The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review
Abstract
1. Introduction
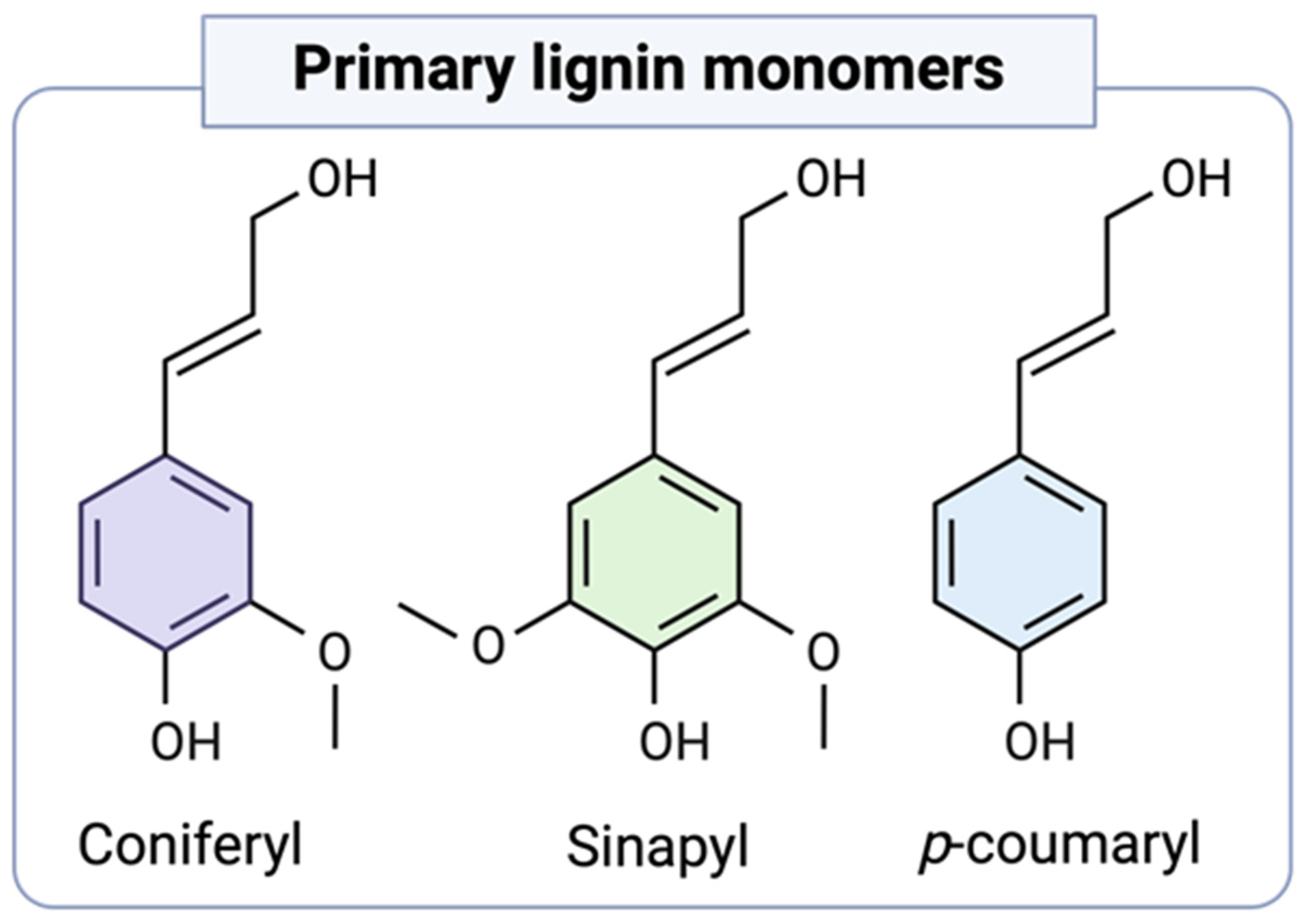
2. Native Lignin Chemical Properties
3. Technical Lignins and Their Antimicrobial Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cazacu, G.; Capraru, M.; Popa, V.I. Advances Concerning Lignin Utilization in New Materials. In Advances in Natural Polymers; Thomas, S., Visakh, P.M., Mathew, A.P., Eds.; Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2013; Volume 18, pp. 255–312. ISBN 978-3-642-20939-0. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; De Jong, E.; Guran, B.; Abächerli, A. Co-Ordination Network for Lignin—Standardisation, Production and Applications Adapted to Market Requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 978-0-444-59561-4. [Google Scholar]
- Lora, J. Industrial Commercial Lignins: Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 225–241. ISBN 978-0-08-045316-3. [Google Scholar]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and Antioxidant Activities of Lignin from Residue of Corn Stover to Ethanol Production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Jha, A.; Kumar, A. Deciphering the Role of Sodium Lignosulfonate against Candida spp. as Persuasive Anticandidal Agent. Int. J. Biol. Macromol. 2018, 107, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Férir, G.; D’huys, T.; Petrova, M.I.; Lebeer, S.; Snoeck, R.; Andrei, G.; Schols, D. The Low-Cost Compound Lignosulfonic Acid (LA) Exhibits Broad-Spectrum Anti-HIV and Anti-HSV Activity and Has Potential for Microbicidal Applications. PLoS ONE 2015, 10, e0131219. [Google Scholar] [CrossRef]
- Flickinger, E.A.; Campbell, J.M.; Schmitt, L.G.; Fahey, G.C. Selected Lignosulfonate Fractions Affect Growth Performance, Digestibility, and Cecal and Colonic Properties in Rats. J. Anim. Sci. 1998, 76, 1626. [Google Scholar] [CrossRef][Green Version]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fernandes, M.M.; Matamá, T.; Loureiro, A.; Gomes, A.C.; Cavaco-Paulo, A. Chitosan–Lignosulfonates Sono-Chemically Prepared Nanoparticles: Characterisation and Potential Applications. Colloids Surf. B Biointerfaces 2013, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural Polymers from Oxidative Coupling of 4-Hydroxyphenyl-Propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Hatfield, R.; Vermerris, W. Lignin Formation in Plants. The Dilemma of Linkage Specificity. Plant Physiol. 2001, 126, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Adler, E. Lignin Chemistry? Past, Present and Future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.S.; Menachem, S.B. Lignin. In Biotechnology in the Pulp and Paper Industry; Eriksson, K.-E.L., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.-O., Eriksson, K.