The Influence of Molecular Weights on Dispersion and Thermoelectric Performance of Alkoxy Side-Chain Polythiophene/Carbon Nanotube Composite Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Polymer/SWCNTs Composite Film
- Ultrasonic dispersion of single-walled carbon nanotubes: Weigh 40 mg of 5–10 μm long single-walled carbon nanotubes into a 100 mL round-bottom flask. Prepare a 1 mg mL−1 dispersion in anhydrous chlorobenzene solution. Then, ultrasonicate the mixture in an ultrasonic cleaner for 5 h.
- Preparation of different ratios of composite solution: Prepare composite solutions with mass ratios of 1:9, 3:7, 5:5, 7:3, and 9:1 for P3ODT/SWCNTs. Label each composite solution accordingly. Subsequently, place the composite solutions in the ultrasonic cleaner and sonicate for 2 h to ensure uniform dispersion.
- Preparation of P3ODT/SWCNTs composite film using the drop-casting method: First, clean a 1 cm × 1 cm glass slide (1 mm thickness) sequentially with dichloromethane, deionized water, and acetone through ultrasonication for half an hour. After drying the glass slide, slowly drop-cast 120 μL of the different mass ratio composite solutions onto the glass slide using a pipette. Allow the chlorobenzene solvent to evaporate naturally at room temperature for half an hour to obtain composite films with different mass ratios of P3ODT/SWCNTs. The labeling of the film samples is based on the percentage of SWCNTs present. For example, if SWCNTs account for 50% of the composite film, it is labeled as P3ODT/SWCNTs-0.5.
2.3. Manufacture and Measurement of a Flexible Thermoelectric Device
- Prepare the required composite solution.
- Cut the flexible polyimide (PI) film into rectangular strips measuring 1 cm × 4 cm. Sequentially ultrasonic with dichloromethane, deionized water, and acetone for half an hour; dry after cleaning.
- Pipette 300 μL of the prepared composite solution onto the PI film. Allow it to naturally evaporate at room temperature.
- Take the 1 cm × 4 cm composite films and adhere them onto an 8 cm × 24 cm PI film using double-sided tape with a 1 cm spacing between each composite film. Then, connect the composite films and the copper foil tape at the intersections using conductive silver paste to ensure good electrical contact and to reduce contact resistance.
