Development of Interpolyelectrolyte Complex Based on Chitosan and Carboxymethylcellulose for Stabilizing Sandy Soil and Stimulating Vegetation of Scots Pine (Pinus sylvestris L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objects and Materials
2.2. Synthesis of IPEC
2.3. Gravimetric Analysis of Complex Formation
2.4. Turbidimetric Analysis of Complex Formation
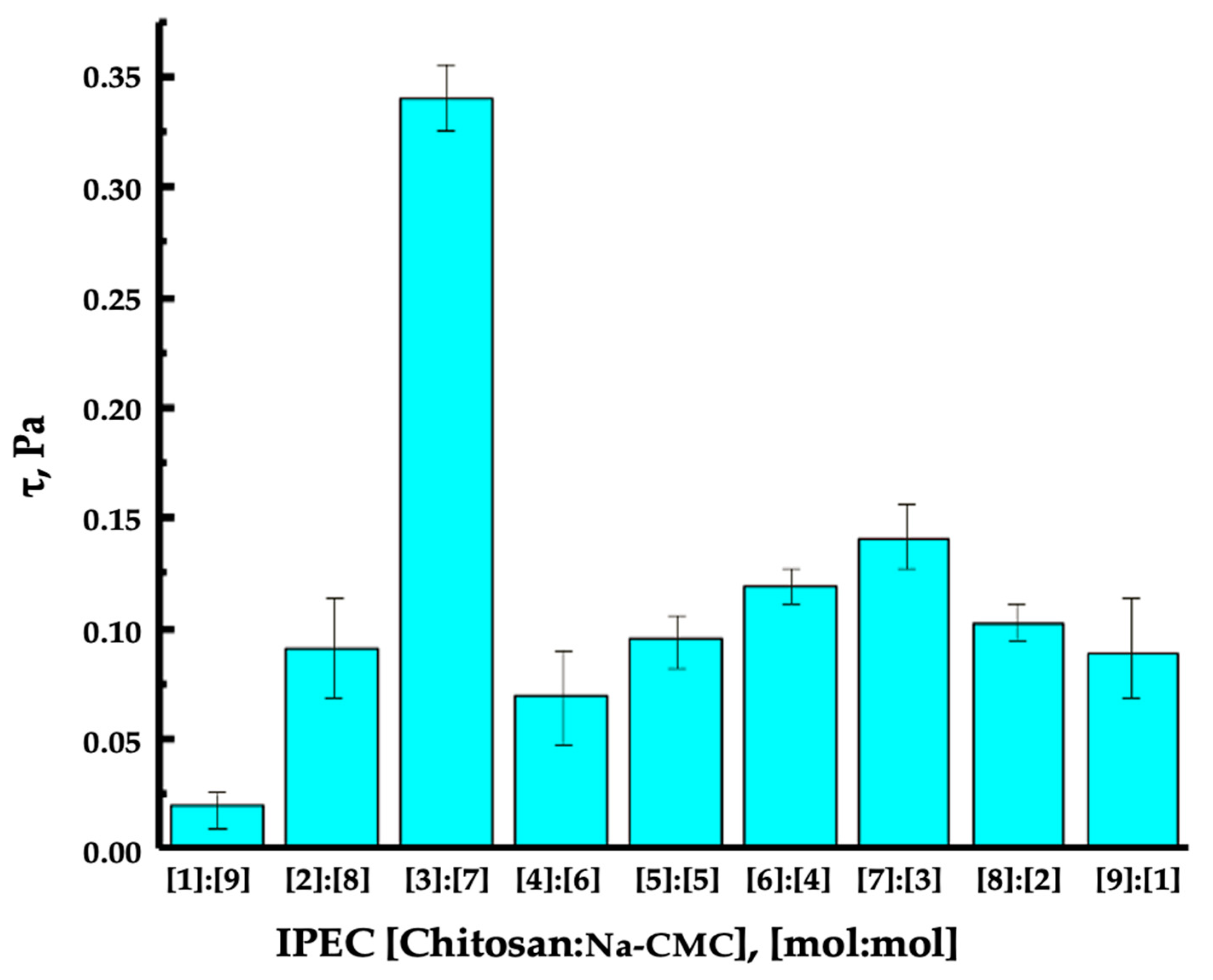
2.5. Rheoviscometric Analysis of Complex Formation
2.6. Soil Sampling and Preparation
2.7. Determination of Agrochemical Soil Indicators
2.8. Determination of the Mechanical Composition of the Soil
2.9. Microscopic Study of the Structure of Soil Aggregates after Treatment with Water and Application of IPEC
2.10. Modeling the Stability of Soil Aggregates against Wind and Water Erosion in Laboratory Conditions
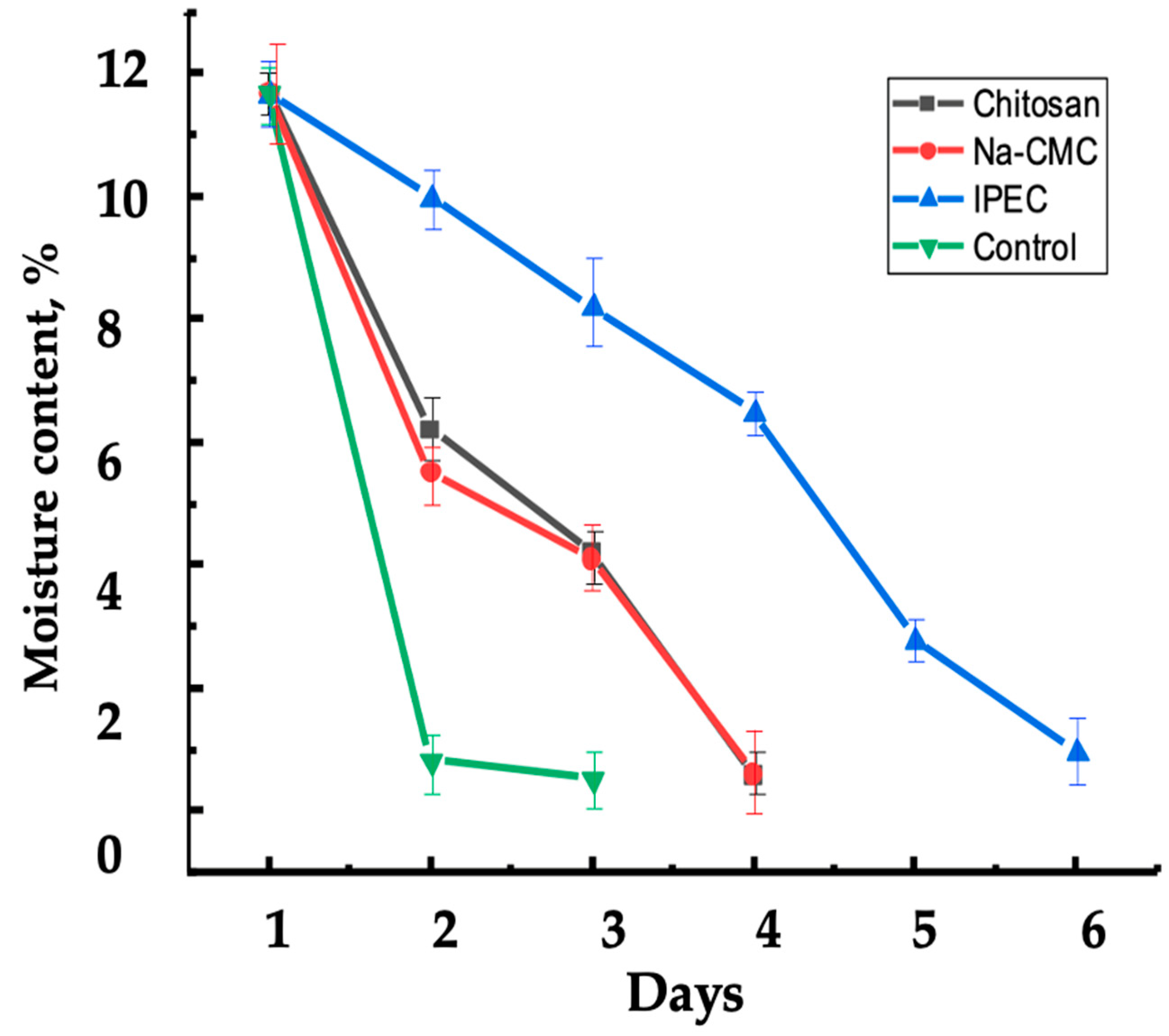
2.11. Determination of IPEC’s Effect on Soil Moisture Retention
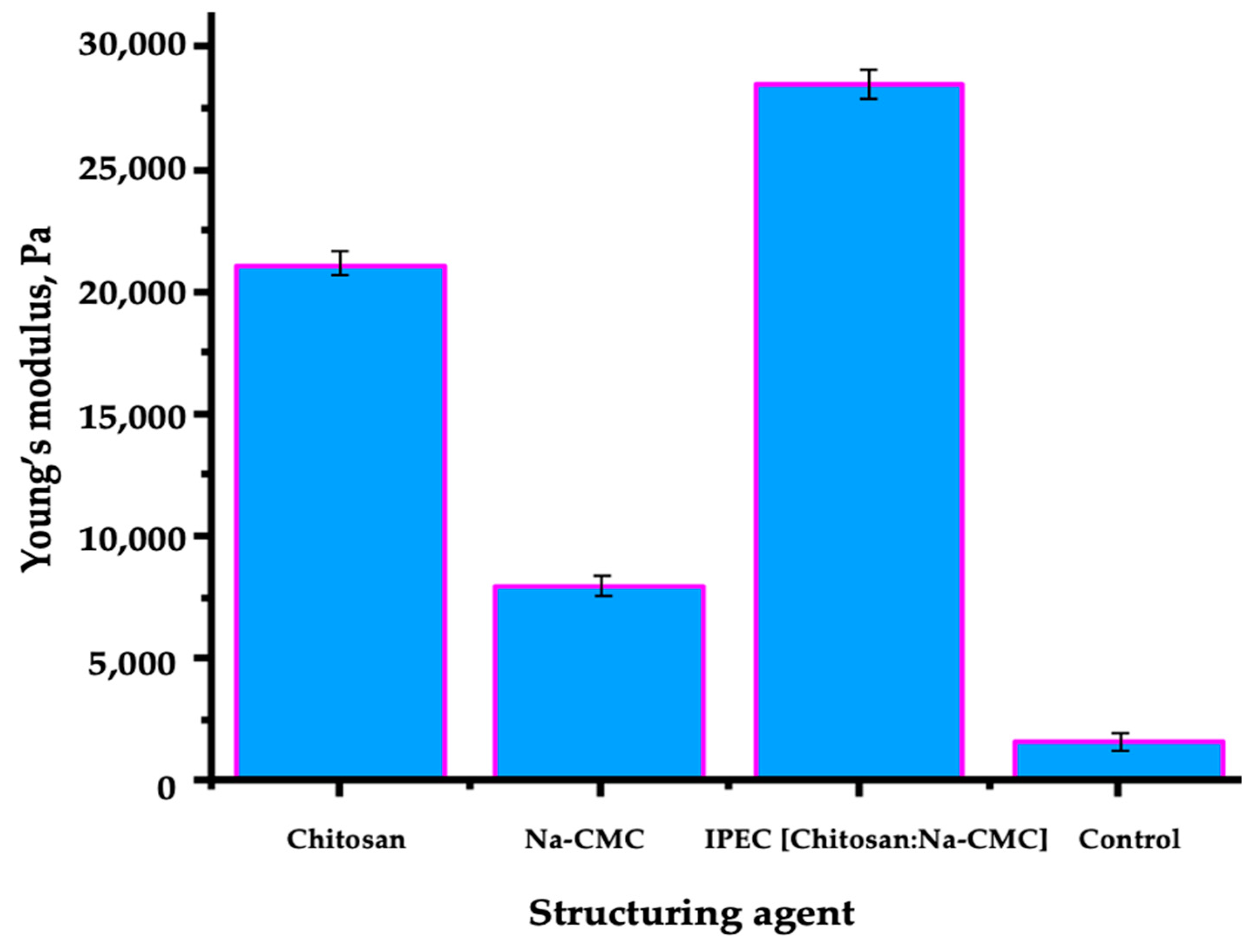
2.12. Determination of Soil Mechanical Strength
2.13. Study of IPEC Bioactivity Using Vegetation Method
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhumadina, S.; Chlachula, J.; Zhaglovskaya-Faurat, A.; Czerniawska, J.; Satybaldieva, G.; Nurbayeva, N.; Mapitov, N.; Myrzagaliyeva, A.; Boribay, E. Environmental dynamics of the ribbon-like pine forests in the parklands of North Kazakhstan. Forests 2022, 13, 2. [Google Scholar] [CrossRef]