-E.L., Fiechter, A., Klibanov, A.M., Mattiasson, B., Primrose, S.B., et al., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 1997; Volume 57, pp. 127–158. ISBN 978-3-540-61868-3. [Google Scholar]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Ralph, J.; Grabber, J.H.; Hatfield, R.D. Lignin-Ferulate Cross-Links in Grasses: Active Incorporation of Ferulate Polysaccharide Esters into Ryegrass Lignins. Carbohydr. Res. 1995, 275, 167–178. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-73843-5. [Google Scholar]
- Terashima, N.; Fukushima, K.; He, L.-F.; Takabe, K. Comprehensive Model of the Lignified Plant Cell Wall. In ASA, CSSA, and SSSA Books; Jung, H.G., Buxton, D.R., Hatfield, R.D., Ralph, J., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 247–270. ISBN 978-0-89118-238-2. [Google Scholar]
- Baucher, M.; Monties, B.; Montagu, M.V.; Boerjan, W. Biosynthesis and Genetic Engineering of Lignin. Crit. Rev. Plant Sci. 1998, 17, 125–197. [Google Scholar] [CrossRef]
- Cadix, A.; James, S. Cementing Additives. In Fluid Chemistry, Drilling and Completion; Elsevier: Amsterdam, The Netherlands, 2022; pp. 187–254. ISBN 978-0-12-822721-3. [Google Scholar]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Smook, G.; Kocurek, M. Handbook for Pulp & Paper Technologists; TAPPI Press: Atlanta, GA, USA, 1982. [Google Scholar]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications. BioRes 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Yun, J.; Wei, L.; Li, W.; Gong, D.; Qin, H.; Feng, X.; Li, G.; Ling, Z.; Wang, P.; Yin, B. Isolating High Antimicrobial Ability Lignin From Bamboo Kraft Lignin by Organosolv Fractionation. Front. Bioeng. Biotechnol. 2021, 9, 683796. [Google Scholar] [CrossRef]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Role of Lignosulphonate in Properties of Fish Gelatin Films. Food Hydrocoll. 2012, 27, 60–71. [Google Scholar] [CrossRef]
- Reyes, D.C.; Annis, S.L.; Rivera, S.A.; Leon-Tinoco, A.Y.; Wu, C.; Perkins, L.B.; Perry, J.J.; Ma, Z.X.; Knight, C.W.; Castillo, M.S.; et al. In Vitro Screening of Technical Lignins to Determine Their Potential as Hay Preservatives. J. Dairy Sci. 2020, 103, 6114–6134. [Google Scholar] [CrossRef]
- Leon-Tinoco, A.Y.; Annis, S.L.; Almeida, S.T.; Guimarães, B.C.; Killerby, M.; Zhang, J.; Wu, C.; Perkins, L.B.; Ma, Z.; Jeong, K.C.; et al. Evaluating the Potential of Lignosulfonates and Chitosans as Alfalfa Hay Preservatives Using in Vitro Techniques. J. Anim. Sci. 2022, 100, skac154. [Google Scholar] [CrossRef]
- Reyes, D.C.; Rivera, S.A.; Ma, Z.X.; Dubuc, H.M.; Leon-Tinoco, A.Y.; Lichtenwalner, A.B.; Romero, J.J. Mitigating Environmental Mastitis Microbes with the Novel Use of Paper Mill Byproducts. J. Dairy Sci. 2019, 102, 182–183. [Google Scholar]
