavaco-Paulo, A. Chitosan–Lignosulfonates Sono-Chemically Prepared Nanoparticles: Characterisation and Potential Applications. Colloids Surf. B Biointerfaces 2013, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural Polymers from Oxidative Coupling of 4-Hydroxyphenyl-Propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Hatfield, R.; Vermerris, W. Lignin Formation in Plants. The Dilemma of Linkage Specificity. Plant Physiol. 2001, 126, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Adler, E. Lignin Chemistry? Past, Present and Future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.S.; Menachem, S.B. Lignin. In Biotechnology in the Pulp and Paper Industry; Eriksson, K.-E.L., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.-O., Eriksson, K.-E.L., Fiechter, A., Klibanov, A.M., Mattiasson, B., Primrose, S.B., et al., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 1997; Volume 57, pp. 127–158. ISBN 978-3-540-61868-3. [Google Scholar]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Ralph, J.; Grabber, J.H.; Hatfield, R.D. Lignin-Ferulate Cross-Links in Grasses: Active Incorporation of Ferulate Polysaccharide Esters into Ryegrass Lignins. Carbohydr. Res. 1995, 275, 167–178. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-73843-5. [Google Scholar]
- Terashima, N.; Fukushima, K.; He, L.-F.; Takabe, K. Comprehensive Model of the Lignified Plant Cell Wall. In ASA, CSSA, and SSSA Books; Jung, H.G., Buxton, D.R., Hatfield, R.D., Ralph, J., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 247–270. ISBN 978-0-89118-238-2. [Google Scholar]
- Baucher, M.; Monties, B.; Montagu, M.V.; Boerjan, W. Biosynthesis and Genetic Engineering of Lignin. Crit. Rev. Plant Sci. 1998, 17, 125–197. [Google Scholar] [CrossRef]
- Cadix, A.; James, S. Cementing Additives. In Fluid Chemistry, Drilling and Completion; Elsevier: Amsterdam, The Netherlands, 2022; pp. 187–254. ISBN 978-0-12-822721-3. [Google Scholar]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Smook, G.; Kocurek, M. Handbook for Pulp & Paper Technologists; TAPPI Press: Atlanta, GA, USA, 1982. [Google Scholar]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications. BioRes 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Yun, J.; Wei, L.; Li, W.; Gong, D.; Qin, H.; Feng, X.; Li, G.; Ling, Z.; Wang, P.; Yin, B. Isolating High Antimicrobial Ability Lignin From Bamboo Kraft Lignin by Organosolv Fractionation. Front. Bioeng. Biotechnol. 2021, 9, 683796. [Google Scholar] [CrossRef]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Role of Lignosulphonate in Properties of Fish Gelatin Films. Food Hydrocoll. 2012, 27, 60–71. [Google Scholar] [CrossRef]
- Reyes, D.C.; Annis, S.L.; Rivera, S.A.; Leon-Tinoco, A.Y.; Wu, C.; Perkins, L.B.; Perry, J.J.; Ma, Z.X.; Knight, C.W.; Castillo, M.S.; et al. In Vitro Screening of Technical Lignins to Determine Their Potential as Hay Preservatives. J. Dairy Sci. 2020, 103, 6114–6134. [Google Scholar] [CrossRef]
