A Comparative Study on the Melt Crystallization of Biodegradable Poly(butylene succinate-co-terephthalate) and Poly(butylene adipate-co-terephthalate) Copolyesters
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. Nonisothermal Melt Crystallization and Melting
3.2. Isothermal Crystallization and Melting Behavior
3.3. Light Transmittance Performance
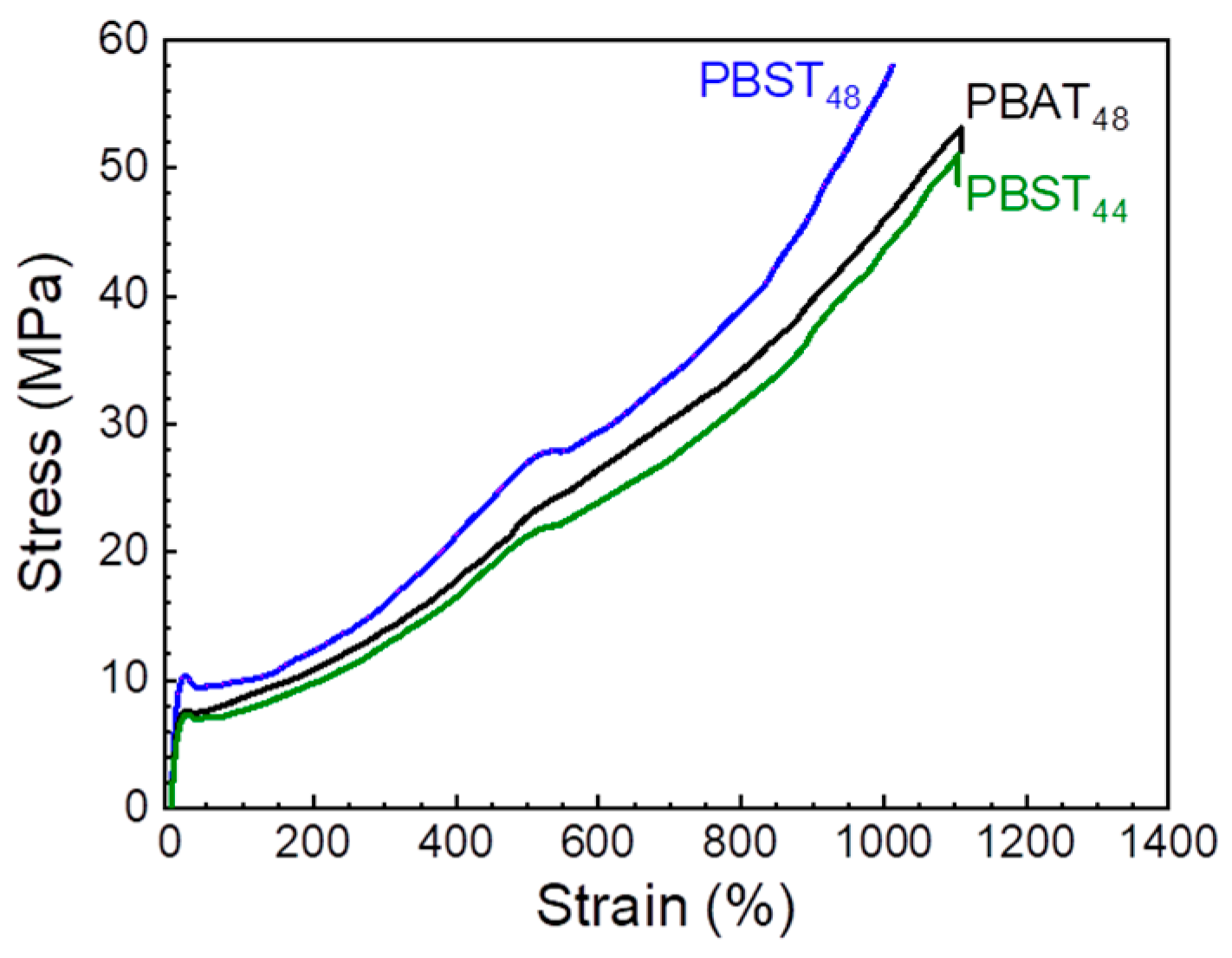
3.4. Tensile Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marten, E.; Müller, R.-J.; Deckwer, W.-D. Studies on the enzymatic hydrolysis of polyesters. II. Aliphatic-aromatic copolyesters. Polym. Degrad. Stab. 2005, 88, 371–381. [Google Scholar] [CrossRef]
- Witt, U.; Müller, R.J.; Deckwer, W.D. Studies on sequence distribution of aliphatic/aromatic copolyesters by high-resolution 13C nuclear magnetic resonance spectroscopy for evaluation of biodegradability. Macromol. Chem. Phys. 1996, 197, 1525–1535. [Google Scholar] [CrossRef]
- Müller, R.J.; Witt, U.; Rantze, E.; Deckwer, W.D. Architecture of biodegradable copolyesters containing aromatic constituents. Polym. Degrad. Stab. 1998, 59, 203–208. [Google Scholar] [CrossRef]
- Müller, R.-J.; Kleeberg, I.; Deckwer, W.-D. Biodegradation of polyesters containing aromatic constituents. J. Biotechnol. 2001, 86, 87–95. [Google Scholar] [CrossRef]
- Siegenthaler, K.O.; Künkel, A.; Skupin, G.; Yamamoto, M. Ecoflex® and Ecovio®: Biodegradable, performance-enabling plastics. In Synthetic Biodegradable Polymers; Springer: Berlin/Heidelberg, Germany, 2011; pp. 91–136. [Google Scholar]
- Jiao, J.; Zeng, X.B.; Huang, X.B. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)-PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An overview on properties and applications of poly(butylene adipate-co-terephthalate)-PBAT based composites. Polym. Eng. Sci. 2017, 59, E7–E15. [Google Scholar] [CrossRef]
- Debeli, D.K.; Huang, F.F.; Wu, L.B. Sulfonated poly(butylene adipate-co-terephthalate)/sodium montmorillonite nanocomposite films with an ultra-high oxygen barrier. Ind. Eng. Chem. Res. 2022, 61, 13283–13293. [Google Scholar] [CrossRef]
- Wei, C.; Guo, P.; Lyu, M.F.; Wang, B.; Li, C.; Sang, L.; Wei, Z.Y. High barrier poly (glycolic acid) modified poly (butylene adipate-co-terephthalate) blown films and accelerated ultraviolet degradability evaluation. ACS Appl. Polym. Mater. 2023, 5, 3457–3467. [Google Scholar] [CrossRef]
- Gao, F.X.; Cai, Y.; Liu, S.J.; Wang, X.H. High-performance biodegradable PBAT/PPC composite film through reactive compatibilizer. Chin. J. Polym. Sci. 2022, 41, 1051–1058. [Google Scholar] [CrossRef]