- Suzuki, H.; Tochikura, T.S.; Iiyama, K.; Yamazaki, S.; Yamamoto, N.; Toda, S. Lignosulfonate, a Water-Solubilized Lignin from the Waste Liquor of the Pulping Process, Inhibits the Infectivity and Cytopathic Effects of Human Immunodeficiency Virus in Vitro. Agric. Biol. Chem. 1989, 53, 3369–3372. [Google Scholar] [CrossRef]
- Nada, A.M.A.; El-Diwany, A.I.; Elshafei, A.M. Infrared and Antimicrobial Studies on Different Lignins. Acta Biotechnol. 1989, 9, 295–298. [Google Scholar] [CrossRef]
- Durmaz, S.; Erisir, E.; Yildiz, U.C.; Kurtulus, O.C. Using Kraft Black Liquor as a Wood Preservative. Procedia Soc. Behav. Sci. 2015, 195, 2177–2180. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential Use of Kraft and Organosolv Lignins as a Natural Additive for Healthcare Products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef]
- Wang, G.; Pang, T.; Xia, Y.; Liu, X.; Li, S.; Parvez, A.M.; Kong, F.; Si, C. Subdivision of Bamboo Kraft Lignin by One-Step Ethanol Fractionation to Enhance Its Water-Solubility and Antibacterial Performance. Int. J. Biol. Macromol. 2019, 133, 156–164. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites Against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial Activity of Carvacrol toward Bacillus Cereus on Rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Roller, S.; Seedhar, P. Carvacrol and Cinnamic Acid Inhibit Microbial Growth in Fresh-Cut Melon and Kiwifruit at 4° and 8 °C. Lett. Appl. Microbiol. 2002, 35, 390–394. [Google Scholar] [CrossRef]
- Zemek, J.; Košíková, B.; Augustín, J.; Joniak, D. Antibiotic Properties of Lignin Components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef]
- Telysheva, G.; Sergeeva, V.; Gavare, L. Fungicidal Properties of Alkali Oxidation Destruction Products of Lignin. Latv. PSR Zinat. Akad. Vestis. Kim. Ser. 1968, 1, 117–122. [Google Scholar]
- De Greef, J.; Van Sumere, C. Effect of Phenolic Aldehydes, Coumarins and Related Compounds on the Growth of Saccharomyces Cerevisiae. Arch. Int. Physiol. Biochem. 1966, 74, 512. [Google Scholar]
- Baranowski, J.D.; Davidson, P.M.; Nagel, C.W.; Branen, A.L. Inhibition of Saccharomyces Cerevisiae by Naturally Occurring Hydroxycinnamates. J. Food Sci. 1980, 45, 592–594. [Google Scholar] [CrossRef]
- Barber, M.S.; McConnell, V.S.; DeCaux, B.S. Antimicrobial Intermediates of the General Phenylpropanoid and Lignin Specific Pathways. Phytochemistry 2000, 54, 53–56. [Google Scholar] [CrossRef]
- Sunthornvarabhas, J.; Liengprayoon, S.; Suwonsichon, T. Antimicrobial Kinetic Activities of Lignin from Sugarcane Bagasse for Textile Product. Ind. Crops Prod. 2017, 109, 857–861. [Google Scholar] [CrossRef]
- Nelson, J.L.; Alexander, J.W.; Gianotti, L.; Chalk, C.L.; Pyles, T. Influence of Dietary Fiber on Microbial Growth in Vitro and Bacterial Translocation after Burn Injury in Mice. Nutrition 1994, 10, 32–36. [Google Scholar]