- Abayeva, K.T.; Rakhimzhanova, G.M.; Myrzabayeva, G.; Zhilkibayeva, E.; Beisekeyeva, A. The Relevance of Sustainable Development of Forest Resource Reproduction in Kazakhstan. Evergreen 2024, 11, 46–55. [Google Scholar] [CrossRef]
- Gutiérrez-Flores, L.; López-Reyes, L.; Hipólito-Romero, E.; Torres-Ramírez, E.; Castañeda-Roldán, E.; Mauricio-Gutiérrez, A. Biological control perspectives in the pine forest (Pinus spp.), an environmentally friendly alternative to the use of pesticides. Mex. J. Phytopathol. 2022, 40, 401–424. [Google Scholar] [CrossRef]
- Adla, K.; Dejan, K.; Neira, D.; Dragana, S. Degradation of ecosystems and loss of ecosystem services. In One Health: Integrated Approach to 21st Century Challenges to Health; Academic Press: Cambridge, MA, USA, 2022; pp. 281–327. [Google Scholar] [CrossRef]
- Abayeva, K.T.; Toktassynova, F.A.; Igembaeva, A.K.; Serikbayeva, A.T.; Rakhimzhanova, G.M. Modern trends in the development of forestry enterprises in Kazakhstan. CAB Rev. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Skutsch, M.; Paneque-Galvez, J.; Ghilardi, A. Remote sensing of forest degradation: A review. Environ. Res. Lett. 2020, 15, 2–18. [Google Scholar] [CrossRef]
- Chlachula, J.; Zhagloskaya, A. Environmental aspects of the black saxaul forests’ distribution in the cold semi-deserts of central asia. SGEM 2017, 17, 821–828. [Google Scholar]
- The Nature of Semipalatinsk. Available online: https://silkadv.com/en/content/semey-ormany-reserve (accessed on 1 June 2024).