- Ran, L.B.; Hong, W.Y.R.; Yu, G.Y.; Du, Q.J.; Guo, S.Y.; Li, C.H. Preparation and improving mechanism of PBAT/PPC-based micro-layer biodegradable mulch film with excellent water resistance and mechanical properties. Polymer 2024, 291, 126614. [Google Scholar] [CrossRef]
- de Matos Costa, A.R.; Crocitti, A.; Hecker de Carvalho, L.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of biodegradable films based on poly (butylene succinate)(PBS) and poly (butylene adipate-co-terephthalate)(PBAT) blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef]
- Ren, P.G.; Liu, X.H.; Ren, F.; Zhong, G.J.; Ji, X.; Xu, L. Biodegradable graphene oxide nanosheets/poly-(butylene adipate-co-terephthalate) nanocomposite film with enhanced gas and water vapor barrier properties. Polym. Test. 2017, 58, 173–180. [Google Scholar] [CrossRef]
- Livi, S.; Bugatti, V.; Marechal, M.; Soares, B.G.; Barra, G.M.O.; Duchet-Rumeau, J.; Gérard, J.-F. Ionic liquids-lignin combination: An innovative way to improve mechanical behaviour and water vapour permeability of eco-designed biodegradable polymer blends. RSC Adv. 2015, 5, 1989–1998. [Google Scholar] [CrossRef]
- Li, F.X.; Xu, X.J.; Hao, Q.H.; Li, Q.B.; Yu, J.Y.; Cao, A.M. Effects of comonomer sequential structure on thermal and crystallization behaviors of biodegradable poly(butylene succinate-co-butylene terephthalate)s. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1635–1644. [Google Scholar] [CrossRef]
- Gang, M.J.; Wang, Y.X.; Zhang, Y.; Liu, L.Z.; Shi, Y. The relationship between microstructure and mechanical properties of PBST two-component crystalline random copolymers with different BT contents. Polymers 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Goto, H.; Sakai, W.; Tsutsumi, N. Synthesis and enzymatic degradation of poly(tetramethylene succinate) copolymers with terephthalic acid. Polymer 2000, 41, 4373–4376. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.W.; Lee, K.H. Properties of potentially biodegradable copolyesters of (succinic acid-1,4-butanediol)/(dimethyl terephthalate-1,4-butanediol). Polym. Int. 1999, 48, 861–867. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wu, L.B.; Bu, Z.Y.; Li, B.G.; Li, N.X.; Dai, J.M. Synthesis and thermomechanical and rheological properties of biodegradable long-chain branched poly(butylene succinate-co-butylene terephthalate) copolyesters. Ind. Eng. Chem. Res. 2014, 53, 10380–10386. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wu, L.B.; Li, N.X.; Dai, J.M. Co-esterification process for synthesis of aliphatic-aromatic copolyester poly(butylene succinate-co-butylene terephalate). Chem. Reac. Eng. Technol. 2016, 32, 78–82. [Google Scholar]