- Leon-Tinoco, A.Y.; Annis, S.L.; Almeida, S.T.; Guimarães, B.C.; Killerby, M.; Zhang, J.; Wu, C.; Perkins, L.B.; Ma, Z.; Jeong, K.C.; et al. Evaluating the Potential of Lignosulfonates and Chitosans as Alfalfa Hay Preservatives Using in Vitro Techniques. J. Anim. Sci. 2022, 100, skac154. [Google Scholar] [CrossRef]
- Reyes, D.C.; Rivera, S.A.; Ma, Z.X.; Dubuc, H.M.; Leon-Tinoco, A.Y.; Lichtenwalner, A.B.; Romero, J.J. Mitigating Environmental Mastitis Microbes with the Novel Use of Paper Mill Byproducts. J. Dairy Sci. 2019, 102, 182–183. [Google Scholar]
- Suzuki, H.; Tochikura, T.S.; Iiyama, K.; Yamazaki, S.; Yamamoto, N.; Toda, S. Lignosulfonate, a Water-Solubilized Lignin from the Waste Liquor of the Pulping Process, Inhibits the Infectivity and Cytopathic Effects of Human Immunodeficiency Virus in Vitro. Agric. Biol. Chem. 1989, 53, 3369–3372. [Google Scholar] [CrossRef]
- Nada, A.M.A.; El-Diwany, A.I.; Elshafei, A.M. Infrared and Antimicrobial Studies on Different Lignins. Acta Biotechnol. 1989, 9, 295–298. [Google Scholar] [CrossRef]
- Durmaz, S.; Erisir, E.; Yildiz, U.C.; Kurtulus, O.C. Using Kraft Black Liquor as a Wood Preservative. Procedia Soc. Behav. Sci. 2015, 195, 2177–2180. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential Use of Kraft and Organosolv Lignins as a Natural Additive for Healthcare Products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef]
- Wang, G.; Pang, T.; Xia, Y.; Liu, X.; Li, S.; Parvez, A.M.; Kong, F.; Si, C. Subdivision of Bamboo Kraft Lignin by One-Step Ethanol Fractionation to Enhance Its Water-Solubility and Antibacterial Performance. Int. J. Biol. Macromol. 2019, 133, 156–164. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites Against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial Activity of Carvacrol toward Bacillus Cereus on Rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Roller, S.; Seedhar, P. Carvacrol and Cinnamic Acid Inhibit Microbial Growth in Fresh-Cut Melon and Kiwifruit at 4° and 8 °C. Lett. Appl. Microbiol. 2002, 35, 390–394. [Google Scholar] [CrossRef]
- Zemek, J.; Košíková, B.; Augustín, J.; Joniak, D. Antibiotic Properties of Lignin Components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef]
- Telysheva, G.; Sergeeva, V.; Gavare, L. Fungicidal Properties of Alkali Oxidation Destruction Products of Lignin. Latv. PSR Zinat. Akad. Vestis. Kim. Ser. 1968, 1, 117–122. [Google Scholar]
- De Greef, J.; Van Sumere, C. Effect of Phenolic Aldehydes, Coumarins and Related Compounds on the Growth of Saccharomyces Cerevisiae. Arch. Int. Physiol. Biochem. 1966, 74, 512. [Google Scholar]
- Baranowski, J.D.; Davidson, P.M.; Nagel, C.W.; Branen, A.L. Inhibition of Saccharomyces Cerevisiae by Naturally Occurring Hydroxycinnamates. J. Food Sci. 1980, 45, 592–594. [Google Scholar] [CrossRef]
- Barber, M.S.; McConnell, V.S.; DeCaux, B.S. Antimicrobial Intermediates of the General Phenylpropanoid and Lignin Specific Pathways. Phytochemistry 2000, 54, 53–56. [Google Scholar] [CrossRef]
- Sunthornvarabhas, J.; Liengprayoon, S.; Suwonsichon, T. Antimicrobial Kinetic Activities of Lignin from Sugarcane Bagasse for Textile Product. Ind. Crops Prod. 2017, 109, 857–861. [Google Scholar] [CrossRef]