- Sibirkina, A.R. Beryllium content in the organs of Pinus sylvestris L. in the belt coniferous forests near the Irtysh River in the republic of Kazakhstan. Contemp. Probl. Ecol. 2012, 5, 208–214. [Google Scholar] [CrossRef]
- Zhensikbayeva, N.Z.; Kabdrakhmanova, N.K.; Yeginbayeva, A.Y.; Beisembayeva, R.S.; Amangeldy, N. Assessment of forest fires factors in eastern Kazakhstan over the last 20 years (2003–2023) using gis technologies. GeoJournal Tour. Geosites 2023, 51, 1803–1811. [Google Scholar] [CrossRef]
- Kazakhstan: Area Burnt by Forest Fires 2023. Available online: https://www.statista.com/statistics/1402205/area-burnt-by-forest-fires-in-kazakhstan/ (accessed on 1 June 2024).
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science:A global perspective. Front Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Gould, N.; Reglinski, T.; Northcott, G.L.; Spiers, M.; Taylor, J.T. Physiological and biochemical responses in Pinus radiata seedlings associated with methyl jasmonate-induced resistance to Diplodia pinea. Physiol. Mol. Plant Pathol. 2009, 74, 121–128. [Google Scholar] [CrossRef]
- Andreeva, E.M.; Stetsenko, S.K.; Terekhov, G.G.; Basistov, D. The agrotechnical measures influence to the growth and state of the 1-year-old pine seedlings root system in forest nursery by soil contamination with pesticides. For. Eng. J. 2022, 12, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, N.N.; Little, K.; Baillie, B.; Rolando, C. An evaluation of the environmental behaviour, fate and risk of key pesticides used in South African forest plantations. South. For. 2022, 84, 83–92. [Google Scholar] [CrossRef]
- Stetsenko, S.K.; Andreeva, E.M.; Terekhov, G.G.; Hurshkainen, T.V.; Kuchin, A.V. On the Regulation of the Joint Use of Growth Stimulants and Pesticides in Forest Growing. Ecol. Ind. Russ. 2019, 23, 66–71. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Chouard, P. Introduction to the knowledge of the mechanism of teratogenesis in plants, Introduction a la connaissance des mécanismes de la teratogenese chez les plantes. Rev. Pathol. Gen. Physiol. Clin. 1953, 53, 1003–1040. [Google Scholar]
- Freiberg, I.A.; Stetsenko, S.K. Teratogenesis of pine seedlings as bioindicator of soil contamination in forest nurseries by pesticides. J. Sci. Rev. Biol. Sci. 2015, 1, 167–168. [Google Scholar]
- Lee, H.; Lim, S.; Paik, H. An assessment of fire-damaged forest using spatial analysis techniques. J. Spat. Sci. 2010, 55, 289–301. [Google Scholar] [CrossRef]
- Halofsky, J.E.; Andrews-Key, S.A.; Edwards, J.E.; Johnston, M.H.; Nelson, H.W.; Peterson, D.L.; Schmitt, K.M.; Swanston, C.W.; Williamson, T.B. Adapting Forest management to climate change: The state of science and applications in Canada and the United States. For. Ecol. Manag. 2018, 94, 84–97. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Shin, H.-D.; Cho, G.-C. Soil treatment using microbial biopolymers for anti-desertification purposes. Geoderma 2015, 253, 39–47. [Google Scholar] [CrossRef]
- Yost, J.L.; Hartemink, A.E. Soil organic carbon in sandy soils: A review. Adv. Agron. 2019, 158, 217–310. [Google Scholar] [CrossRef]
- Huang, J.; Hartemink, A.E. Soil and environmental issues in sandy soils. Earth Sci. Rev. 2020, 208, 103295. [Google Scholar] [CrossRef]
- Falah, M.W.; Muteb, H. Applying different soil stabilization mechanisms: A review. Arch. Civ. Eng. 2023, 69, 339–358. [Google Scholar] [CrossRef]
- Ouyang, W.; Skidmore, A.K.; Hao, F.; Wang, T. Soil erosion dynamics response to landscape pattern. Sci. Total Environ. 2010, 408, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Panova, I.G.; Ilyasov, L.O.; Yaroslavov, A.A. Polycomplex Formulations for the Protection of Soils against Degradation. Polym. Sci. Ser. C 2021, 3, 237–248. [Google Scholar] [CrossRef]
- Malachovskienė, E.; Bridžiuvienė, D.; Ostrauskaitė, J.; Vaičekauskaitė, J.; Žalūdienė, G. A Comparative Investigation of the Biodegradation Behaviour of Linseed Oil-Based Cross-Linked Composites Filled with Industrial Waste Materials in Two Different Soils. J. Renew. Mater. 2023, 11, 1255–1269. [Google Scholar] [CrossRef]