- Lu, J.; Wu, L.B.; Li, B.G. Long chain branched poly(butylene succinate-co-terephthalate) copolyesters using pentaerythritol as branching agent: Synthesis, thermo-mechanical, and rheological properties. J. Appl. Polym. Sci. 2016, 133, 44544. [Google Scholar] [CrossRef]
- Hu, L.X.; Wu, L.B.; Song, F.C.; Li, B.G. Kinetics and modeling of melt polycondensation of poly(butylene succinate-co-terephthalate), 1-esterification. Macromol. React. Eng. 2010, 4, 621–632. [Google Scholar] [CrossRef]
- Liu, T.Q.; Gu, X.P.; Li, N.X.; Wu, L.B.; Wang, J.J.; Feng, L.F. Modeling of coesterification process for biodegradable poly(butylene succinate-co-butylene terephthalate) copolyesters. Macromol. React. Eng. 2019, 13, 1800069. [Google Scholar] [CrossRef]
- Song, H.; Sang, Y.L. Production of succinic acid by bacterial fermentation. Enzym. Microb. Tech. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Succinic acid: A new platform chemical for biobased polymers from renewable resources. Chem. Eng. Technol. 2008, 31, 647–654. [Google Scholar] [CrossRef]
- Qin, P.K.; Wu, L.B.; Li, B.-G.; Li, N.X.; Pan, X.H.; Dai, J.M. Superior gas barrier properties of biodegradable PBST vs. PBAT copolyesters: A comparative study. Polymers 2021, 13, 3449. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Z.; Tu, Z.; Liang, J.M.; Wei, Z.Y. Barrier properties of biodegradable aliphatic–aromatic copolyesters (PBXT series). Macromol. Chem. Phys. 2024, 225, 2400051. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.G.; Luo, B.J.; Cui, Y.; Shu, S.L.; Chen, W.; Wang, L. Effect of styrene-maleic anhydride copolymer on properties of PBST/PLA blends. Polymers 2023, 15, 952. [Google Scholar] [CrossRef]
- Chen, P.; Gao, X.L.; Zhao, L.; Xu, Z.M.; Li, N.X.; Pan, X.H.; Dai, J.M.; Hu, D.D. Preparation of biodegradable PBST/PLA microcellular foams under supercritical CO2: Heterogeneous nucleation and anti-shrinkage effect of PLA. Polym. Degrad. Stab. 2022, 197, 109844. [Google Scholar] [CrossRef]
- Yan, X.Y.; Xie, R.H.; Pan, H.W.; Zhao, T.; Han, L.J.; Bian, J.J.; Yang, H.L.; Zhao, Y.; Wu, G.F.; Zhang, H.L. Effect of 1,4-bis(tert-butyl peroxy isopropyl) benzene on the rheological, mechanical, thermal and barrier properties of poly(butylene succinate-co-terephthalate)/poly(lactic acid) blends and blown films. Mater. Today Commun. 2022, 31, 103830. [Google Scholar] [CrossRef]
- Yan, X.Y.; Liu, C.K.; He, L.T.; Li, C.T.; Wang, D.M.; Wu, G.F.; Bian, J.J.; Zhao, Y.; Zhang, H.L. Biodegradable blends of poly(butylene succinate-co-terephthalate) and stereocomplex polylactide with enhanced rheological, mechanical properties, heat resistance and hydrolytic degradation. J. Mater. Scie. 2023, 58, 6391–6404. [Google Scholar] [CrossRef]