3. Results and Discussion
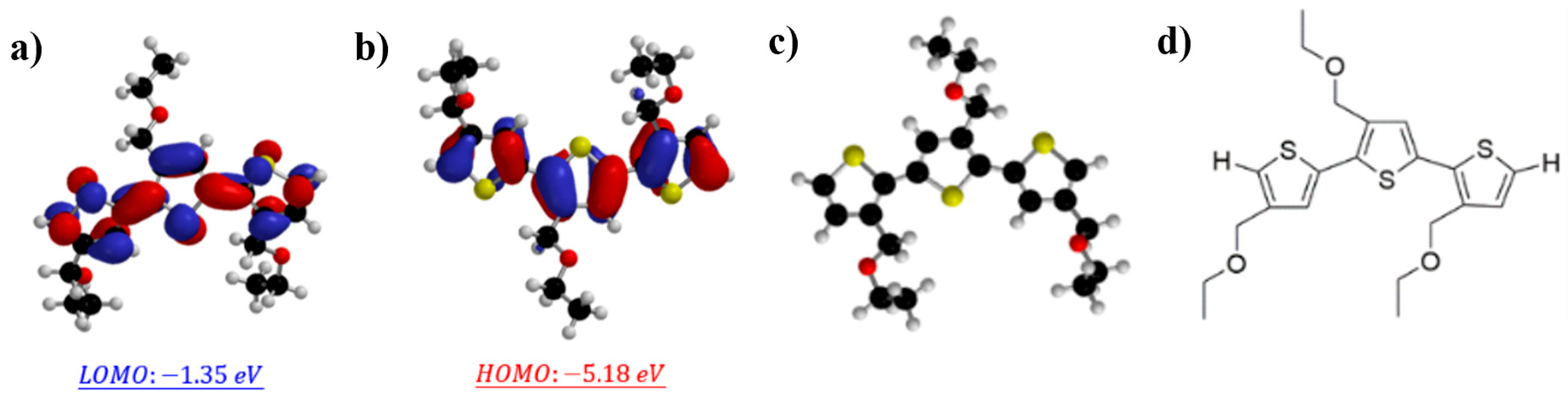
3.1. Synthesis and Evaluation of Polymers
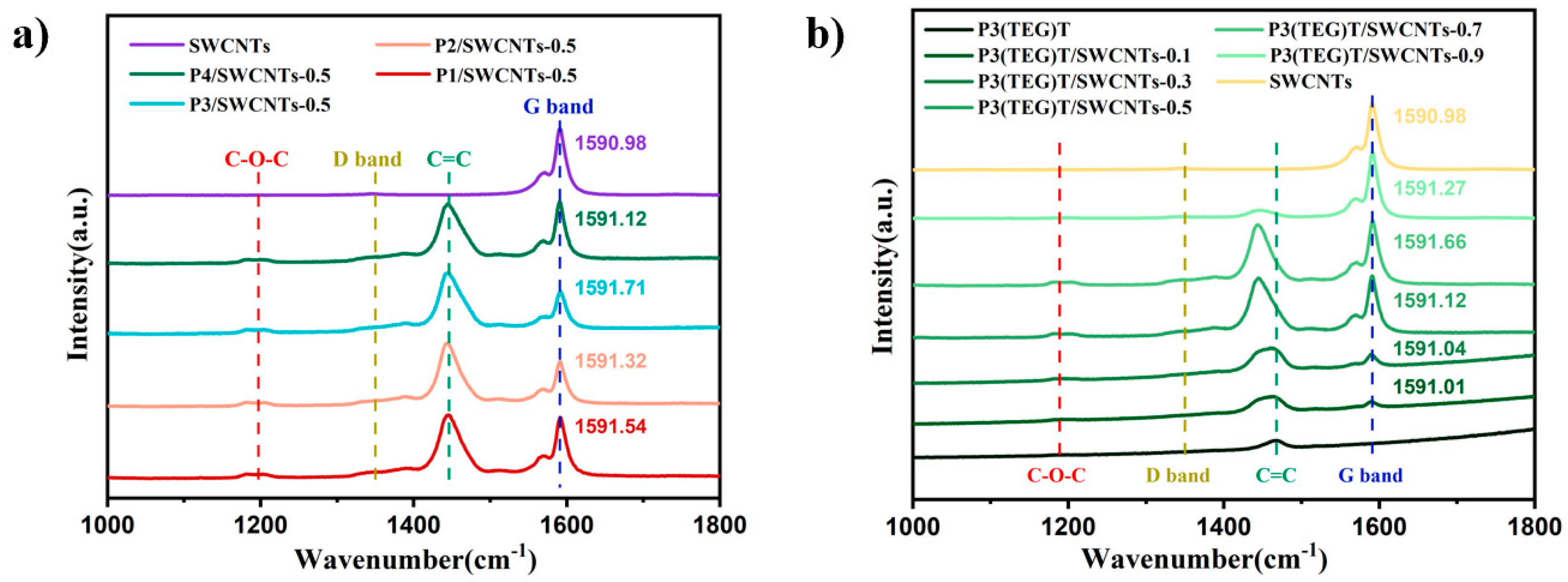
3.2. Spectroscopic Properties of Composites
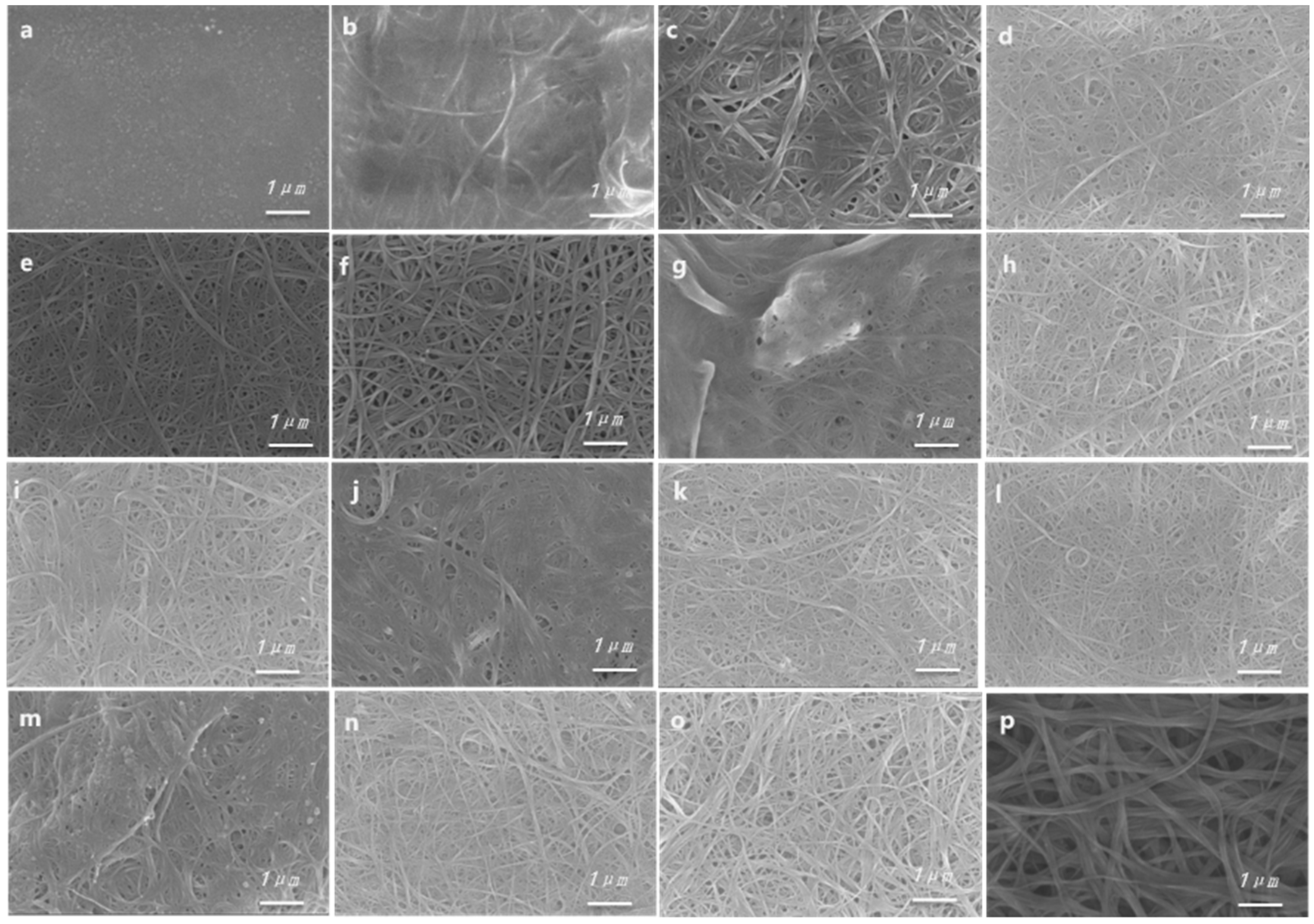
3.3. Morphological Properties of Composites
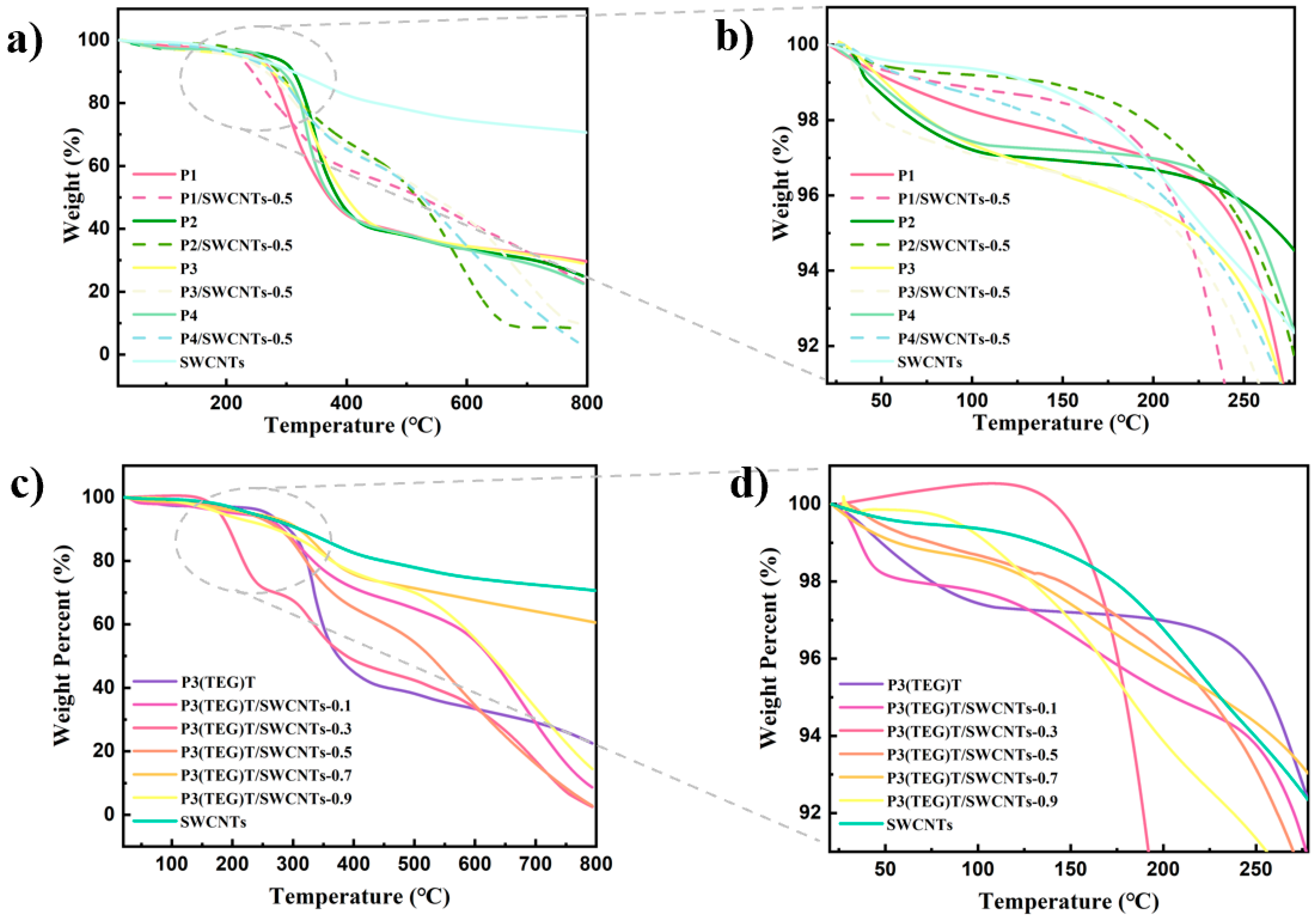
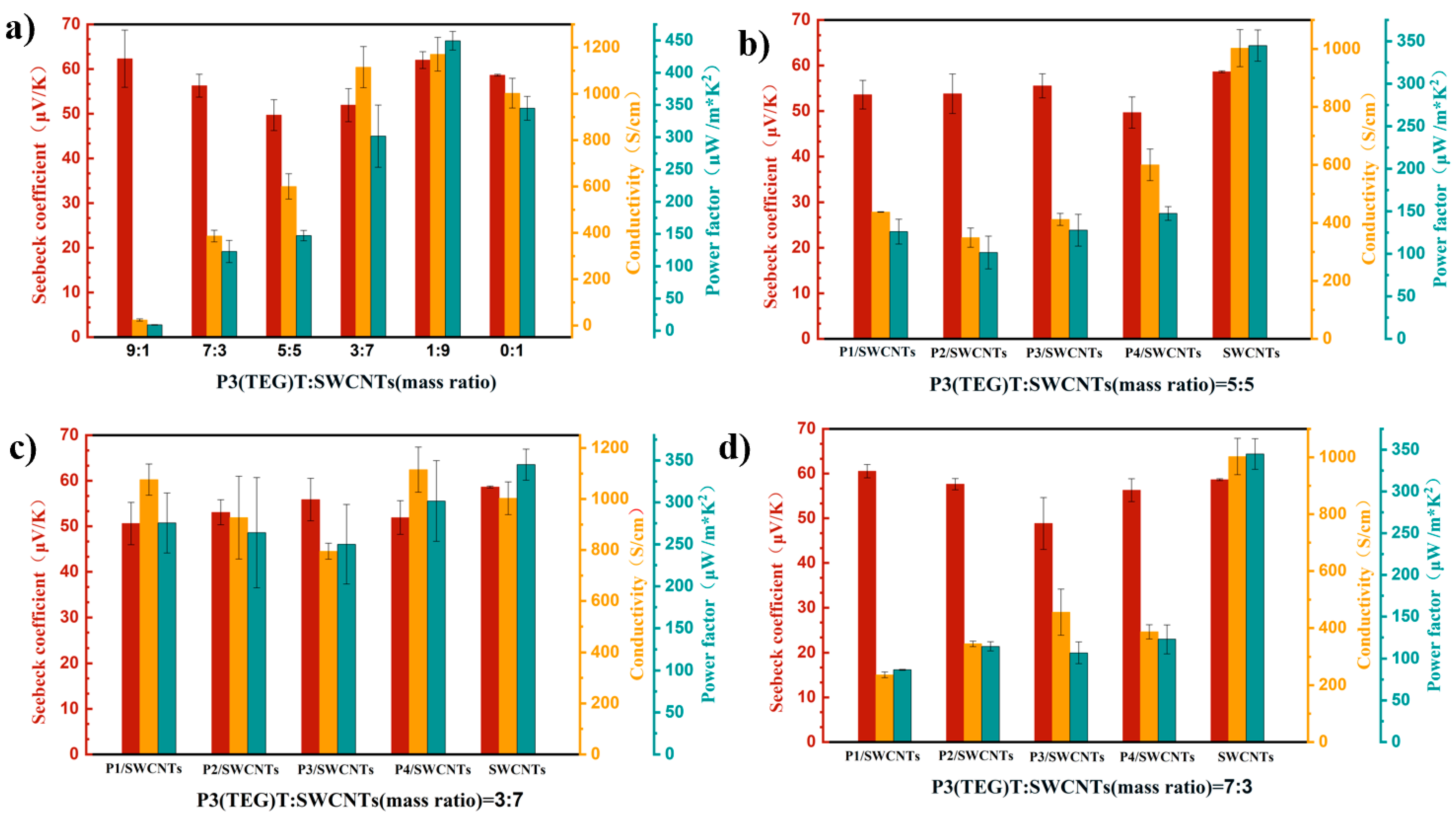
3.4. Thermoelectric Properties of Composite Thin Films
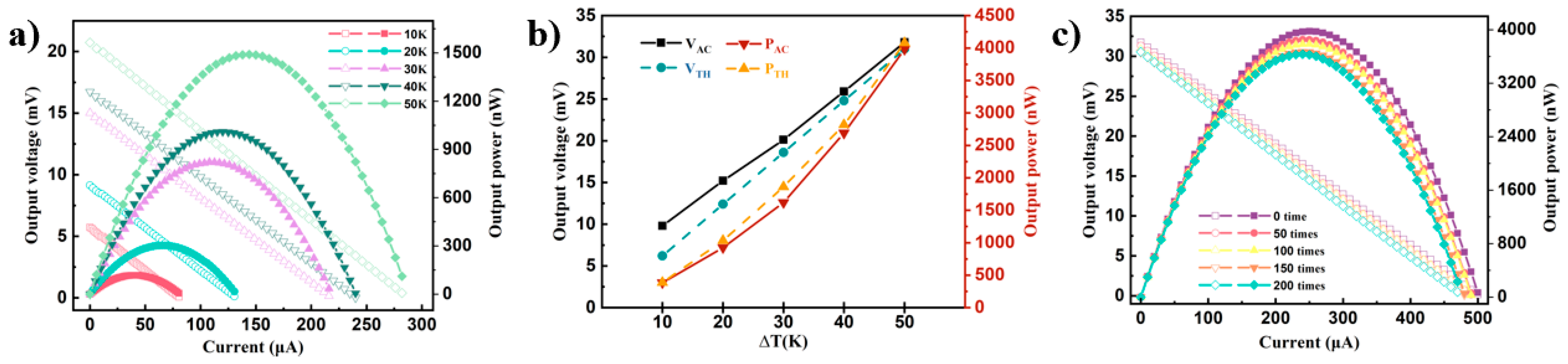
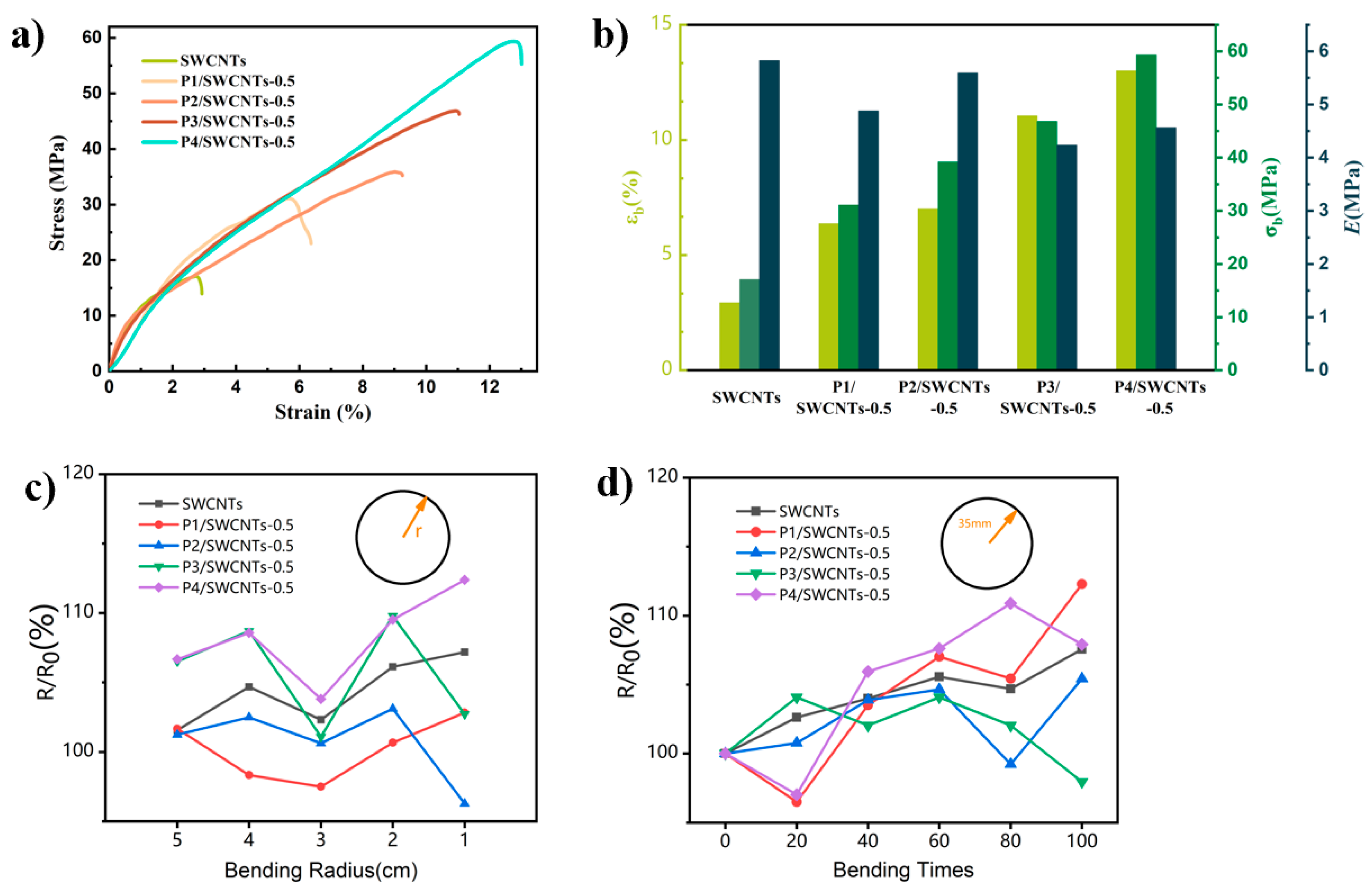
3.5. Evaluation of Flexible P3(TEG)/SWCNTs Composite Thermoelectric Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajimsha, R.S.; Mahapatra, A.; Das, A.K.; Sahu, V.K.; Misra, P. High Output Power Density Owing to Enhanced Charge Transfer in ZnO-Based Triboelectric Nanogenerator. Energy 2023, 263, 125646. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Zhang, X. Ultrarobust, Hierarchically Anisotropic Structured Piezoelectric Nanogenerators for Self-Powered Sensing. Nano Energy 2022, 99, 107379. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; He, X.; Xue, H.; Wang, X.; Dong, H.; Zhu, J.; Mao, W.; Xu, X.; Li, X. Roles of Oxygen Vacancy and Ferroelectric Polarization in Photovoltaic Effects of BiFeO3 Based Devices. Solid State Commun. 2023, 360, 115042. [Google Scholar] [CrossRef]
- Liang, L.; Fan, J.; Wang, M.; Chen, G.; Sun, G. Ternary Thermoelectric Composites of Polypyrrole/PEDOT:PSS/Carbon Nanotube with Unique Layered Structure Prepared by One-Dimensional Polymer Nanostructure as Template. Compos. Sci. Technol. 2020, 187, 107948. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic Thermoelectric Materials: Emerging Green Energy Materials Converting Heat to Electricity Directly and Efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qiu, P.; Chen, L.; Shi, X. Recent Developments in Flexible Thermoelectric Devices. Small Sci. 2021, 1, 2100005. [Google Scholar] [CrossRef]
- Yan, Q.; Kanatzidis, M.G. High-Performance Thermoelectrics and Challenges for Practical Devices. Nat. Mater. 2022, 21, 503–513. [Google Scholar] [CrossRef]
- Scheele, M.; Oeschler, N.; Meier, K.; Kornowski, A.; Klinke, C.; Weller, H. Synthesis and Thermoelectric Characterization of Bi2Te3 Nanoparticles. Adv. Funct. Mater. 2009, 19, 3476–3483. [Google Scholar] [CrossRef]
- Zou, Q.; Shang, H.; Huang, D.; Xie, B.; Zhang, L.; Wang, K.; Dong, H.; Li, C.; Gu, H.; Ding, F. Bi2Te3-Based Flexible Thermoelectric Generator for Wearable Electronics. Appl. Phys. Lett. 2022, 120, 023903. [Google Scholar] [CrossRef]
- Ikeda, T.; Collins, L.A.; Ravi, V.A.; Gascoin, F.S.; Haile, S.M.; Snyder, G.J. Self-Assembled Nanometer Lamellae of Thermoelectric PbTe and Sb2Te3 with Epitaxy-like Interfaces. Chem. Mater. 2007, 19, 763–767. [Google Scholar] [CrossRef]
- Olvera, A.A.; Moroz, N.A.; Sahoo, P.; Ren, P.; Bailey, T.P.; Page, A.A.; Uher, C.; Poudeu, P.F.P. Partial Indium Solubility Induces Chemical Stability and Colossal Thermoelectric Figure of Merit in Cu2Se. Energy Environ. Sci. 2017, 10, 1668–1676. [Google Scholar] [CrossRef]