- Takahashi, M.; Kosaka, I.; Ohta, S. Water Retention Characteristics of Superabsorbent Polymers (SAPs) Used as Soil Amendments. Soil Syst. 2023, 7, 58. [Google Scholar] [CrossRef]
- Inbar, A.; Ben-Hur, M.; Sternberg, M.; Lado, M. Using polyacrylamide to mitigate post-fire soil erosion. Geoderma 2015, 239, 107–114. [Google Scholar] [CrossRef]
- Coello, J.; Ameztegui, A.; Rovira, P.; Fuentes, C.; Piqué, M. Innovative soil conditioners and mulches for forest restoration in semiarid conditions in northeast Spain. Ecol. Eng. 2018, 118, 52–65. [Google Scholar] [CrossRef]
- Smagin, A.V.; Sadovnikova, N.B.; Belyaeva, E.A.; Krivtsova, V.N.; Shoba, S.A.; Smagina, M.V. Gel-Forming Soil Conditioners of Combined Action: Field Trials in Agriculture and Urban Landscaping. Polymers 2022, 14, 5131. [Google Scholar] [CrossRef]
- Shariatmadari, N.; Reza, M.; Tasuji, A.; Ghadir, P.; Javadi, A.A. Experimental study on the effect of chitosan biopolymer on sandy soil stabilization. In Proceedings of the 4th European Conference on Unsaturated Soils, Lisboa, Portugal, 19–21 October 2020. [Google Scholar] [CrossRef]
- Korbecka-Glinka, G.; Piekarska, K.; Wiśniewska-Wrona, M. The Use of Carbohydrate Biopolymers in Plant Protection against Pathogenic Fungi. Polymers 2022, 14, 2854. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Cheng, Y.; Jiang, T.; Sun, D.; Saberian, M. Water-retention behaviour and microscopic analysis of two biopolymer-improved sandy soils. Constr. Build Mater. 2023, 403, 133202. [Google Scholar] [CrossRef]
- Islam, M.S.; Rashid, N.; Dev, B.; Sayeed, Y.; Alam, R.; Mahmud, R.U.; Rahman, M.Z. Progress in sustainable applications of polymers and biopolymers. Ref. Modul. Mater. Sci. Mater. Eng. 2024, 13, 523–554. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 2, 569–588. [Google Scholar] [CrossRef]
- Novoskoltseva, O.A.; Belov, A.A.; Loiko, N.G.; Nikolaev, Y.A.; Panova, I.G.; Yaroslavov, A.A. Biodegradable Interpolycomplexes for Anti-Erosion Stabilization of Soil and Sand. Polymers 2020, 14, 5383. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting sustainability of fungiside seed treatments for field crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Bello, S.K. Biological control of soil-borne pathogens in arid lands: A review. J. Plant Dis. Prot. 2024, 131, 293–313. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Zhang, P.; Zhou, J.; Lu, X.; Tian, W. Carbohydrate polymers exhibit great potential as effective elicitors in organic agriculture: A review. J. Carbohydr. Polym. 2020, 230, 115637. [Google Scholar] [CrossRef]
- Mutchler, C.K.; Murphree, C.E.; McGregor, K.C. Laboratory and field plots for erosion research. In Soil Erosion Research Methods; CRC Press: Boca Raton, FL, USA, 2017; pp. 11–38. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Cho, G.C. Soil strengthening using thermo-gelation biopolymers. Constr. Build. Mater. 2015, 77, 430–438. [Google Scholar] [CrossRef]
- Ilman, B.; Balkis, A.P. Review on Biopolymer Binders as Renewable, Sustainable Stabilizers for Soils. Int. J. Geosynth. Ground Eng. 2023, 9, 49. [Google Scholar] [CrossRef]
- Ham, S.-M.; Chang, I.; Noh, D.-H.; Kwon, T.-H.; Muhunthan, B. Improvement of surface erosion resistance of sand by microbial biopolymer formation. J. Geotech. Geoenviron. Eng. 2018, 144, 06018004. [Google Scholar] [CrossRef]
- Amiri Tasuji, M.; Ghadir, P.; Hosseini, A.; Javadi, A.A.; Habibnejad Korayem, A.; Ranjbar, N. Experimental investigation of sandy soil stabilization using chitosan biopolymer. Transp. Geotech. 2024, 46, 101266. [Google Scholar] [CrossRef]
- Wang, S.; Wei, D.; Yang, X.; Song, S.; Sun, L.; Xin, X.; Zheng, G.; Wang, R.; Liu, L.L.; Sun, J.; et al. Study on a new type of environment-friendly polymer and its preliminary application as soil consolidation agent during tree transplanting. Sci. Rep. 2021, 11, 5575. [Google Scholar] [CrossRef]
- Hataf, N.; Ghadir, P.; Ranjbar, N. Investigation of soil stabilization using chitosan biopolymer. J. Clean. Prod. 2019, 170, 1493–1500. [Google Scholar] [CrossRef]