- Baurhoo, B.; Letellier, A.; Zhao, X.; Ruiz-Feria, C.A. Cecal Populations of Lactobacilli and Bifidobacteria and Escherichia Coli Populations After In Vivo Escherichia Coli Challenge in Birds Fed Diets with Purified Lignin or Mannanoligosaccharides. Poult. Sci. 2007, 86, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, H.; Umezawa, T.; Yoshikawa, T.; Koyama, Y.; Fumoto, E.; Sato, S.; Nakasaka, Y.; Masuda, T. Antifungal Activity of Simply Fractionated Organosolv Lignin against Trametes Versicolor. J. Biotechnol. 2023, 364, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Sakagami, H.; Konno, K.; Sato, T.; Osawa, N.; Fujimaki, M.; Komatsu, N. Induction of Antimicrobial Activity by Antitumor Substances from Pine Cone Extract of Pinus Parviflora Sieb. et Zucc. Anticancer. Res. 1988, 8, 581–587. [Google Scholar] [PubMed]
- Oh-Hara, T.; Sakagami, H.; Kawazoe, Y.; Kaiya, T.; Komatsu, N.; Ohsawa, N.; Fujimaki, M.; Tanuma, S.; Konno, K. Antimicrobial Spectrum of Lignin-Related Pine Cone Extracts of Pinus Parviflora Sieb. et Zucc. Vivo 1990, 4, 7–12. [Google Scholar]
- Lai, P.K.; Donovan, J.; Takayama, H.; Sakagami, H.; Tanaka, A.; Konno, K.; Nonoyama, M. Modification of Human Immunodeficiency Viral Replication by Pine Cone Extracts. AIDS Res. Hum. Retroviruses 1990, 6, 205–217. [Google Scholar] [CrossRef]
- Sakagami, H.; Satoh, K.; Fukamachi, H.; Ikarashi, T.; Shimizu, A.; Yano, K.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Hasegawa, H.; et al. Anti-HIV and Vitamin C-Synergized Radical Scavenging Activity of Cacao Husk Lignin Fractions. Vivo 2008, 22, 327–332. [Google Scholar]
- Lee, E.; Song, Y.; Lee, S. Crosslinking of Lignin/Poly(Vinyl Alcohol) Nanocomposite Fiber Webs and Their Antimicrobial and Ultraviolet-Protective Properties. Text. Res. J. 2019, 89, 3–12. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K.; Sharma, P. Antioxidant and Antibacterial Activities of Sugarcane Bagasse Lignin and Chemically Modified Lignins. Sugar Tech 2017, 19, 675–680. [Google Scholar] [CrossRef]
- García, A.; Spigno, G.; Labidi, J. Antioxidant and Biocide Behaviour of Lignin Fractions from Apple Tree Pruning Residues. Ind. Crops Prod. 2017, 104, 242–252. [Google Scholar] [CrossRef]
- Rahouti, M.; Steiman, R.; Seigle-Murandi, F.; Christov, L.P. Growth of 1044 Strains and Species of Fungi on 7 Phenolic Lignin Model Compounds. Chemosphere 1999, 38, 2549–2559. [Google Scholar] [CrossRef]
- Coral Medina, J.D.; Woiciechowski, A.L.; Zandona Filho, A.; Bissoqui, L.; Noseda, M.D.; De Souza Vandenberghe, L.P.; Zawadzki, S.F.; Soccol, C.R. Biological Activities and Thermal Behavior of Lignin from Oil Palm Empty Fruit Bunches as Potential Source of Chemicals of Added Value. Ind. Crops Prod. 2016, 94, 630–637. [Google Scholar] [CrossRef]
- ASTM E 2149-10; Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents under Dynamic Contact Conditions. Astm International: West Conshohocken, PA, USA, 2020.