- Li, M.; Cortie, D.L.; Liu, J.; Yu, D.; Islam, S.M.K.N.; Zhao, L.; Mitchell, D.R.G.; Mole, R.A.; Cortie, M.B.; Dou, S.; et al. Ultra-High Thermoelectric Performance in Graphene Incorporated Cu2Se: Role of Mismatching Phonon Modes. Nano Energy 2018, 53, 993–1002. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Nan, P.; Xiong, F.; Lin, S.; Zhang, X.; Chen, Y.; Chen, L.; Ge, B.; Pei, Y. Lattice Strain Advances Thermoelectrics. Joule 2019, 3, 1276–1288. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shen, S.Z.; Yang, W.; Donelson, R.; Cai, K.; Casey, P.S. Simultaneous Increase in Conductivity and Seebeck Coefficient in a Polyaniline/Graphene Nanosheets Thermoelectric Nanocomposite. Synth. Met. 2012, 161, 2688–2692. [Google Scholar] [CrossRef]
- Zevalkink, A.; Smiadak, D.M.; Blackburn, J.L.; Ferguson, A.J.; Chabinyc, M.L.; Delaire, O.; Wang, J.; Kovnir, K.; Martin, J.; Schelhas, L.T.; et al. A Practical Field Guide to Thermoelectrics: Fundamentals, Synthesis, and Characterization. Appl. Phys. Rev. 2018, 5, 021303. [Google Scholar] [CrossRef]
- Thermoelectric Fabrics: Toward Power Generating Clothing|Scientific Reports. Available online: https://www.nature.com/articles/srep06411 (accessed on 12 September 2023).