- Aguilar, R.; Nakamatsu, J.; Ramírez, E.; Elgegren, M.; Ayarza, J.; Kim, S.; Pando, M.A.; Ortega-San-Martin, L. The potential use of chitosan as a biopolymer additive for enhanced mechanical properties and water resis- tance of earthen construction. Constr. Build. Mater. 2016, 114, 625–637. [Google Scholar] [CrossRef]
- Gumilar, T.A.; Prihastanti, E.; Haryanti, S.; Subagio, A.; Ngadiwiyana, A. Utilization of waste silica and chitosan as fertilizer nano chisil to improve corn production in Indonesia. Adv. Sci. Lett. 2017, 23, 2447–2449. [Google Scholar] [CrossRef]
- Yakimenko, O.S.; Ziganshina, A.R.; Stepanov, A.A.; Panova, I.G.; Yaroslavov, A.A. Effect of Polyelectrolytes on Soil Organic Matter in Model Experiments. Eurasian Soil Sci. 2022, 55, 988–997. [Google Scholar] [CrossRef]
- Kassymova, Z.S.; Orazzhanova, L.K.; Klivenko, A.N.; Mussabayeva, B.K.; Aserzhanov, D.K. Preparation and properties of Interpolymer complexes capable of soil structuring. Russ. J. Appl. Chem. 2019, 92, 208–217. [Google Scholar] [CrossRef]
- Orazzhanova, L.K.; Kassymova, Z.S.; Mussabayeva, B.K.; Klivenko, A.N. Soil Structuring in the Presence of the Chitosan–Polyacrylic Acid Interpolymer Complex. Eurasian Soil Sci. 2020, 53, 1773–1781. [Google Scholar] [CrossRef]
- Kannan, G.; O’Kelly, B.C.; Sujatha, E.R. Effect of chitin, chitosan and NaCMC biopolymers on the consistency limits of organic silt. Int. J. Environ. Sci. Technol. 2024, 21, 2121–2128. [Google Scholar] [CrossRef]
- Klivenko, A.; Orazzhanova, L.; Mussabayeva, B.; Yelemessova, G.; Kassymova, Z. Soil structuring using interpolyelectrolyte complexes of water-soluble polysaccharides. Polym. Adv. Technol. 2020, 31, 3292–3301. [Google Scholar] [CrossRef]
- Nghiem, D.T.; Nguyen, T.C.; Do, M.T.; Lam, T.H.; Tran, D.H.; Tran-Dung, L.; Le, V.Q.; Vu, Q.T.; Nguyen, D.T.; Thai, H. Influence of the Preparation Method on Some Characteristics of Alginate/Chitosan/Lovastatin Composites. Adv. Polym. Technol. 2020, 12, 7879368. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Keldibekova, R.; Suleimenova, S.; Nurgozhina, G.; Kopishev, E. Interpolymer Complexes Based on Cellulose Ethers: Application. Polymers 2023, 15, 3326. [Google Scholar] [CrossRef] [PubMed]
- Izumrudov, V.A.; Mussabayeva, B.K.; Kassymova, Z.S.; Klivenko, A.N.; Orazzhanova, L.K. Interpolyelectrolyte complexes: Advances and prospects of application. Russ. Chem. Rev. 2019, 88, 1046–1062. [Google Scholar] [CrossRef]
- Zinchenko, A.; Sakai, T.; Morikawa, K.; Nakano, M. Efficient stabilization of soil, sand, and clay by a polymer network of biomass-derived chitosan and carboxymethyl cellulose. J. Environ. Chem. Eng. 2022, 10, 107084. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.H. Review on interfacial bonding mechanism of functional polymer coating on glass in atomistic modeling perspective. Polymers 2021, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Bekturov, E.A.; Kudaibergenov, S.E.; Rafikov, S.R. The properties of solutions and complex formation reactions of amphoteric polyelectrolytes. Russ. Chem. Rev. 1991, 60, 410–419. [Google Scholar] [CrossRef]
- Inagamov, S.Y.; Mukhamedov, G.I. Structure and physical-mechanical properties of interpolymeric complexes based on sodium carboxymethylcellulose. J. Appl. Polym. Sci. 2011, 122, 1749–1757. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Nurhat, N. Intra- and Interpolyelectrolyte Complexes of Polyampholytes. Polymers 2018, 10, 3390. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Kabanov, V.A. Interpolyelectrolyte and block ionomer complexes for gene delivery: Physico-chemical aspects. Adv. Drug Deliv. Rev. 1998, 30, 49–60. [Google Scholar] [CrossRef]
- Panova, I.G.; Sybachin, A.V.; Spiridonov, V.V.; Kydralieva, K.; Jorobekova, S.; Zezin, A.B.; Yaroslavov, A.A. Non-stoichiometric interpolyelectrolyte complexes: Promising candidates for protection of soils. Geoderma 2017, 307, 91–97. [Google Scholar] [CrossRef]