- Nelson, J.L.; Alexander, J.W.; Gianotti, L.; Chalk, C.L.; Pyles, T. Influence of Dietary Fiber on Microbial Growth in Vitro and Bacterial Translocation after Burn Injury in Mice. Nutrition 1994, 10, 32–36. [Google Scholar]
- Baurhoo, B.; Letellier, A.; Zhao, X.; Ruiz-Feria, C.A. Cecal Populations of Lactobacilli and Bifidobacteria and Escherichia Coli Populations After In Vivo Escherichia Coli Challenge in Birds Fed Diets with Purified Lignin or Mannanoligosaccharides. Poult. Sci. 2007, 86, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, H.; Umezawa, T.; Yoshikawa, T.; Koyama, Y.; Fumoto, E.; Sato, S.; Nakasaka, Y.; Masuda, T. Antifungal Activity of Simply Fractionated Organosolv Lignin against Trametes Versicolor. J. Biotechnol. 2023, 364, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Sakagami, H.; Konno, K.; Sato, T.; Osawa, N.; Fujimaki, M.; Komatsu, N. Induction of Antimicrobial Activity by Antitumor Substances from Pine Cone Extract of Pinus Parviflora Sieb. et Zucc. Anticancer. Res. 1988, 8, 581–587. [Google Scholar] [PubMed]
- Oh-Hara, T.; Sakagami, H.; Kawazoe, Y.; Kaiya, T.; Komatsu, N.; Ohsawa, N.; Fujimaki, M.; Tanuma, S.; Konno, K. Antimicrobial Spectrum of Lignin-Related Pine Cone Extracts of Pinus Parviflora Sieb. et Zucc. Vivo 1990, 4, 7–12. [Google Scholar]
- Lai, P.K.; Donovan, J.; Takayama, H.; Sakagami, H.; Tanaka, A.; Konno, K.; Nonoyama, M. Modification of Human Immunodeficiency Viral Replication by Pine Cone Extracts. AIDS Res. Hum. Retroviruses 1990, 6, 205–217. [Google Scholar] [CrossRef]
- Sakagami, H.; Satoh, K.; Fukamachi, H.; Ikarashi, T.; Shimizu, A.; Yano, K.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Hasegawa, H.; et al. Anti-HIV and Vitamin C-Synergized Radical Scavenging Activity of Cacao Husk Lignin Fractions. Vivo 2008, 22, 327–332. [Google Scholar]
- Lee, E.; Song, Y.; Lee, S. Crosslinking of Lignin/Poly(Vinyl Alcohol) Nanocomposite Fiber Webs and Their Antimicrobial and Ultraviolet-Protective Properties. Text. Res. J. 2019, 89, 3–12. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K.; Sharma, P. Antioxidant and Antibacterial Activities of Sugarcane Bagasse Lignin and Chemically Modified Lignins. Sugar Tech 2017, 19, 675–680. [Google Scholar] [CrossRef]
- García, A.; Spigno, G.; Labidi, J. Antioxidant and Biocide Behaviour of Lignin Fractions from Apple Tree Pruning Residues. Ind. Crops Prod. 2017, 104, 242–252. [Google Scholar] [CrossRef]
- Rahouti, M.; Steiman, R.; Seigle-Murandi, F.; Christov, L.P. Growth of 1044 Strains and Species of Fungi on 7 Phenolic Lignin Model Compounds. Chemosphere 1999, 38, 2549–2559. [Google Scholar] [CrossRef]
- Coral Medina, J.D.; Woiciechowski, A.L.; Zandona Filho, A.; Bissoqui, L.; Noseda, M.D.; De Souza Vandenberghe, L.P.; Zawadzki, S.F.; Soccol, C.R. Biological Activities and Thermal Behavior of Lignin from Oil Palm Empty Fruit Bunches as Potential Source of Chemicals of Added Value. Ind. Crops Prod. 2016, 94, 630–637. [Google Scholar] [CrossRef]
- ASTM E 2149-10; Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents under Dynamic Contact Conditions. Astm International: West Conshohocken, PA, USA, 2020.