- Yao, H.; Fan, Z.; Cheng, H.; Guan, X.; Wang, C.; Sun, K.; Ouyang, J. Recent Development of Thermoelectric Polymers and Composites. Macromol. Rapid Commun. 2018, 39, 1700727. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.-J.; Mori, T. Polymer Based Thermoelectric Nanocomposite Materials and Devices: Fabrication and Characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Zhang, F.; Di, C.; Zhu, D. Advances in Organic Thermoelectric Materials and Devices for Smart Applications. SmartMat 2021, 2, 426–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Chen, G. Carbon and Carbon Composites for Thermoelectric Applications. Carbon Energy 2020, 2, 408–436. [Google Scholar] [CrossRef]
- Yun, J.-S.; Choi, S.; Im, S.H. Advances in Carbon-Based Thermoelectric Materials for High-Performance, Flexible Thermoelectric Devices. Carbon Energy 2021, 3, 667–708. [Google Scholar] [CrossRef]
- Statz, M.; Schneider, S.; Berger, F.J.; Lai, L.; Wood, W.A.; Abdi-Jalebi, M.; Leingang, S.; Himmel, H.-J.; Zaumseil, J.; Sirringhaus, H. Charge and Thermoelectric Transport in Polymer-Sorted Semiconducting Single-Walled Carbon Nanotube Networks. ACS Nano 2020, 14, 15552–15565. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.V.; Jang, S.-H.; Shchegolkov, A.V.; Rodionov, Y.V.; Glivenkova, O.A. Multistage Mechanical Activation of Multilayer Carbon Nanotubes in Creation of Electric Heaters with Self-Regulating Temperature. Materials 2021, 14, 4654. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Kurosawa, T.; Fu, T.; Chiu, Y.-C.; Mun, J.; Wang, G.-J.N.; Gu, X.; Shaw, L.; Kneller, J.W.E.; Kreouzis, T.; et al. Taming Charge Transport in Semiconducting Polymers with Branched Alkyl Side Chains. Adv. Funct. Mater. 2017, 27, 1701973. [Google Scholar] [CrossRef]
- Lin, P.-S.; Shoji, Y.; Afraj, S.N.; Ueda, M.; Lin, C.-H.; Inagaki, S.; Endo, T.; Tung, S.-H.; Chen, M.-C.; Liu, C.-L.; et al. Controlled Synthesis of Poly[(3-Alkylthio)Thiophene]s and Their Application to Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 31898–31909. [Google Scholar] [CrossRef]
- Liang, A.; Zhou, X.; Zhou, W.; Wan, T.; Wang, L.; Pan, C.; Wang, L. Side-Chain Effects on the Thermoelectric Properties of Fluorene-Based Copolymers. Macromol. Rapid Commun. 2017, 38, 1600817. [Google Scholar] [CrossRef]
- Kroon, R.; Kiefer, D.; Stegerer, D.; Yu, L.; Sommer, M.; Müller, C. Polar Side Chains Enhance Processability, Electrical Conductivity, and Thermal Stability of a Molecularly p-Doped Polythiophene. Adv. Mater. 2017, 29, 1700930. [Google Scholar] [CrossRef]
- Kiefer, D.; Giovannitti, A.; Sun, H.; Biskup, T.; Hofmann, A.; Koopmans, M.; Cendra, C.; Weber, S.; Anton Koster, L.J.; Olsson, E.; et al. Enhanced N-Doping Efficiency of a Naphthalenediimide-Based Copolymer through Polar Side Chains for Organic Thermoelectrics. ACS Energy Lett. 2018, 3, 278–285. [Google Scholar] [CrossRef]
- Lin, P.-S.; Inagaki, S.; Liu, J.-H.; Chen, M.-C.; Higashihara, T.; Liu, C.-L. The Role of Branched Alkylthio Side Chain on Dispersion and Thermoelectric Properties of Regioregular Polythiophene/Carbon Nanotubes Nanocomposites. Chem. Eng. J. 2023, 458, 141366. [Google Scholar] [CrossRef]
- Hao, L.; Kang, J.; Shi, J.; Xu, J.; Cao, J.; Wang, L.; Liu, Y.; Pan, C. Enhanced Thermoelectric Performance of Poly(3-Substituted Thiophene)/Single-Walled Carbon Nanotube Composites via Polar Side Chain Modification. Compos. Sci. Technol. 2020, 199, 108359. [Google Scholar] [CrossRef]
- Yemata, T.; Zheng, Y.; Kyaw, A.; Wang, X.; Song, J.; Chin, W.; Xu, J. Improved Thermoelectric Properties and Environmental Stability of Conducting PEDOT:PSS Films Post-Treated With Imidazolium Ionic Liquids. Front. Chem. 2020, 7, 870. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A. Structural (n, m) Determination of Isolated Single Wall Carbon Nanotubes by Resonant Raman Scattering. In AIP Conference Proceedings; AIP: Kamakura, Japan, 2001; Volume 590, pp. 129–132. [Google Scholar]