- Panova, I.G.; Demidov, V.V.; Shulga, P.S.; Ilyasov, L.O.; Butilkina, M.A.; Yaroslavov, A.A. Interpolyelectrolyte. complexes as effective structure-forming agents for Chernozem soil. LDD 2020, 32, 1022–1033. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A state-of-the-art review of polymers used in soil stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Israelachvili, J. Chapter 13—Van der Waals Forces between Particles and Surfaces. In Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 253–289. [Google Scholar]
- Feldstein, M.M.; Dormidontova, E.E.; Khokhlov, A.R. Pressure sensitive adhesives based on interpolymer complexes. Prog. Polym. Sci. 2015, 42, 79–153. [Google Scholar] [CrossRef]
- Kojima, A.; Yamada, K.; Fujii, T.; Hirata, M. Improvement in adhesive-free adhesion by the use of electrostatic interactions between polymer chains grafted onto polyethylene plates. J. Appl. Polym. Sci. 2006, 101, 2632–2638. [Google Scholar] [CrossRef]
- Mussabayeva, B.K.; Kassymova, Z.S.; Orazzhanova, L.K.; Klivenko, A.N.; Sabitova, A.N.; Bayakhmetova, B.B. Interpolyelectrolyte Complex Chitosan—Alginate for Soil Structuring. Bull. Univ. Karaganda Chem. 2022, 107, 102–114. [Google Scholar] [CrossRef]
- Zezin, A.; Mikheikin, S.V.; Rogacheva, V.B.; Zansokhova, M.F.; Sybachin, A.V.; Yaroslavov, A.A. Polymeric stabilizers for protection of soil and ground against wind and water erosion. Adv Colloid Interf. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Aidarova, S.; Bekturganova, N.; Kerimkulova, M.; Musabekov, K.; Sharipova, A. Structure formation of the surface layer of soil as a way to prevent a wind and water erosion. Eurasian Chem.-Technol. J. 2012, 14, 321–325. [Google Scholar] [CrossRef]
- Available online: https://weatherandclimate.com/kazakhstan/east-kazakhstan-region/semey# (accessed on 1 May 2024).
- Moussa, I.; Khiari, R.; Moussa, A.; Belgacem, N.; Mhenni, M.F. Preparation and Characterization of Carboxymethyl Cellulose with a High Degree of Substitution from Agricultural Wastes. Fibers Polym. 2019, 20, 933–943. [Google Scholar] [CrossRef]
- Markin, V.I.; Bazarnova, N.G.; Kolosov, P.V.; Cherpasova, M.Y.; Moskova, Y.S. Carboxymethylation of pine wood subjected to microwave irradiation after pretreatment in an “acetic acid-hydrogen peroxide-water-catalyst” system. Russ. J. Bioorg. Chem. 2013, 39, 699–703. [Google Scholar] [CrossRef]
- Tsitson, T.K. Seed quality characteristics of Pinus halepensis—Seed germination strategy and early seedling growth. Web Ecol. 2009, 9, 72–76. [Google Scholar] [CrossRef]
- Schramm, G. Fundamentals of Practical Rheology and Rheometry, 3rd ed.; Moscow: KolosS, Russia, 2003; p. 312. [Google Scholar]
- Kim, H.T. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 77–89. [Google Scholar] [CrossRef]
- Akimzhanova, K.G.; Sabitova, A.N.; Mussabayeva, B.K.; Bayakhmetova, B.B. Inorganic Composition and Physico-Chemical Properties of the Peloid of the Salt Lake Moiyldy (Kazakhstan) As a Natural Source of Biologically Active Substances. Chem. Eng. Trans. 2023, 103, 433–438. [Google Scholar] [CrossRef]
- Kapar, B.; Kurmangozhinov, A.; Osserkhan, B.; Ayan, S. The effect of different biological products applied with different doses and exposure time on the germination of Scots pine seeds. Casp. J. Environ. Sci. 2024, 22, 23–29. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Smyslov, R.Y.; Yakimansky, A.V. Modern Methods for Studying Polymer Complexes in Aqueous and Organic Solutions. Polym. Sci. Ser. A 2018, 60, 553–576. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Malkin, A.Y. The Role of Structure in Polymer Rheology: Review. Polymers 2022, 14, 1262. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.; Vasilyev, G.; Vilensky, R.; Cohen, Y.; Zussman, E. Structure and Rheology of Polyelectrolyte Complexes in the Presence of a Hydrogen-Bonded Co-Solvent. Polymers 2019, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, D.D. Effect of superabsorbent polymer on soil and plants on steep surfaces. Water Environ. J. 2018, 32, 158–163. [Google Scholar] [CrossRef]
