A Dynamic Mechanical Analysis on the Compatibilization Effect of Two Different Polymer Waste-Based Compatibilizers in the Fifty/Fifty Polypropylene/Polyamide 6 Blend
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Procedures
3. Results and Discussion
3.1. Background
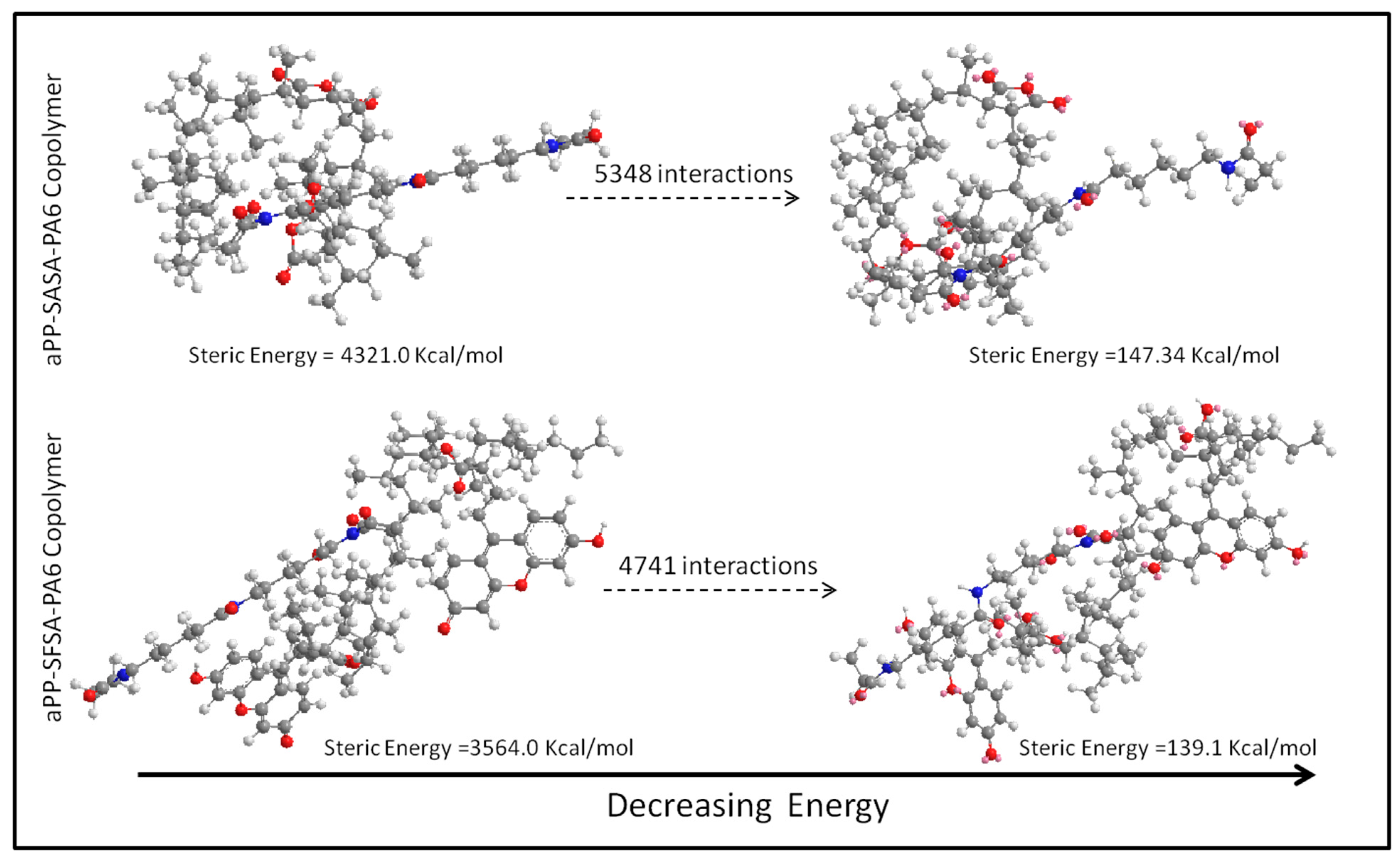
3.1.1. Molecular Modeling
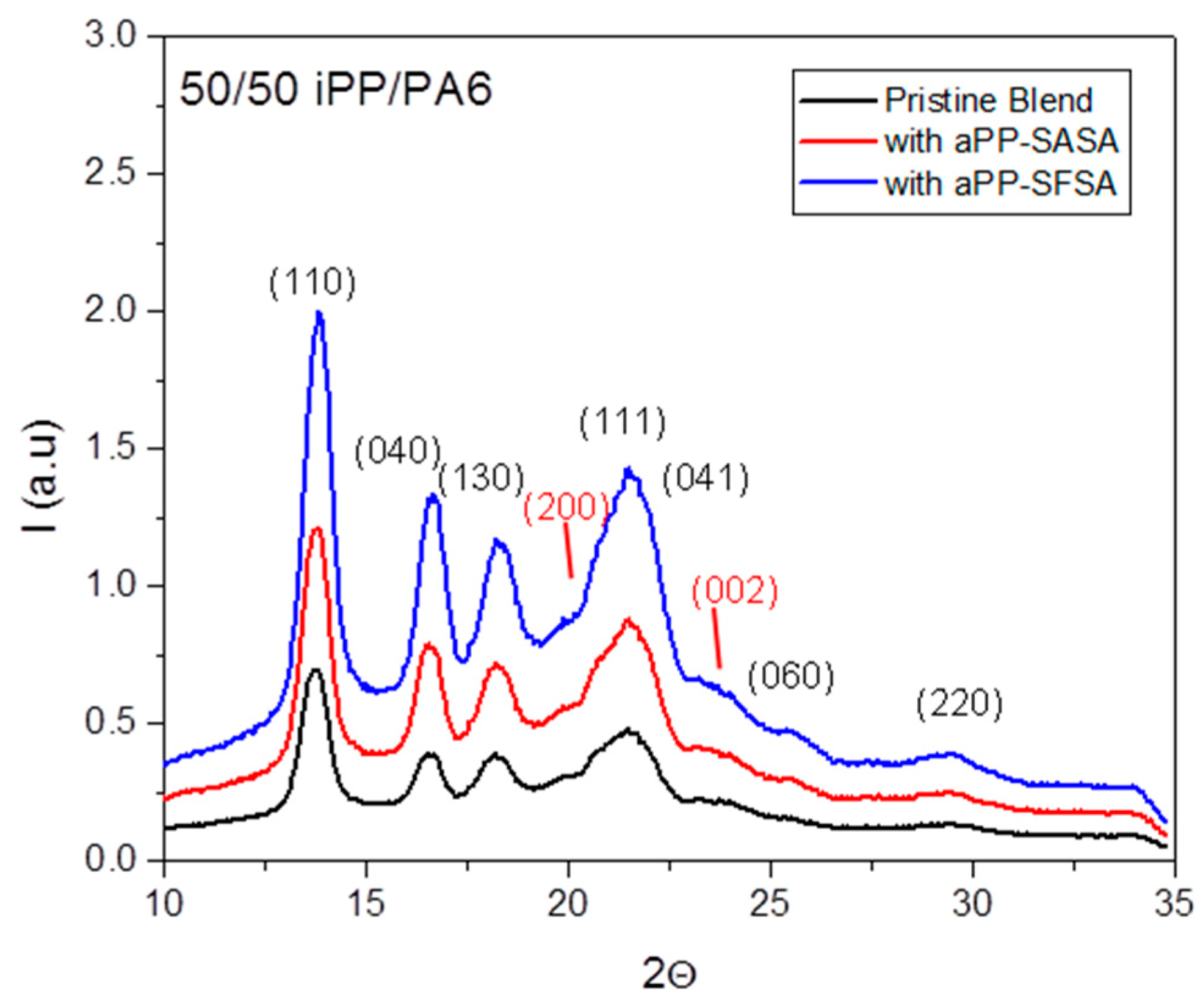
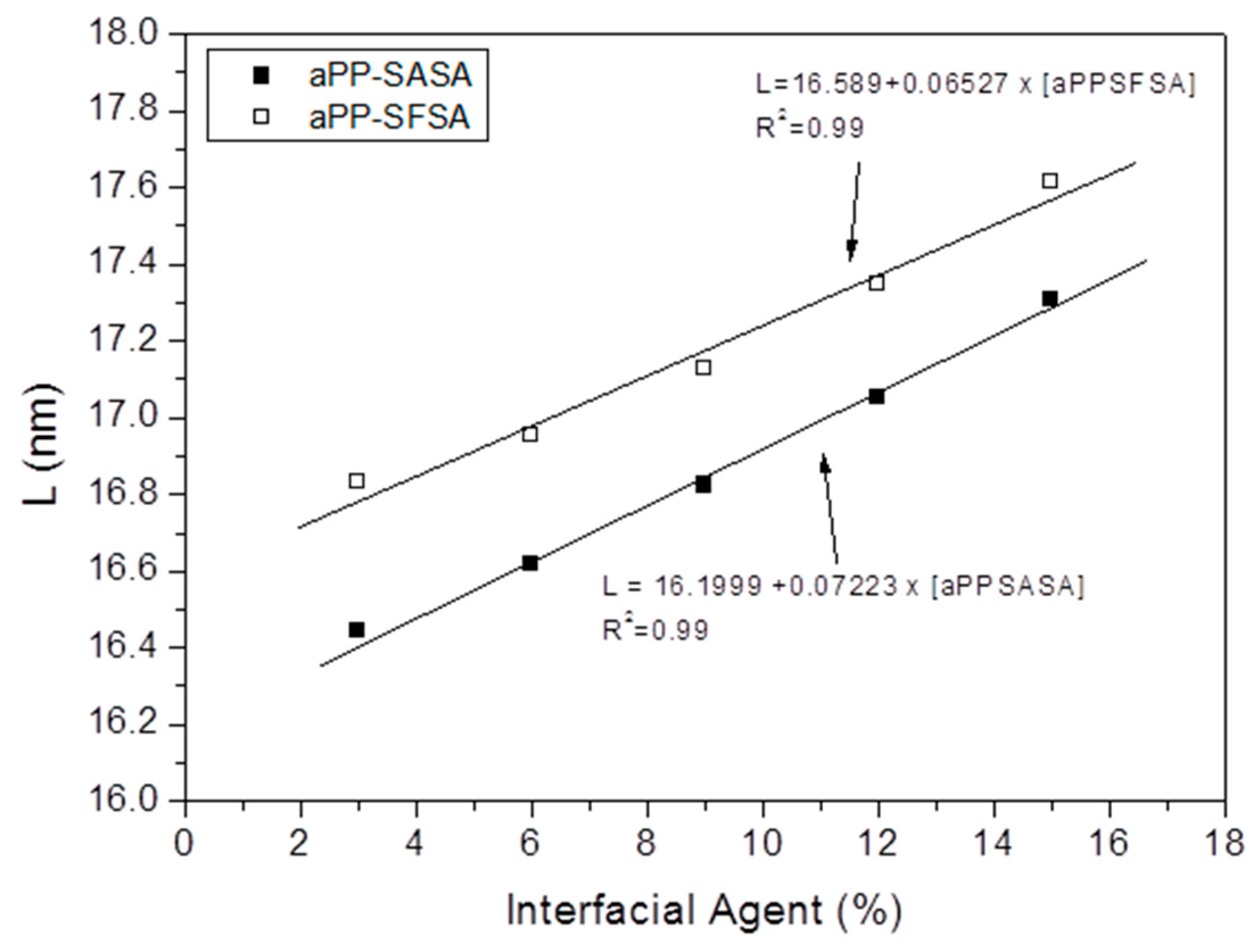
3.1.2. X-ray Diffraction Studies
3.1.3. Tensile Properties
3.2. Dynamic Mechanical Analysis
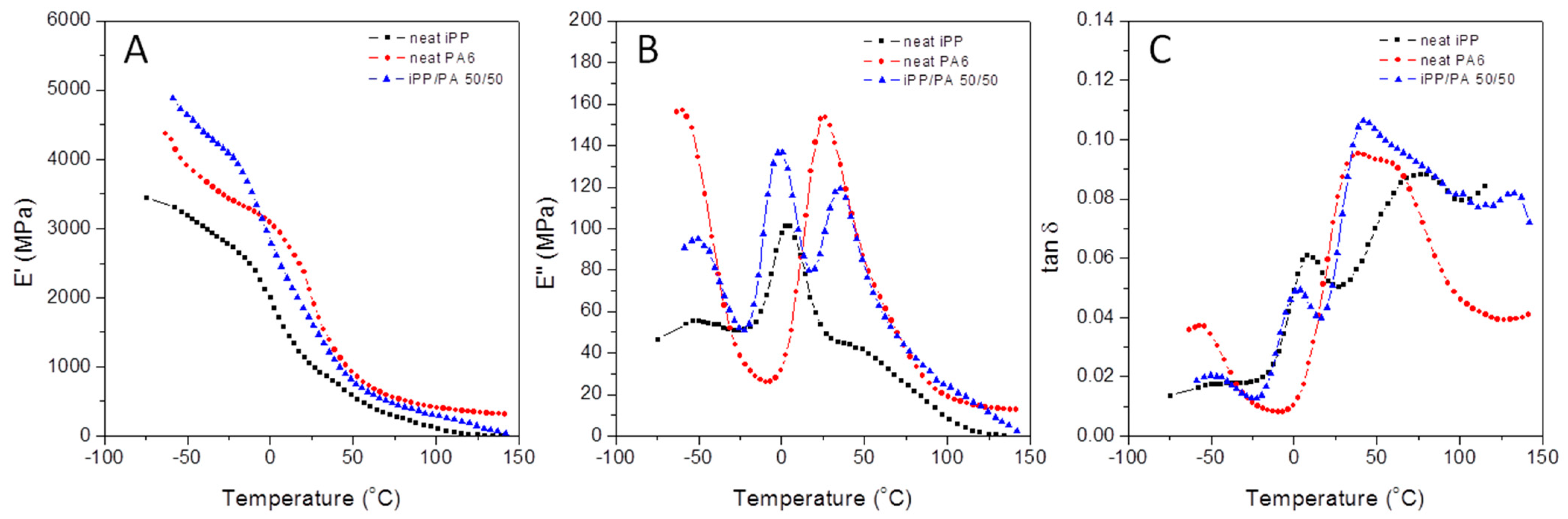
3.2.1. Unmodified iPP, PA6, and iPP/PA Blends
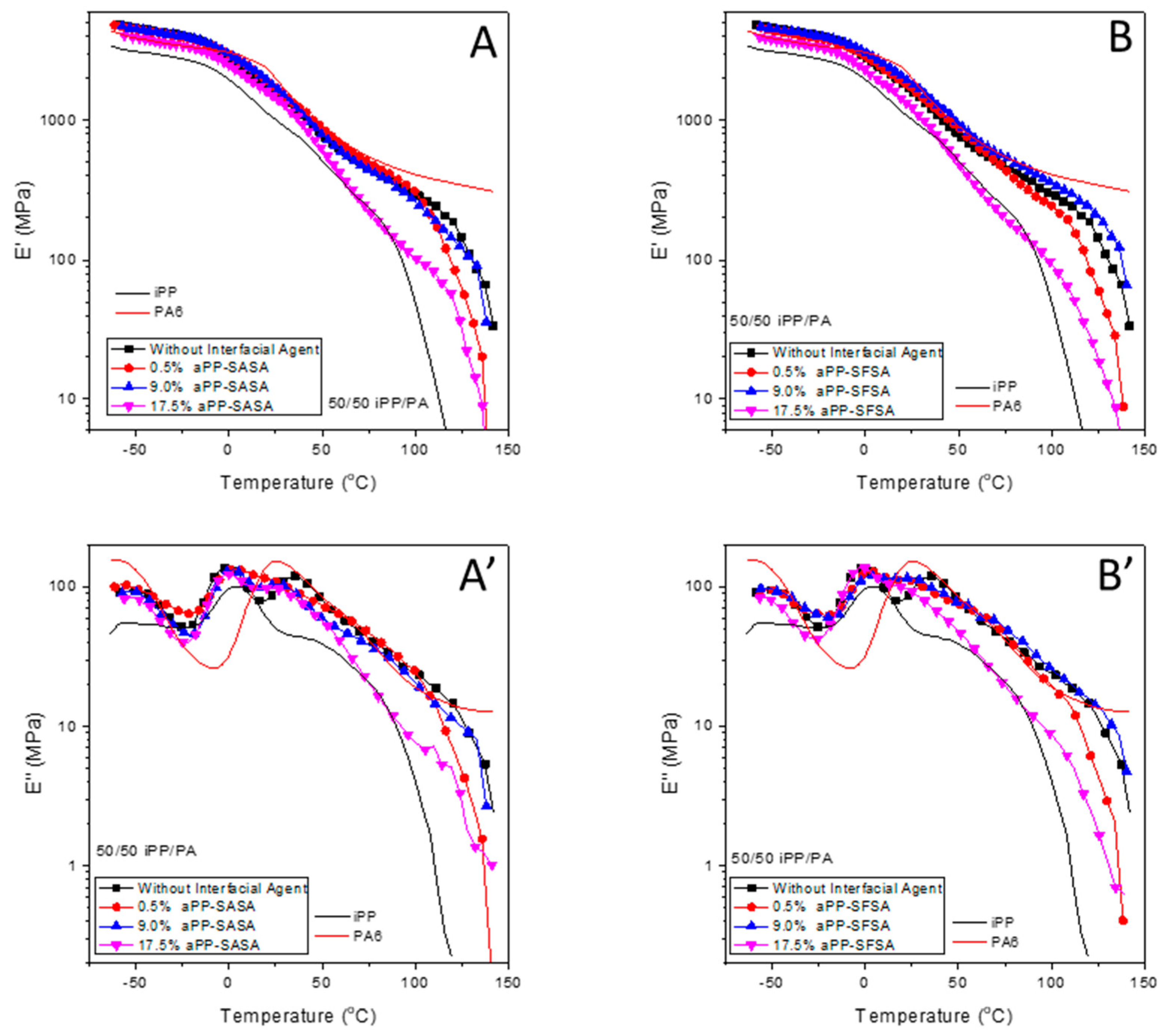
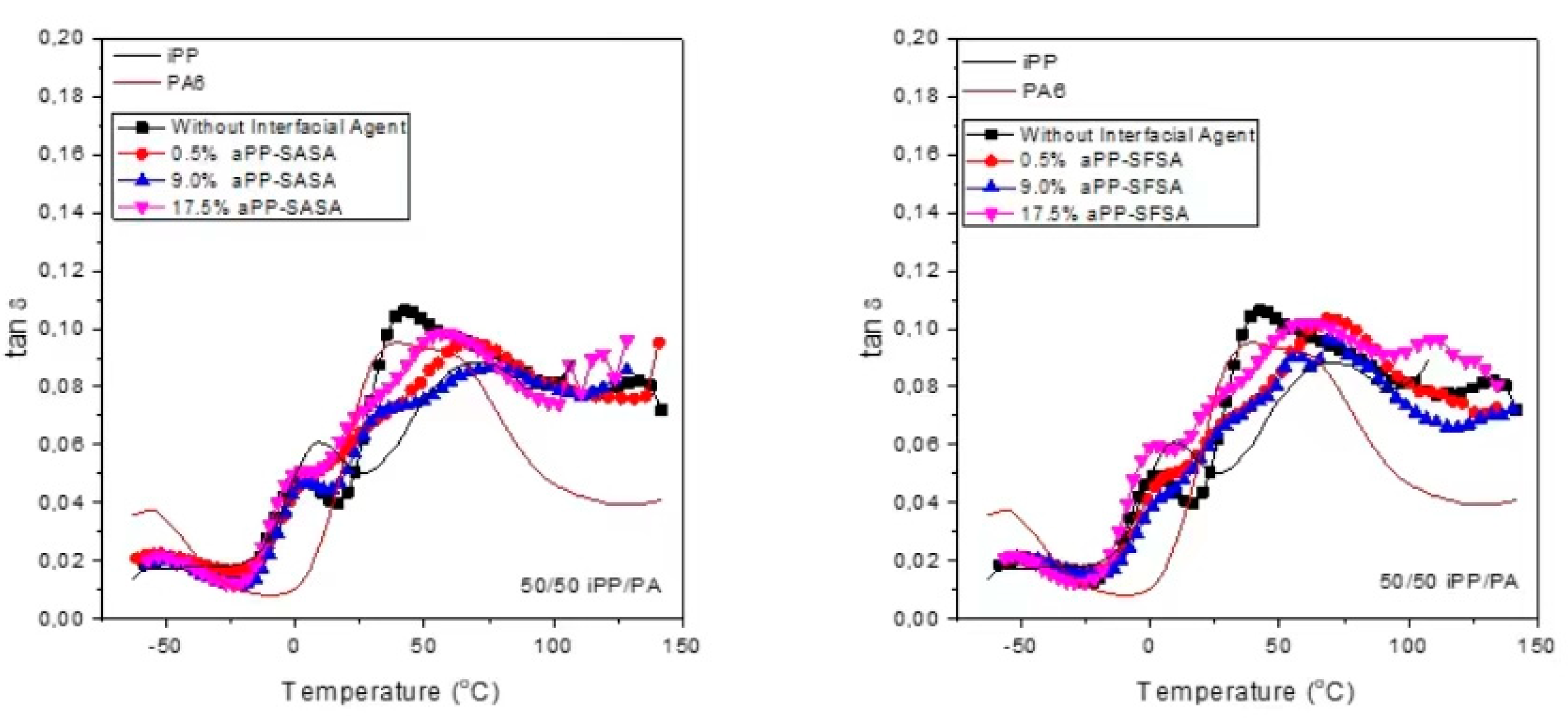
3.2.2. DMA of the Modified 50/50 iPP/PA6 Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pernot, H.; Baumert, M.; Court, F.; Leibler, L. Design and properties of co-continuous nanostructured polymers by reactive blending. Nat. Mater. 2002, 1, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Kornfield, J.A.; Kumaraswamy, G.; Issaian, A.M. Recent advances in understanding flow effects on polymer crystallization. Ind. Eng. Chem. Res. 2002, 41, 6383–6392. [Google Scholar] [CrossRef]
- Utracki, L.A. Polymer Alloys and Blends; Hanser: Munich, Germany, 1989. [Google Scholar]