- Xue, K.; Chen, P.; Yang, C.; Xu, Z.M.; Zhao, L.; Hu, D.D. Low-shrinkage biodegradable PBST/PBS foams fabricated by microcellular foaming using CO2 & N2 as co-blowing agents. Polym. Degrad. Stab. 2022, 206, 110182. [Google Scholar]
- Yan, X.Y.; Chen, L.; Tian, H.L.; Jia, S.L.; Wang, X.Y.; Pan, H.W.; Han, L.J.; Bian, J.J.; Yang, H.L.; Wu, G.F.; et al. Enhancement of the compatibility, mechanical properties, and heat resistance of poly(butylene succinate-co-terephthalate)/poly(butylene succinate) blends by the addition of chain extender and nucleating agent. J. Polym. Res. 2023, 30, 111. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.X.; Yu, J.Y. Non-isothermal crystallization behavior of biodegrdable poly(butylene succinate-co-terephthalate) (PBST) copolyesters. Therm. Sci. 2012, 16, 1480–1483. [Google Scholar] [CrossRef]
- Zheng, C.; Zhu, G.X.; Shi, Y.; Liu, L.Z.; Ren, M.Q.; Zhang, W.; Han, L. Crystallization, structures and properties of biodegradable poly(butylene succinate-co-butylene terephthalate) with a symmetric composition. Mater. Chem. Phys. 2021, 260, 124183. [Google Scholar] [CrossRef]
- Heidarzadeh, N.; Rafizadeh, M.; Taromi, F.A.; Puiggalí, J.; Del Valle, L.J.; Franco, L. Preparation of random poly(butylene alkylate-co-terephthalate)s with different methylene group contents: Crystallization and degradation kinetics. J. Polym. Res. 2017, 24, 163. [Google Scholar] [CrossRef]
- Xing, Q.Q.; Ruch, D.; Dubois, P.; Wu, L.B.; Wang, W.J. Biodegradable and High-Performance Poly(butylene adipate-coterephthalate)−Lignin UV-Blocking Films. ACS Sustain. Chem. Eng. 2017, 5, 10342–10351. [Google Scholar] [CrossRef]
- Xing, Q.Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.B.; Wang, W.J. Biodegradable UV-Blocking Films through Core−Shell Lignin−Melanin Nanoparticles in Poly(butylene adipate-co-terephthalate). ACS Sustainable Chem. Eng. 2019, 7, 4147–4157. [Google Scholar] [CrossRef]
- Li, F.X.; Luo, S.L.; Yu, J.Y. Mechanical, thermal properties and isothermal crystallization kinetics of biodegradable poly(butylene succinate-co-terephthalate) (PBST) fibers. J. Polym. Res. 2010, 17, 279–287. [Google Scholar] [CrossRef]
- Righettig, M.C.; Piuoli, M. Crystallization kinetics and melting behavior of poly(buty1ene adipate), poly(buty1ene isophthalate) and their copolymers. Macromol. Chem. Phys. 1998, 199, 2063–2070. [Google Scholar] [CrossRef]
- Cranston, E.; Kawada, J.; Raymond, S.; Morin, F.G.; Marchessault, R.H. Cocrystallization model for synthetic biodegradable poly(butylene adipate-co-butylene terephthalate). Biomacromolecules 2003, 4, 995–999. [Google Scholar] [CrossRef]