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials; Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-54959-9. [Google Scholar]
- Gonçalves, S.; Ferra, J.; Paiva, N.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Lignosulphonates as an Alternative to Non-Renewable Binders in Wood-Based Materials. Polymers 2021, 13, 4196. [Google Scholar] [CrossRef] [PubMed]
- Vainio, U.; Lauten, R.A.; Haas, S.; Svedström, K.; Veiga, L.S.I.; Hoell, A.; Serimaa, R. Distribution of Counterions around Lignosulfonate Macromolecules in Different Polar Solvent Mixtures. Langmuir 2012, 28, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Merianos, J. Quaternary Ammonium Antimicrobial Compounds. J Disinfect. 1991, 225–255. [Google Scholar]
- Russell, A.D.; Hugo, W.B. (Eds.) Principles and Practice of Disinfection, Preservation and Sterilisation; Blackwell: Oxford, UK, 1982; ISBN 978-0-632-00547-5. [Google Scholar]
- Lange, H.; Decina, S.; Crestini, C. Oxidative Upgrade of Lignin—Recent Routes Reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. BioRes 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Koljonen, K.; Österberg, M.; Kleen, M.; Fuhrmann, A.; Stenius, P. Precipitation of Lignin and Extractives on Kraft Pulp: Effect on Surface Chemistry, Surface Morphology and Paper Strength. Cellulose 2004, 11, 209–224. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, Binary and Multi-Component Adsorption of Copper and Cadmium from Aqueous Solutions on Kraft Lignin—A Biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef]
- Mansouri, N.-E.E.; Salvadó, J. Structural Characterization of Technical Lignins for the Production of Adhesives: Application to Lignosulfonate, Kraft, Soda-Anthraquinone, Organosolv and Ethanol Process Lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-Chemical Characterization of Lignins from Different Sources for Use in Phenol–Formaldehyde Resin Synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, H.-J.; Ramaswamy, S. Reaction Kinetics of the Hydrothermal Treatment of Lignin. Appl. Biochem. Biotechnol. 2008, 147, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass-Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2007; Volume PNNL-16983, pp. 29–30. [Google Scholar]
- Dizhbite, T. Characterization of the Radical Scavenging Activity of Lignins–Natural Antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Baurhoo, B.; Ruiz-Feria, C.A.; Zhao, X. Purified Lignin: Nutritional and Health Impacts on Farm Animals—A Review. Anim. Feed Sci. Technol. 2008, 144, 175–184. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E.; Berlin, A. Isolation and Analysis of Lignin–Carbohydrate Complexes Preparations with Traditional and Advanced Methods: A Review. In Stud. Nat. Prod. Chem. 2014, 42, 83–115. [Google Scholar] [CrossRef]
- Sakagami, H.; Kushida, T.; Oizumi, T.; Nakashima, H.; Makino, T. Distribution of Lignin–Carbohydrate Complex in Plant Kingdom and Its Functionality as Alternative Medicine. Pharmacol. Ther. 2010, 128, 91–105. [Google Scholar] [CrossRef]
- Abe, M.; Okamoto, K.; Konno, K.; Sakagami, H. Induction of Antiparasite Activity by Pine Cone Lignin-Related Substances. Vivo 1989, 3, 359–362. [Google Scholar]
- Lee, H.; Aoki, K.; Sakagami, H.; Yoshida, T.; Kuroiwa, Y. Interaction of Pine Cone Extract Fraction VI with Mutagens. Mutat. Res./Rev. Genet. Toxicol. 1993, 297, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Watanabe, S. Beneficial Effects of Mulberry on Human Health. In Phytotherapeutics and Human Health: Pharmacological and Molecular Aspects; Nova Science Publishers Inc: Hauppauge, NY, USA, 2011. [Google Scholar]