- Tadmor, C.G.; Gogos, C.G. Principles of Polymer Processing, 2nd ed.; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Cristini, V.; Hooper, R.W.; Macosko, C.W.; Simeone, M.; Guido, S. A numerical and experimental investigation of lamellar blend morphologies. Ind. Eng. Chem. Res. 2002, 41, 6305–6311. [Google Scholar] [CrossRef]
- Van Puyvelde, P.; Vananroye, A.; Cardinaels, R.; Moldenaers, P. Review on morphology development of immiscible blends in confined shear flow. Polymer 2009, 49, 5363–5372. [Google Scholar] [CrossRef]
- Camesasca, M.; Manas-Zloczower, I. Danckwerts revisited-The use of entropy to define scale and intensity of segregation. Macromol. Theory Simul. 2009, 18, 87–96. [Google Scholar] [CrossRef]
- Berezkin, A.V.; Guseva, D.V.; Kudrayavtsev, V. Formation of linear and graft copolymers at a Polymer/polymer interface: How copolymer brush and microdomain morphology control heterogeneous reactions. Macromolecules 2012, 45, 8910–8920. [Google Scholar] [CrossRef]
- de Gennes, P.G. Conformation of polymers attached to an interface. Macromolecules 1980, 13, 1069–1075. [Google Scholar] [CrossRef]
- Baschnagel, J.; Meyer, H.; Varnik, F.; Metzger, S.; Aichele, M.; Müller, M.; Binder, K. Computer simulations of Polymers close to solid interfaces: Some selected topics. Interf. Sci. 2003, 11, 159–173. [Google Scholar] [CrossRef]
- Tonzani, S. The renaissance of Polyolefins. J. Appl. Polym. Sci. 2013, 127, 837. [Google Scholar] [CrossRef]