| Sample | φTPA a (mol%) | ϕBT b (mol%) | ϕw,BT c (wt%) | Ln,BX d | Ln,BT e | R f | IV g (dL/g) |
|---|---|---|---|---|---|---|---|
| PBAT48 | 46.0 | 47.8 | 50.2 | 2.07 | 1.89 | 1.01 | 1.15 |
| PBST48 | 46.0 | 48.4 | 54.5 | 2.03 | 1.97 | 1.00 | 1.21 |
| PBST44 | 42.0 | 44.4 | 50.5 | 2.24 | 1.81 | 1.00 | 1.08 |
| Sample | Tc b (°C) | ΔHc c (J/g) | t1/2 d (s) | Tg (°C) | Tcc (°C) | ΔHcc e (J/g) | Tm (°C) | ΔHm f (J/g) |
|---|---|---|---|---|---|---|---|---|
| Cooling at 10 °C/min | 2nd heating at 10 °C/min | |||||||
| PBAT48 | 78 | 19.8 | 43 | −31 | nd | nd | 122 | 19.2 |
| PBST48 | 78 | 20.4 | 45 | −13 | nd | nd | 128 | 19.7 |
| PBST44 | 47 | 17.3 | 98 | −16 | 23 | 1.2 | 112 | 18.8 |
| PBAT g | 82, 80 | 8.3, 13.1 | / | −33, −35 | / | / | 120, 119 | 5.7, 8.5 |
| Cooling at 40 °C/min | 2nd heating at 10 °C/min | |||||||
| PBAT48 | 63 | 20.1 | 15 | −31 | nd | nd | 118 | 20.6 |
| PBST48 | 51 | 16.9 | 30 | −14 | 21 | 4.7 | 127 | 21.6 |
| PBST44 | nd | nd | nd | −5 | 24 | 15.4 | 111 | 19.2 |
| Sample | Tc (°C) | n | K (min−n) | t1/2 (min) |
|---|---|---|---|---|
| PBAT48 | 78 | 3.2 | 3.3 | 0.61 |
| PBST48 | 78 | 3.2 | 3.3 | 0.61 |
| PBST44 | 47 | 3.1 | 2.3 | 0.68 |
| Sample | Tm1 (°C) | ΔHm1 (J/g) | Tm2 (°C) | ΔHm2 (J/g) | ΔHm,sum a (J/g) |
|---|---|---|---|---|---|
| PBAT48 | 88 | 2.6 | 123 | 16.2 | 18.8 |
| PBST48 | 88 | 2.4 | 130 | 19.5 | 21.9 |
| PBST44 | 59 | 1.9 | 115 | 18.2 | 20.1 |
| Sample | Haze (%) | Trans (%) | E a (MPa) | σy b (MPa) | σb c (MPa) | εy d (%) | εb e (%) |
|---|---|---|---|---|---|---|---|
| PBAT48 | 98.4 | 45.0 | 107 ± 1 | 7.7 ± 0.1 | 55 ± 1 | 19.8 ± 0.1 | 1110 ± 10 |
| PBST48 | 98.7 | 51.3 | 144 ± 5 | 9.9 ± 0.2 | 56 ± 2 | 19.3 ± 0.4 | 995 ± 12 |
| PBST44 | 98.5 | 53.7 | 97 ± 1 | 7.5 ± 0.2 | 49 ± 3 | 22.4 ± 0.3 | 1040 ± 42 |
| PBAT f | / | / | 46 ± 6 | / | 29 ± 4 | / | 655 ± 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, P.; Wu, L. A Comparative Study on the Melt Crystallization of Biodegradable Poly(butylene succinate-co-terephthalate) and Poly(butylene adipate-co-terephthalate) Copolyesters. Polymers 2024, 16, 2445. https://doi.org/10.3390/polym16172445
Qin P, Wu L. A Comparative Study on the Melt Crystallization of Biodegradable Poly(butylene succinate-co-terephthalate) and Poly(butylene adipate-co-terephthalate) Copolyesters. Polymers. 2024; 16(17):2445. https://doi.org/10.3390/polym16172445
Chicago/Turabian StyleQin, Pengkai, and Linbo Wu. 2024. "A Comparative Study on the Melt Crystallization of Biodegradable Poly(butylene succinate-co-terephthalate) and Poly(butylene adipate-co-terephthalate) Copolyesters" Polymers 16, no. 17: 2445. https://doi.org/10.3390/polym16172445