- Situ, Y.; Yang, Y.; Huang, C.; Liang, S.; Mao, X.; Chen, X. Effects of several superabsorbent polymers on soil exchangeable cations and crop growth. Environ. Technol. Innov. 2023, 30, 103126. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef]
- Jafarzadeh-Haghighi, A.H.; Shamshuddin, J.; Hamdan, J.; Zainuddin, N. Structural composition of organic matter in particle-size fractions of soils along a climo-biosequence in the main range of Peninsular Malaysia. Open Geosci. 2016, 8, 503–513. [Google Scholar] [CrossRef]
- Ayeldeen, M.; Negm, A.; El Sawwaf, M.; Gädda, T. Laboratory study of using biopolymer to reduce wind erosion. Int. J. Geotech. Eng. 2018, 12, 228–240. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Briggs, S.P.H.; Knox, J.P. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003, 26, 977–989. [Google Scholar] [CrossRef]
- Novoskoltseva, O.A.; Loiko, N.G.; Nikolaev, Y.A.; Lisin, A.O.; Panova, I.G.; Yaroslavov, A.A. Interpolyelectrolyte complexes based on hydrolyzed polyacrylonitrile for anti-erosion stabilization of soils and ground. Polym. Int. 2022, 71, 697–705. [Google Scholar] [CrossRef]
- Panova, I.; Shevaleva, E.; Gritskova, I.; Arzhakov, M.; Yaroslavov, A. Mixtures of Cationic Linear Polymer and Anionic Polymeric Microspheres for Stabilization of Sand: Physicochemical, Structural and Mechanical Study. Appl. Sci. 2022, 13, 4311. [Google Scholar] [CrossRef]
- Novoskoltseva, O.A.; Panova, I.G.; Loiko, N.G.; Nikolaev, Y.A.; Litmanovich, E.A.; Yaroslavov, A.A. Polyelectrolytes and Polycomplexes for Stabilizing Sandy Grounds. Polym. Sci. Ser. B 2021, 63, 488–495. [Google Scholar] [CrossRef]
- Jacob, M.E.; Nair, D.S.; Sreekala, G.S.; Anith, K.N.; Alex, S.; Sajitha Rani, T.; Viji, M.M. Chitosan seed priming enhanced germination and seedling growth in Ashwagandha (Withania somnifera L.) Dunal. Med. Plants 2024, 1, 119–127. [Google Scholar] [CrossRef]
- Akinshina, N.G.; Rashidova, D.K.; Azizov, A.A. Seed encapsulation in chitosan and its derivatives restores levels of chlorophyll and photosynthesis in wilt-affected cotton (Gossypium L., 1753) plants. Agric. Biol. 2016, 51, 696–704. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcinska, M.; Bogusiewicz, A.; Szkop, M.; Drozdowski, S. Effect of chitosan on disease control and growth of scots pine (Pinus sylvestris L.) in a forest nursery. Forests 2015, 6, 3165–3176. [Google Scholar] [CrossRef]
- Sołtys, A.; Studnicki, M.; Zawadzki, G.; Aleksandrowicz-Trzcińska, M. The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in scots pine in a forest nursery. Iforest 2020, 13, 441–446. [Google Scholar] [CrossRef]
- Syacic-Nikolic, M.; Vilotic, D.; Milovanovic, J.; Veselinovic, M.; Stankovic, D. Application of superabsorbent polymers in the production of scotch pine (Pinus sylvestris L.) and Austrian pine (Pinus nigra ARN.) seedlings. Fresenius Environ. Bull. 2010, 6, 1180–1185. [Google Scholar]
- Pamuk, G.S.; Olsson, T.; Bergsten, U.; Lindberg, H. Evaluation of polymer coating on Scots Pine (Pinus sylvestris L.) seeds using scanning electron microscopy (SEM). Seed Sci. Technol. 2002, 30, 167–176. [Google Scholar]
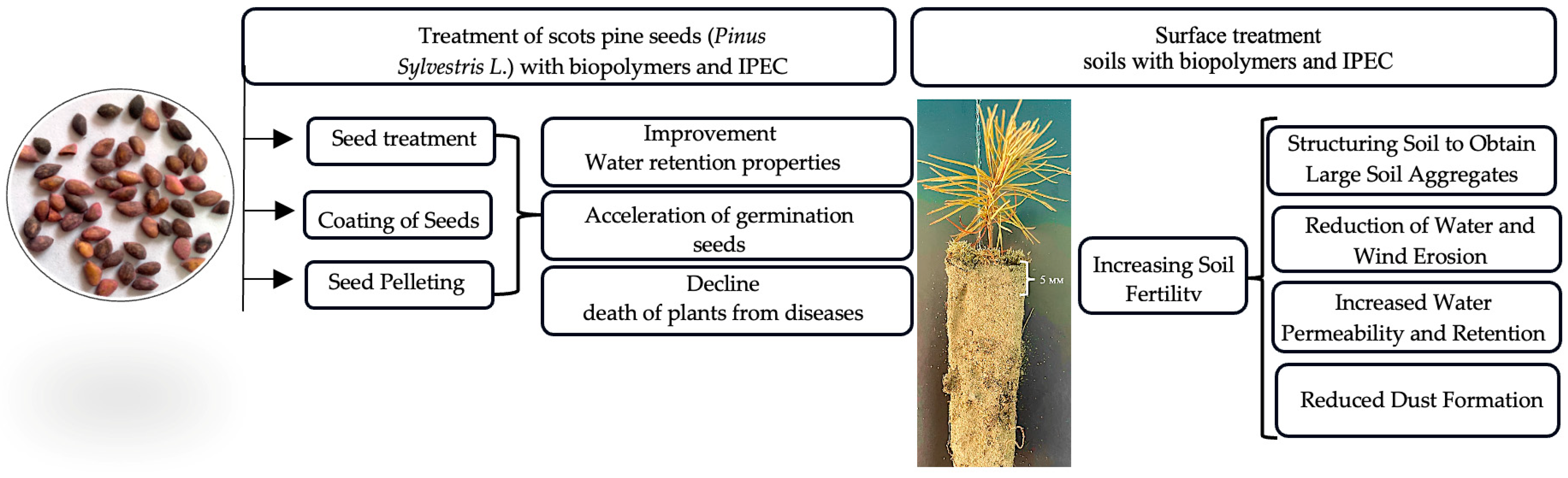

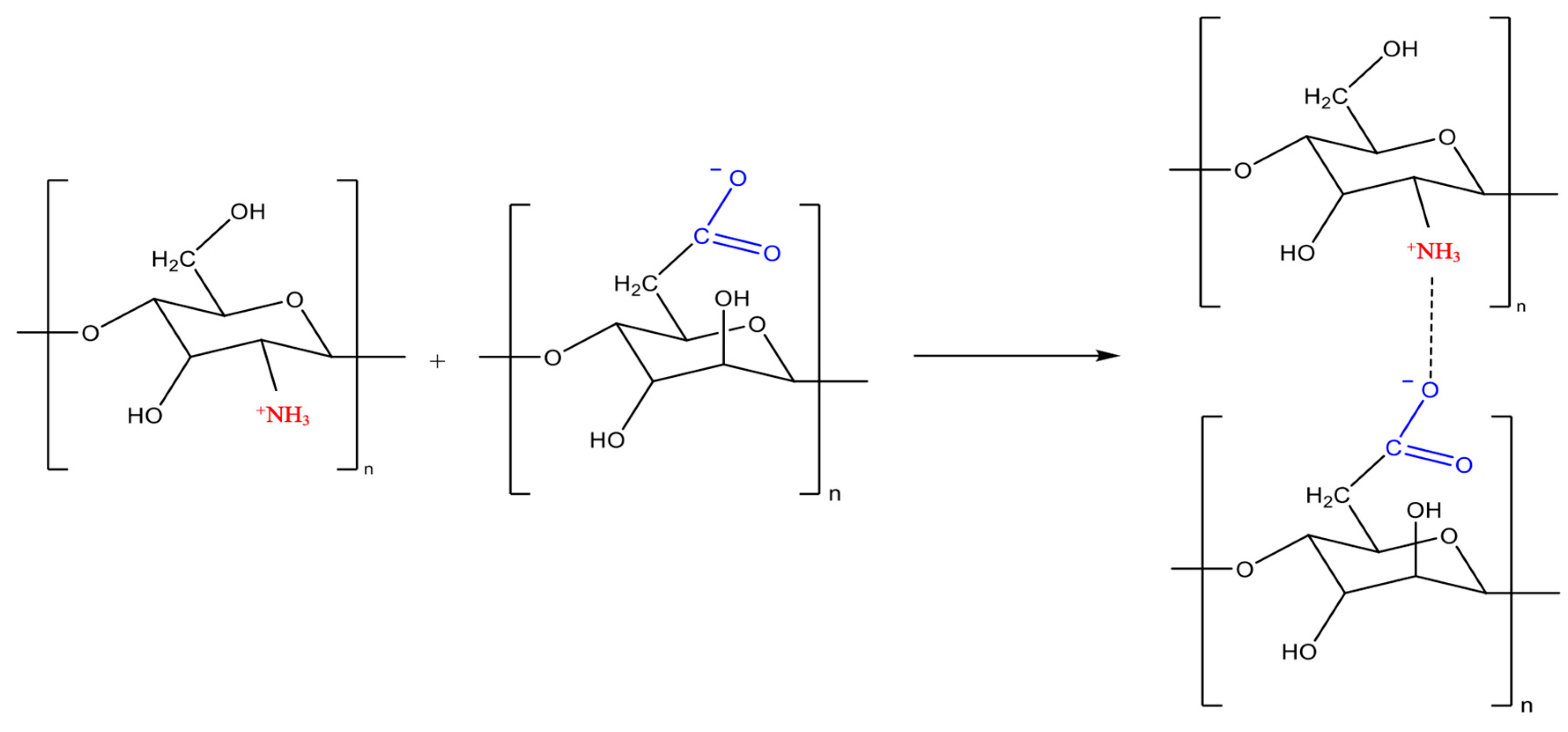


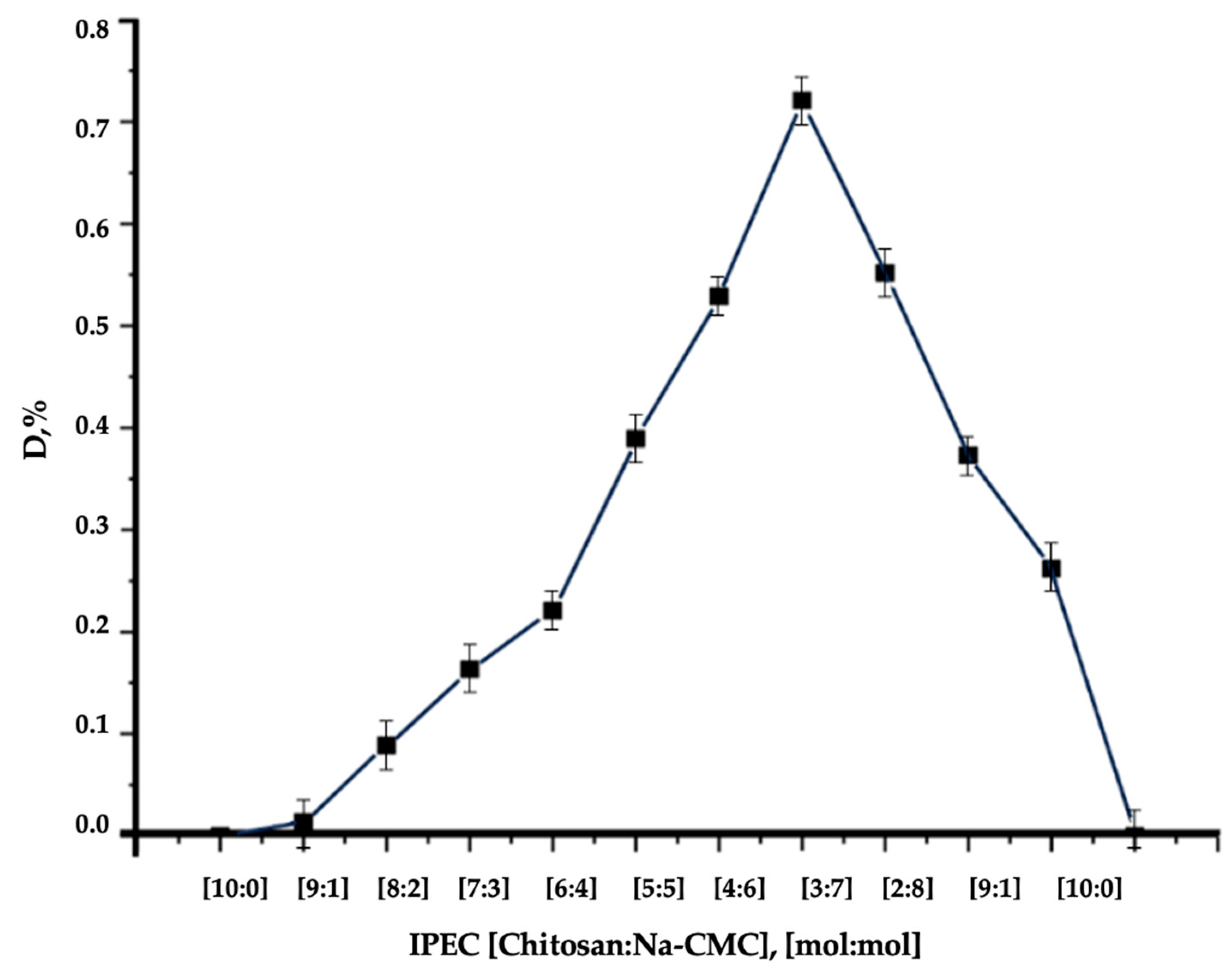
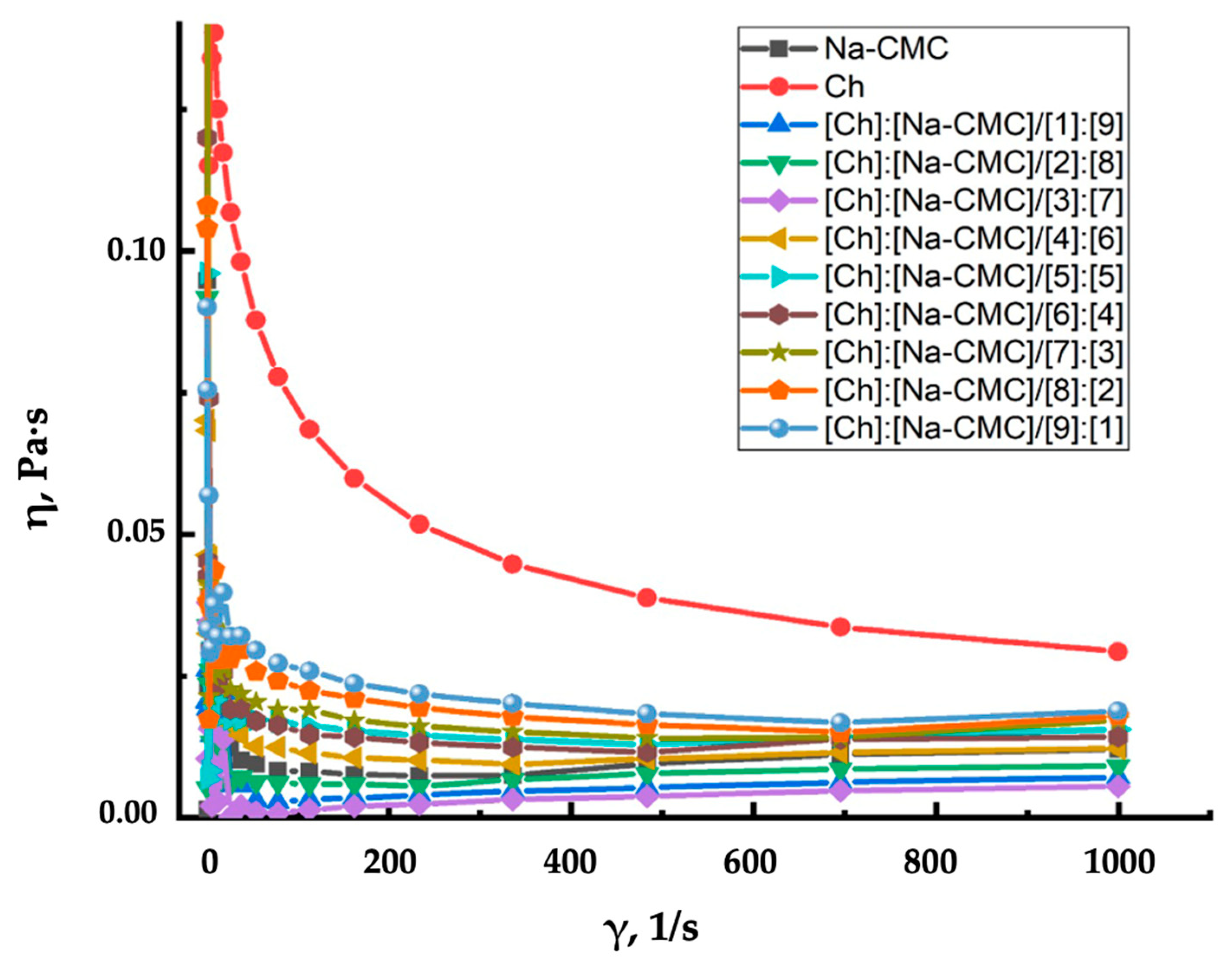



| Polymer | Soil Type | Result | Reference |
|---|---|---|---|
| Epoxidized linseed oil + wastes | Two types of forest soils: deciduous and coniferous | The tested biocomposites demonstrated promising potential for application as mulching films in agriculture or forestry as ecofriendly alternatives to polyethylene films. | [28] |
| Acrylic acid/Acrylamide based superabsorbent polymers (SAP) | Sandy soils | The performance of SAPs depends on the pore space and a saline environment in the soil, and low SAP application rates are suitable for maximizing the water available to plants in sandy soils. | [29] |
| PAM | Calcareous montmorillonitic sandy clay loam, non-calcareous kaolinitic sandy loam | The application of 25 and 50 kg ha−1 of granular PAM reduced soil erosion by 23 and 57%, respectively, compared to the untreated control. | [30] |