- Galli, P.; Vecellio, G. Polyolefins: The Most Promising Large-Volume Materials for the 21st Century. J. Polym. Sci. Polym. Chem. 2004, 42, 396–415. [Google Scholar] [CrossRef]
- Gardiner, F. Plastics and Environment; Smithers Rapra: Shrewsbury, UK, 2010. [Google Scholar]
- Collar, E.P.; García-Martínez, J.M. Environment, Health and Safety: Regulatory and Legislative Issues. In Handbook of Thermoplastics, 2nd ed.; Olabisi, O., Adewale, K., Eds.; CRC Press: Boca Raton, FL, USA; Hoboken, NJ, USA, 2016; Volume 28, pp. 939–954. ISBN 9781466577237. [Google Scholar]
- García-Martínez, J.M.; Collar, E.P. Recycling of Thermoplastics. In Handbook of Thermoplastics, 2nd ed.; Olabisi, O., Adewale, K., Eds.; CRC Press: Boca Raton, FL, USA; Hoboken, NJ, USA, 2016; Volume 27, pp. 919–940. ISBN 9781466577220. [Google Scholar]
- Anjum, N.; Gulrez SK, H.; Singh, H.; Gupta, B. Development of antimicrobial polypropylene sutures by graft polymerization. I. Influence of grafting conditions and characterization. J. Appl. Polym. Sci. 2006, 101, 3895–3901. [Google Scholar] [CrossRef]
- Toth, R.; Ferrone, M.; Miertus, S.; Chiellini, E.; Fermeglia, M.; Priel, S. Structure and energetics of biocompatible polymer nanocomposite systems: A molecular dynamics study. Biomacromolecules 2006, 7, 1714–1719. [Google Scholar] [CrossRef]
- Poon, B.C.; Chum, S.P.; Hiltner, A.; Baer, E. Modifying adhesion of linear low-density polyethylene to polypropylene by blending with a homogeneous ethylene copolymer. J. Appl. Polym. Sci. 2004, 92, 109–115. [Google Scholar] [CrossRef]