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials; Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-54959-9. [Google Scholar]
- Gonçalves, S.; Ferra, J.; Paiva, N.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Lignosulphonates as an Alternative to Non-Renewable Binders in Wood-Based Materials. Polymers 2021, 13, 4196. [Google Scholar] [CrossRef] [PubMed]
- Vainio, U.; Lauten, R.A.; Haas, S.; Svedström, K.; Veiga, L.S.I.; Hoell, A.; Serimaa, R. Distribution of Counterions around Lignosulfonate Macromolecules in Different Polar Solvent Mixtures. Langmuir 2012, 28, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Merianos, J. Quaternary Ammonium Antimicrobial Compounds. J Disinfect. 1991, 225–255. [Google Scholar]
- Russell, A.D.; Hugo, W.B. (Eds.) Principles and Practice of Disinfection, Preservation and Sterilisation; Blackwell: Oxford, UK, 1982; ISBN 978-0-632-00547-5. [Google Scholar]
- Lange, H.; Decina, S.; Crestini, C. Oxidative Upgrade of Lignin—Recent Routes Reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. BioRes 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Koljonen, K.; Österberg, M.; Kleen, M.; Fuhrmann, A.; Stenius, P. Precipitation of Lignin and Extractives on Kraft Pulp: Effect on Surface Chemistry, Surface Morphology and Paper Strength. Cellulose 2004, 11, 209–224. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, Binary and Multi-Component Adsorption of Copper and Cadmium from Aqueous Solutions on Kraft Lignin—A Biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef]
- Mansouri, N.-E.E.; Salvadó, J. Structural Characterization of Technical Lignins for the Production of Adhesives: Application to Lignosulfonate, Kraft, Soda-Anthraquinone, Organosolv and Ethanol Process Lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-Chemical Characterization of Lignins from Different Sources for Use in Phenol–Formaldehyde Resin Synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, H.-J.; Ramaswamy, S. Reaction Kinetics of the Hydrothermal Treatment of Lignin. Appl. Biochem. Biotechnol. 2008, 147, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass-Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2007; Volume PNNL-16983, pp. 29–30. [Google Scholar]
- Dizhbite, T. Characterization of the Radical Scavenging Activity of Lignins–Natural Antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Baurhoo, B.; Ruiz-Feria, C.A.; Zhao, X. Purified Lignin: Nutritional and Health Impacts on Farm Animals—A Review. Anim. Feed Sci. Technol. 2008, 144, 175–184. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E.; Berlin, A. Isolation and Analysis of Lignin–Carbohydrate Complexes Preparations with Traditional and Advanced Methods: A Review. In Stud. Nat. Prod. Chem. 2014, 42, 83–115. [Google Scholar] [CrossRef]
- Sakagami, H.; Kushida, T.; Oizumi, T.; Nakashima, H.; Makino, T. Distribution of Lignin–Carbohydrate Complex in Plant Kingdom and Its Functionality as Alternative Medicine. Pharmacol. Ther. 2010, 128, 91–105. [Google Scholar] [CrossRef]
- Abe, M.; Okamoto, K.; Konno, K.; Sakagami, H. Induction of Antiparasite Activity by Pine Cone Lignin-Related Substances. Vivo 1989, 3, 359–362. [Google Scholar]
- Lee, H.; Aoki, K.; Sakagami, H.; Yoshida, T.; Kuroiwa, Y. Interaction of Pine Cone Extract Fraction VI with Mutagens. Mutat. Res./Rev. Genet. Toxicol. 1993, 297, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Watanabe, S. Beneficial Effects of Mulberry on Human Health. In Phytotherapeutics and Human Health: Pharmacological and Molecular Aspects; Nova Science Publishers Inc: Hauppauge, NY, USA, 2011. [Google Scholar]