- Guldi, D.M.; Taieb, H.; Rahman, G.M.A.; Tagmatarchis, N.; Prato, M. Novel Photoactive Single-Walled Carbon Nanotube–Porphyrin Polymer Wraps: Efficient and Long-Lived Intracomplex Charge Separation. Adv. Mater. 2005, 17, 871–875. [Google Scholar] [CrossRef]
- Luo, J.; Billep, D.; Waechtler, T.; Otto, T.; Toader, M.; Gordan, O.; Sheremet, E.; Martin, J.; Hietschold, M.; Zahn, D.R.T.; et al. Enhancement of the Thermoelectric Properties of PEDOT:PSS Thin Films by Post-Treatment. J. Mater. Chem. A 2013, 1, 7576–7583. [Google Scholar] [CrossRef]
- Tonga, M.; Wei, L.; Wilusz, E.; Korugic-Karasz, L.; Karasz, F.E.; Lahti, P.M. Solution-Fabrication Dependent Thermoelectric Behavior of Iodine-Doped Regioregular and Regiorandom P3HT/Carbon Nanotube Composites. Synth. Met. 2018, 239, 51–58. [Google Scholar] [CrossRef]
- Bounioux, C.; Díaz-Chao, P.; Campoy-Quiles, M.; Martín-González, M.S.; Goñi, A.R.; Yerushalmi-Rozen, R.; Müller, C. Thermoelectric Composites of Poly(3-Hexylthiophene) and Carbon Nanotubes with a Large Power Factor. Energy Environ. Sci. 2013, 6, 918. [Google Scholar] [CrossRef]
- Lee, W.; Hong, C.T.; Kwon, O.H.; Yoo, Y.; Kang, Y.H.; Lee, J.Y.; Cho, S.Y.; Jang, K.S. Enhanced Thermoelectric Performance of Bar-Coated Swcnt/P3ht Thin Films. ACS Appl. Mater. Interfaces 2015, 7, 6550–6556. [Google Scholar] [CrossRef]
- Hong, C.T.; Lee, W.; Kang, Y.H.; Yoo, Y.; Ryu, J.; Cho, S.Y.; Jang, K.-S. Effective Doping by Spin-Coating and Enhanced Thermoelectric Power Factors in Swcnt/P3ht Hybrid Films. J. Mater. Chem. A 2015, 3, 12314–12319. [Google Scholar] [CrossRef]
- Kang, Y.H.; Lee, U.-H.; Jung, I.H.; Yoon, S.C.; Cho, S.Y. Enhanced Thermoelectric Performance of Conjugated Polymer/Cnt Nanocomposites by Modulating the Potential Barrier Difference between Conjugated Polymer and Cnt. ACS Appl. Electron. Mater. 2019, 1, 1282–1289. [Google Scholar] [CrossRef]
- Liu, C.; Yin, X.; Chen, Z.; Gao, C.; Wang, L. Improving the Thermoelectric Performance of Solution-Processed Polymer Nanocomposites by Introducing Platinum Acetylides with Tailored Intermolecular Interactions. Chem. Eng. J. 2021, 419. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, C.; Liang, A.; Wang, L.; Wong, W.-Y. Thermoelectric Properties of Composite Films Prepared with Benzodithiophene Derivatives and Carbon Nanotubes. Compos. Sci. Technol. 2017, 145, 40–45. [Google Scholar] [CrossRef]
| Polymer | Mn (kDa) | Mw (kDa) | PDI | DPn |
|---|---|---|---|---|
| P1 | 18.4 | 28.4 | 1.53 | 71 |
| P2 | 22.9 | 31.7 | 1.38 | 89 |
| P3 | 27.3 | 43.4 | 1.37 | 105 |
| P4 | 29.8 | 49.3 | 1.65 | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, S.; Wang, D.; Qiu, Y.; Chen, Z.; Yang, H.; Yang, Q.; Yin, Z.; Pan, C. The Influence of Molecular Weights on Dispersion and Thermoelectric Performance of Alkoxy Side-Chain Polythiophene/Carbon Nanotube Composite Materials. Polymers 2024, 16, 2444. https://doi.org/10.3390/polym16172444
Chen X, Chen S, Wang D, Qiu Y, Chen Z, Yang H, Yang Q, Yin Z, Pan C. The Influence of Molecular Weights on Dispersion and Thermoelectric Performance of Alkoxy Side-Chain Polythiophene/Carbon Nanotube Composite Materials. Polymers. 2024; 16(17):2444. https://doi.org/10.3390/polym16172444
Chicago/Turabian StyleChen, Xiaogang, Shihong Chen, Dagang Wang, Yongfu Qiu, Zhongming Chen, Haixin Yang, Qing Yang, Zijian Yin, and Chengjun Pan. 2024. "The Influence of Molecular Weights on Dispersion and Thermoelectric Performance of Alkoxy Side-Chain Polythiophene/Carbon Nanotube Composite Materials" Polymers 16, no. 17: 2444. https://doi.org/10.3390/polym16172444
APA StyleChen, X., Chen, S., Wang, D., Qiu, Y., Chen, Z., Yang, H., Yang, Q., Yin, Z., & Pan, C. (2024). The Influence of Molecular Weights on Dispersion and Thermoelectric Performance of Alkoxy Side-Chain Polythiophene/Carbon Nanotube Composite Materials. Polymers, 16(17), 2444. https://doi.org/10.3390/polym16172444