- Poon, B.C.; Chum, S.P.; Hiltner, A.; Baer, E. Adhesion to polyethylene blends to polypropylene. Polymer 2004, 45, 893–903. [Google Scholar] [CrossRef]
- Hiltner, A.; Liu RY, F.; Hu, Y.S.; Baer, E. Oxygen transport as a solid state structure probe for polymeric materials: A review. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1047–1063. [Google Scholar] [CrossRef]
- Jarus, D.; Hiltner, A.; Baer, E. Barrier properties of polypropylene/polyamide blends produced by microlayer extrusion. Polymer 2002, 43, 2401–2408. [Google Scholar] [CrossRef]
- Jang, B.N.; Costache, M.; Wilkie, C.A. The relationship between thermal degradation behavior of polymer and the fire retardancy of polymer/nanoclay nanocomposites. Polymer 2005, 46, 10678–10687. [Google Scholar] [CrossRef]
- Song, P.; Shen, Y.; Du, B.; Peng, M.; Shen, L.; Fang, Z. Effects of Reactive Compatibilization on the morphological, thermal, mechanical, and rheological properties of intumescent flame retardant polypropylene. Appl. Mater. Interfaces 2009, 1, 452–459. [Google Scholar] [CrossRef]
- Lomakin, S.M.; Zaikov, G.E.; Koverzanova, E.V. Thermal degradation and combustibility of polypropylene filled with magnesium hydroxide micro-filler and polypropylene nano-filled aluminosilicate composite. Oxid. Commun. 