- Duval, A.; Lawoko, M. A Review on Lignin-Based Polymeric, Micro- and Nano-Structured Materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Nadif, A.; Hunkeler, D.; Kauper, P. Sulfur-Free Lignins from Alkaline Pulping Tested in Mortar for Use as Mortar Additives. Bioresour. Technol. 2002, 84, 49–55. [Google Scholar] [CrossRef]
- Cruz, R.; Dopico, D.; Figueredo, J.; Rodriguez, M.; Martinez, G. Uso de La Lignina de Bagazo Con Fines Medicinales. Rev. Peru. De Med. Exp. Y Salud Publica 1997, 14, 67–71. [Google Scholar]
- Xu, F.; Sun, J.-X.; Sun, R.; Fowler, P.; Baird, M.S. Comparative Study of Organosolv Lignins from Wheat Straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Ayyachamy, M.; Cliffe, F.E.; Coyne, J.M.; Collier, J.; Tuohy, M.G. Lignin: Untapped Biopolymers in Biomass Conversion Technologies. Biomass Conv. Bioref. 2013, 3, 255–269. [Google Scholar] [CrossRef]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of Purified Lignin and Mannan Oligosaccharides on Intestinal Integrity and Microbial Populations in the Ceca and Litter of Broiler Chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Marx, T.; Lora, J.; Phillip, L.E.; McAllister, T.A. Effects of Purified Lignin on in Vitro Ruminal Fermentation and Growth Performance, Carcass Traits and Fecal Shedding of Escherichia Coli by Feedlot Lambs. Anim. Feed Sci. Technol. 2009, 151, 21–31. [Google Scholar] [CrossRef]
- Ørskov, E.R.; Flatt, W.P.; Moe, P.W. Fermentation Balance Approach to Estimate Extent of Fermentation and Efficiency of Volatile Fatty Acid Formation in Ruminants. J. Dairy Sci. 1968, 51, 1429–1435. [Google Scholar] [CrossRef]
- Reay, D.; Smith, P.; van Amstel, A. (Eds.) Methane and Climate Change; Earthscan: London, UK; Washington, DC, USA, 2010; ISBN 978-1-84407-823-3. [Google Scholar]

| Technical Lignin | Pathogens Tested | Antimicrobial Test Method | Reference |
|---|---|---|---|
| Sodium lignosulfonate | Candida dubliniensis C. tropicalis C. albicans C. glabrata C. parasilopsis | MIC 1; Disk diffusion assay | [7] |
| Sodium lignosulfonate | D. hansenii Aspergillus niger Penicillium expansum | Disk diffusion assay | [30] |
| Sodium lignosulfonate; magnesium lignosulfonate; alkali kraft lignin; southern pine kraft lignin (LBKL); LBKL acetone-insoluble; | Aspergillus amoenus Mucor circinelloides Penicillium solitum Debaromyces hansenii | Broth antimicrobial assay; MIC at different pH levels | [31] |
| Sodium lignosulfonate | A. amoenus M. circinelloides P. solitum D. hansenii | MIC and MFC 2 | [32] |
| Lignosulfonate nanoparticles | Staphylococcus aureus Bacillus subtilis Escherichia coli | Turbidimetric method | [11] |
| Sodium lignosulfonate; magnesium lignosulfonate; alkali kraft lignin; LBKL | Streptococcus uberis Staphylococcus hyicus E. coli Klebsiella pneumoniae Pseudomonas aeruginosa | MIC and MBC 3 | [33] |
| Lignosulfonate | HIV 4 | Virus antigen expression; cytopathic effect evaluation; cell-to-cell infection; reverse transcriptase assay | [34] |
| Lignosulfonic acid | HIV HSV 5 | Virus replication assay; virus time-of-drug-addition assay; virus inactivation assay; in vivo antiviral activity in mice | [8] |
| Kraft lignins; soda lignins | E. coli Bacillus mycoides B. subtillis A. niger | Disk diffusion assay | [35] |
| Kraft black liquor | Coniophora puteana Poria placenta | Wood protection from fungal degradation | [36] |
| Alkali kraft lignin | Candida lipolytica S. aureus Listeria monocytogenes | MIC | [6] |
| Kraft spruce lignins; Kraft eucalyptus lignins | A. niger B. thuringiensis E. coli Enterobacter aerogenes Proteus microbilis P. vulgaris S. aureus | Fungal growth inhibition test; disk diffusion assay | [37] |