- Duval, A.; Lawoko, M. A Review on Lignin-Based Polymeric, Micro- and Nano-Structured Materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Nadif, A.; Hunkeler, D.; Kauper, P. Sulfur-Free Lignins from Alkaline Pulping Tested in Mortar for Use as Mortar Additives. Bioresour. Technol. 2002, 84, 49–55. [Google Scholar] [CrossRef]
- Cruz, R.; Dopico, D.; Figueredo, J.; Rodriguez, M.; Martinez, G. Uso de La Lignina de Bagazo Con Fines Medicinales. Rev. Peru. De Med. Exp. Y Salud Publica 1997, 14, 67–71. [Google Scholar]
- Xu, F.; Sun, J.-X.; Sun, R.; Fowler, P.; Baird, M.S. Comparative Study of Organosolv Lignins from Wheat Straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Ayyachamy, M.; Cliffe, F.E.; Coyne, J.M.; Collier, J.; Tuohy, M.G. Lignin: Untapped Biopolymers in Biomass Conversion Technologies. Biomass Conv. Bioref. 2013, 3, 255–269. [Google Scholar] [CrossRef]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of Purified Lignin and Mannan Oligosaccharides on Intestinal Integrity and Microbial Populations in the Ceca and Litter of Broiler Chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Marx, T.; Lora, J.; Phillip, L.E.; McAllister, T.A. Effects of Purified Lignin on in Vitro Ruminal Fermentation and Growth Performance, Carcass Traits and Fecal Shedding of Escherichia Coli by Feedlot Lambs. Anim. Feed Sci. Technol. 2009, 151, 21–31. [Google Scholar] [CrossRef]
- Ørskov, E.R.; Flatt, W.P.; Moe, P.W. Fermentation Balance Approach to Estimate Extent of Fermentation and Efficiency of Volatile Fatty Acid Formation in Ruminants. J. Dairy Sci. 1968, 51, 1429–1435. [Google Scholar] [CrossRef]
- Reay, D.; Smith, P.; van Amstel, A. (Eds.) Methane and Climate Change; Earthscan: London, UK; Washington, DC, USA, 2010; ISBN 978-1-84407-823-3. [Google Scholar]

| Technical Lignin | Pathogens Tested | Antimicrobial Test Method | Reference |
|---|---|---|---|
| Sodium lignosulfonate | Candida dubliniensis C. tropicalis C. albicans C. glabrata C. parasilopsis | MIC 1; Disk diffusion assay | [7] |
| Sodium lignosulfonate | D. hansenii Aspergillus niger Penicillium expansum | Disk diffusion assay | [30] |
| Sodium lignosulfonate; magnesium lignosulfonate; alkali kraft lignin; southern pine kraft lignin (LBKL); LBKL acetone-insoluble; | Aspergillus amoenus Mucor circinelloides Penicillium solitum Debaromyces hansenii | Broth antimicrobial assay; MIC at different pH levels | [31] |
| Sodium lignosulfonate | A. amoenus M. circinelloides P. solitum D. hansenii | MIC and MFC 2 | [32] |
| Lignosulfonate nanoparticles | Staphylococcus aureus Bacillus subtilis Escherichia coli | Turbidimetric method | [11] |
| Sodium lignosulfonate; magnesium lignosulfonate; alkali kraft lignin; LBKL | Streptococcus uberis Staphylococcus hyicus E. coli Klebsiella pneumoniae Pseudomonas aeruginosa | MIC and MBC 3 | [33] |
| Lignosulfonate | HIV 4 | Virus antigen expression; cytopathic effect evaluation; cell-to-cell infection; reverse transcriptase assay | [34] |
| Lignosulfonic acid | HIV HSV 5 | Virus replication assay; virus time-of-drug-addition assay; virus inactivation assay; in vivo antiviral activity in mice | [8] |
| Kraft lignins; soda lignins | E. coli Bacillus mycoides B. subtillis A. niger | Disk diffusion assay | [35] |
| Kraft black liquor | Coniophora puteana Poria placenta | Wood protection from fungal degradation | [36] |
| Alkali kraft lignin | Candida lipolytica S. aureus Listeria monocytogenes | MIC | [6] |
| Kraft spruce lignins; Kraft eucalyptus lignins | A. niger B. thuringiensis E. coli Enterobacter aerogenes Proteus microbilis P. vulgaris S. aureus | Fungal growth inhibition test; disk diffusion assay | [37] |