2005, 28, 451–464. [Google Scholar]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Ebert, K.; Fritsch, D.; Ohlrogge, K.; Paul, D.; Peinemann, K.-V.; Pereira-Nunes, S.; Scharnagl, N.; et al. Developments in membrane research: From material via process design to industrial application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Presting, H.; König, U. Future nanotechnology developments for automotive applications. Mater. Sci. Eng. 2003, C23, 737–741. [Google Scholar] [CrossRef]
- Gahleitner, M. Polyolefins for the 21st century. Express Polym. Lett. 2011, 5, 936. [Google Scholar] [CrossRef]
- Joanny, J.F. Polymer at Interfaces. Interface Sci. 2003, 11, 157–158. [Google Scholar] [CrossRef]
- Huang, J.; Ichinose, I.; Kunitake, T. Nanocoating of natural cellulose fibers with conjugated polymers: Hierarchical polypyrrole composite materials. Chem. Commun. 2005, 13, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.P. Nanoscience and engineering. J. Phys. Chem. Solids 2004, 65, 1501–1506. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Areso, S.; Taranco, J.; Collar, E.P. Heterogeneous Materials Based on Polypropylene. In Polyolefin Blends; Kyu, T., Nwabunma, D., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2008; Volume 13, pp. 379–410. [Google Scholar]