| Bamboo kraft lignin (BKL); BKL 95% ethanol soluble fraction; BKL 95% ethanol insoluble fraction | S. aureus B. subtilis E. coli Salmonella enterica | MIC; agar diffusion assay | [38] |
| BKL; BKL acetone fraction; BKL hexane fraction; BKL non-evaporated fraction | E. coli S. aureus Streptococcus pyogenes S. enterica | Agar diffusion assay; microdilution assay; extracellular protein assay; in vivo antimicrobial activity with mice | [29] |
| Kraft lignin fractions | S. aureus L. monocytogenes E. coli | Disk diffusion assay | [39] |
| Carvacrol | B. cereus | Antimicrobial activity in rice | [40] |
| Carvacrol; cinnamaldehyde | Spoilage microbial flora | Antimicrobial activity in melon and kiwifruit | [41] |
| Isoeugenol; ferulic acid | S. cerevisiae C. albicans A. niger | MIC | [42] |
| Vanillin; eugenol; cinnamaldehyde | Fusarium spp. | Antifungal activity | [43] |
| Ferulic acid | S. cerevisiae | Antimicrobial activity | [44] |
| Ferulic acid | S. cerevisiae | Antimicrobial activity | [45] |
| Hydroxycinnamaldehydes; hydroxycinnamic acids; hydroxycinnamyl alcohols | S. cerevisiae Schizosaccharomyces pombe Sporobolomyces roseus B. subtilis E. coli Pseudomonas syringae | MIC | [46] |
| Soda lignin | Staphylococcus epidermidis | MIC and MBC | [47] |
| Alcell lignin | Gut microflora | Antimicrobial activity in vitro; Antimicrobial activity in mice | [48] |
| Alcell lignin | E. coli Lactobacilli Bifidobacteria | Antimicrobial activities in broilers | [49] |
| n-hexane-soluble fraction of organosolv lignin | Trametes versicolor (white rot fungi) | Disk diffusion assay | [50] |
| Lignin–carbohydrate complexes | E. coli | Antimicrobial activity in mice | [51] |
| Lignin–carbohydrate complexes | S. aureus E. coli P. aeruginosa K. pneumoniae C. albicans S. enteriditis | Antimicrobial activity in mice | [52] |
| Lignin–carbohydrate complexes | HIV | Antiviral assay in cell lines | [53] |
| Lignin–carbohydrate complexes | HIV | Antiviral assay in cell lines | [54] |
| Lignin-based hydrogels | S. aureus Proteus mirabilis | Bacterial adherence resistance | [10] |
| Kraft lignin nanocomposite fibers | S. aureus E. coli | ASTM E 2149-10 6 | [55] |
| Kraft lignin nanoparticles | Pseudomonas syringae Xanthomonas axonopodis Xanthomonas arboricola | Spot diffusion assay; bacterial growth in broth | [28] |
| Acetylated, epoxy, and hydroxymethyl lignin | Bacillus aryabhattai Klebsiella spp. | Disk diffusion assay; MIC | [56] |
| Technical lignins obtained by autohydrolysis, organosolv treatment with acid, or ethanol, and soda hydrolysis | A. niger S. cerevisiae | Broth antifungal assay | [57] |
| Phenolic lignin compounds | 1044 strains and species of fungi | Growth inhibition assay | [58] |
| Lignin extracted with a sequential acid–alkaline pretreatment | C. albicans A. niger B. subtilis E. coli S. aureus | Disk diffusion assay Broth antimicrobial assay | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, D.C.; Ma, Z.; Romero, J.J. The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers 2024, 16, 2181. https://doi.org/10.3390/polym16152181
Reyes DC, Ma Z, Romero JJ. The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers. 2024; 16(15):2181. https://doi.org/10.3390/polym16152181
Chicago/Turabian StyleReyes, Diana Carolina, Zhengxin Ma, and Juan Jose Romero. 2024. "The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review" Polymers 16, no. 15: 2181. https://doi.org/10.3390/polym16152181
APA StyleReyes, D. C., Ma, Z., & Romero, J. J. (2024). The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers, 16(15), 2181. https://doi.org/10.3390/polym16152181