| TerraCottem arbor® PAM-free water absorbent polymer | Calcisol with a sandy loam texture | The benefits of the new soil conditioner were highest when applied at doses of 40 or 80 g per seedling. | [31] |
| PAM/polyacrylic acid salts | Carbonate Loamy–Sandy Arenosol from the Emirate of Dubai Loamy–Sandy Retisol from the Moscow Region, Russia Loamy Serozem from the Tashkent Region, Uzbekistan | Gel-forming polymer conditioners and new technologies of their application increase the productivity of plant crops and the quality of biomass by 30–50%, with a 1.3–2-fold saving of water resources and reliable protection of the topsoil from pathogens and secondary salinization. | [32] |
| № | Agrochemical Parameters | Control | IPEC [Chitosan]:[Na-CMC] [3:7] |
|---|---|---|---|
| 1 | pHH2O | 6.1 | 6.0 |
| 2 | pHKCl | 6.5 | 6.3 |
| 3 | Hygroscopic moisture, % | 1.86 | 2.23 |
| 4 | Humus, % | 0.95 | 1.12 |
| 5 | NO3 content, mg/kg | 1.45 | 3.50 |
| 6 | P2O5 content, mg/kg | 12.90 | 14.55 |
| 7 | K2O content, mg/kg | 90.80 | 92.50 |
| 8 | Salinity, mg/kg | ||
| 8.1 | K+ | 22.26 | 24.52 |
| 8.2 | Na+ | 20.03 | 23.42 |
| 8.3 | NH4+ | 2.75 | 3.56 |
| 8.4 | Mg2+ | 8.84 | 10.32 |
| 8.5 | Ca2+ | 32.59 | 33.43 |
| 8.6 | Cl− | 1.15 | 1.16 |
| 8.7 | SO42− | 10.30 | 11.70 |
| Options | Control | IPEC [Chitosan]:[Na-CMC] [3:7] |
|---|---|---|
| k air-dry | ||
| >10 mm | 0 | 0 |
| 10–0.25 mm | 35.4 | 69.5 |
| <0.25 mm | 61.2 | 30.2 |
| water-stable | ||
| >1 mm | 0.2 | 0.6 |
| >0.25 mm | 35.4 | |
| indicators of soil structure | ||
| Kstr | 0.58 | 2.30 |
| K, % | 0.05 | 0.80 |
| W, % | 80.5 | 92.0 |
| IPEC | Type of Soil | Mechanical Strength | Wind Erosion Resistance | Water Erosion Resistance | Increasing of Water Retention Capacity | Reference |
|---|---|---|---|---|---|---|
| HYPAN- PDADMAC * | Sand Soil | 16 MPa 10MPa | 100% 100% | 60% 99% | 1.5 time | [92] |
| ALG-QHECE ** | Sand Soil | 30 MPa 165 MPa | - - | 90% 99% | - - | [38] |
| BSM-PDADMAC *** | Soil | 0.8–45 MPa | - | 100% | [93] | |
| Chitosan-Sodium alginate | Dark chestnut soil | 70 kPa | - | 97% | - | [72] |
| PDADMAC- HYPAN | Sand | 19 MPa | - | 90% | - | [94] |
| Chitosan- Na-CMC | Sandy soil | 28 kPa | >99% | 97% | 6 time | Present work |
| Options | Germinated Seeds, pcs (Total of 100 pcs) | Germination Energy, % | Germination Rate, % | Uniformity, % | Root Length, mm | |||
|---|---|---|---|---|---|---|---|---|
| Days | ||||||||
| 5 | 7 | 10 | 15 | |||||
| Chitosan | 0 | 6 | 6 | 3 | 12 | 15 | 1.5 | 0.10 ± 0.02 |
| Na-CMC | 4 | 70 | 11 | 6 | 85 | 91 | 9.1 | 0.40 ± 0.05 |
| IPEC | 0 | 2 | 5 | 7 | 7 | 14 | 1.4 | 0.10 ± 0.03 |
| Control | 0 | 60 | 13 | 2 | 73 | 75 | 7.5 | 0.30 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berikbol, N.; Klivenko, A.; Markin, V.; Orazzhanova, L.; Yelemessova, G.; Kassymova, Z. Development of Interpolyelectrolyte Complex Based on Chitosan and Carboxymethylcellulose for Stabilizing Sandy Soil and Stimulating Vegetation of Scots Pine (Pinus sylvestris L.). Polymers 2024, 16, 2373. https://doi.org/10.3390/polym16162373
Berikbol N, Klivenko A, Markin V, Orazzhanova L, Yelemessova G, Kassymova Z. Development of Interpolyelectrolyte Complex Based on Chitosan and Carboxymethylcellulose for Stabilizing Sandy Soil and Stimulating Vegetation of Scots Pine (Pinus sylvestris L.). Polymers. 2024; 16(16):2373. https://doi.org/10.3390/polym16162373
Chicago/Turabian StyleBerikbol, Nazira, Alexey Klivenko, Vadim Markin, Lazzyat Orazzhanova, Gulnur Yelemessova, and Zhanar Kassymova. 2024. "Development of Interpolyelectrolyte Complex Based on Chitosan and Carboxymethylcellulose for Stabilizing Sandy Soil and Stimulating Vegetation of Scots Pine (Pinus sylvestris L.)" Polymers 16, no. 16: 2373. https://doi.org/10.3390/polym16162373
APA StyleBerikbol, N., Klivenko, A., Markin, V., Orazzhanova, L., Yelemessova, G., & Kassymova, Z. (2024). Development of Interpolyelectrolyte Complex Based on Chitosan and Carboxymethylcellulose for Stabilizing Sandy Soil and Stimulating Vegetation of Scots Pine (Pinus sylvestris L.). Polymers, 16(16), 2373. https://doi.org/10.3390/polym16162373