- Collar, E.P.; García-Martínez, J.M. On Chemical Modified Polyolefins by Grafting of Polar Monomers. A Survey Based on Recent Patents Literature. Recent Pat. Mater. Sci. 2010, 3, 76–91. [Google Scholar] [CrossRef]
- Ruiz-Pérez, L.; Royston, J.; Patrick, J.; Fairclough, A.; Ryan, A.J. Toughening by nanostructure. Polymer 2008, 49, 4475–4488. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from Preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Allegra, G.; Raos, G.; Vacatello, M. Theories and simulations of polymer-based nanocomposites: From chain statistics to reinforcement. Prog. Polym. Sci. 2008, 33, 683–731. [Google Scholar] [CrossRef]
- Collar, E.P.; Areso, S.; Taranco, J.; García-Martínez, J.M. Understanding the Morphological Changes in the Polypropylene/Polyamide 6 Fifty/Fifty Blends by InterfacialModifiers Based on Grafted AtacticPolypropylenes: Microscopic, Mechanical, and Thermal Characterization. J. Polym. 2015, 2015, 620362. [Google Scholar] [CrossRef]
- García Martínez, J.M.; Areso, S.; Collar, E.P. The transient nature of maximum maleic anhydride grafting on polypropylene: A mechanistic approach based on a consecutive reaction model. I. Batch solution process. J. Appl. Polym. Sci. 2006, 102, 1182–1190. [Google Scholar] [CrossRef]
- García Martínez, J.M.; Areso, S.; Collar, E.P. The transient nature of maximum maleic anhydride grafting on polypropylene: A mechanistic approach based on a consecutive reaction model. II. A comparison between the batch solution and molten state process. J. Appl. Polym. Sci. 2007, 104, 345–351. [Google Scholar] [CrossRef]