| Bamboo kraft lignin (BKL); BKL 95% ethanol soluble fraction; BKL 95% ethanol insoluble fraction | S. aureus B. subtilis E. coli Salmonella enterica | MIC; agar diffusion assay | [38] |
| BKL; BKL acetone fraction; BKL hexane fraction; BKL non-evaporated fraction | E. coli S. aureus Streptococcus pyogenes S. enterica | Agar diffusion assay; microdilution assay; extracellular protein assay; in vivo antimicrobial activity with mice | [29] |
| Kraft lignin fractions | S. aureus L. monocytogenes E. coli | Disk diffusion assay | [39] |
| Carvacrol | B. cereus | Antimicrobial activity in rice | [40] |
| Carvacrol; cinnamaldehyde | Spoilage microbial flora | Antimicrobial activity in melon and kiwifruit | [41] |
| Isoeugenol; ferulic acid | S. cerevisiae C. albicans A. niger | MIC | [42] |
| Vanillin; eugenol; cinnamaldehyde | Fusarium spp. | Antifungal activity | [43] |
| Ferulic acid | S. cerevisiae | Antimicrobial activity | [44] |
| Ferulic acid | S. cerevisiae | Antimicrobial activity | [45] |
| Hydroxycinnamaldehydes; hydroxycinnamic acids; hydroxycinnamyl alcohols | S. cerevisiae Schizosaccharomyces pombe Sporobolomyces roseus B. subtilis E. coli Pseudomonas syringae | MIC | [46] |
| Soda lignin | Staphylococcus epidermidis | MIC and MBC | [47] |
| Alcell lignin | Gut microflora | Antimicrobial activity in vitro; Antimicrobial activity in mice | [48] |
| Alcell lignin | E. coli Lactobacilli Bifidobacteria | Antimicrobial activities in broilers | [49] |
| n-hexane-soluble fraction of organosolv lignin | Trametes versicolor (white rot fungi) | Disk diffusion assay | [50] |
| Lignin–carbohydrate complexes | E. coli | Antimicrobial activity in mice | [51] |
| Lignin–carbohydrate complexes | S. aureus E. coli P. aeruginosa K. pneumoniae C. albicans S. enteriditis | Antimicrobial activity in mice | [52] |
| Lignin–carbohydrate complexes | HIV | Antiviral assay in cell lines | [53] |
| Lignin–carbohydrate complexes | HIV | Antiviral assay in cell lines | [54] |
| Lignin-based hydrogels | S. aureus Proteus mirabilis | Bacterial adherence resistance | [10] |
| Kraft lignin nanocomposite fibers | S. aureus E. coli | ASTM E 2149-10 6 | [55] |
| Kraft lignin nanoparticles | Pseudomonas syringae Xanthomonas axonopodis Xanthomonas arboricola | Spot diffusion assay; bacterial growth in broth | [28] |
| Acetylated, epoxy, and hydroxymethyl lignin | Bacillus aryabhattai Klebsiella spp. | Disk diffusion assay; MIC | [56] |
| Technical lignins obtained by autohydrolysis, organosolv treatment with acid, or ethanol, and soda hydrolysis | A. niger S. cerevisiae | Broth antifungal assay | [57] |
| Phenolic lignin compounds | 1044 strains and species of fungi | Growth inhibition assay | [58] |
| Lignin extracted with a sequential acid–alkaline pretreatment | C. albicans A. niger B. subtilis E. coli S. aureus | Disk diffusion assay Broth antimicrobial assay | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, D.C.; Ma, Z.; Romero, J.J. The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers 2024, 16, 2181. https://doi.org/10.3390/polym16152181
Reyes DC, Ma Z, Romero JJ. The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers. 2024; 16(15):2181. https://doi.org/10.3390/polym16152181
Chicago/Turabian StyleReyes, Diana Carolina, Zhengxin Ma, and Juan Jose Romero. 2024. "The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review" Polymers 16, no. 15: 2181. https://doi.org/10.3390/polym16152181
APA StyleReyes, D. C., Ma, Z., & Romero, J. J. (2024). The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers, 16(15), 2181. https://doi.org/10.3390/polym16152181