- García Martínez, J.M.; Areso, S.; Laguna, O.; Collar, E.P. FT-IR Quantitative characterization of chemically modified polypropylenes containing succinic grafted groups. J. Appl. Polym. Sci. 1999, 73, 2837–2847. [Google Scholar] [CrossRef]
- García Martínez, J.M.; Areso, S.; Laguna, O.; Collar, E.P. Changes on glass transition temperature in atactic polypropylene containing polar groups. J. Therm. Anal. Calorim. 1999, 58, 541–550. [Google Scholar]
- O’Shaughnessy, B.; Sawhney, U. Reaction kinetics at polymer-polymer interfaces. Macromolecules 1996, 29, 7230–7239. [Google Scholar] [CrossRef]
- O’Shaughnessy, B.; Vavylonis, D. Irreversibility and polymer adsorption. Phys. Rev. Lett. 2003, 90, 56103–56107. [Google Scholar] [CrossRef] [PubMed]
- Sadiku-Agboola, O.; Sadiku, E.R.; Adegbola, A.T.; Biotidara, O.F. Rheological properties of polymers: Structure and morphology of molten polymer blends. Mater. Sci. Appl. 2011, 2, 30–41. [Google Scholar] [CrossRef]
- Cardinaels, R.; Vananroye, A.; Puyvelde, P.V.; Moldenaers, P. Breakup criteria for confined droplets: Effects of compatibilization and component viscoelasticity. Macromol. Mater. Eng. 2011, 296, 214–222. [Google Scholar] [CrossRef]
- Ajji, A.; Utracki, L.A. Interphase and compatibilization of Polymer Blends. Polym. Eng. Sci. 1996, 36, 1574–1585. [Google Scholar] [CrossRef]
- Van Puyvelde, P.; Oommen, Z.; Koets, P.; Groeninckx, G.; Moldenaers, P. Effect of reactive compatibilization on the interfacial slip in nylon-6/EPR blends. Polym. Eng. Sci. 2003, 43, 71–77. [Google Scholar] [CrossRef]
- Shi, D.; Ke, Z.; Yang, J.; Gao, Y.; Wu, J.; Yin, J. Rheology and morphology of reactively compatibilized PP/PA6 blends. Macromolecules 2002, 35, 8005–8012. [Google Scholar] [CrossRef]
- Duvall, J.; Sellitti, C.; Myers, C.; Hiltner, A.; Baer, E. Effect of compatibilization on the properties of PP/PA66 (75/25 wt/wt) blends. J. Appl. Polym. Sci. 1994, 52, 195–206. [Google Scholar] [CrossRef]
- Cheng, M.H.; Balazs, A.C.; Yeung, C.; Ginzburg, V.V. Modeling reactive compatibilization of a binary blend with interacting particles. J. Chem. Phys. 2003, 118, 9044–9052. [Google Scholar] [CrossRef]
- Chow, W.S.; Abu Bakar, A.; Mohd Ishak, A.A.; Karger-Kocsis, J.; Ishiaku, U.S. Effect of maleic anhydride-grafted ethylene-propylene rubber on the mechanical, rheological and morphological properties of organoclay reinforced polyamide 6/polypropylene nanocomposites. Eur. Polym. J. 2005, 41, 687–696. [Google Scholar] [CrossRef]
- Ciardelli, F.; Coiai, S.; Passaglia, E.; Pucci, A.; Ruggeri, G. Nanocomposites based on polyolefins and functional thermoplastic materials. Polym. Int. 2008, 57, 805–836. [Google Scholar] [CrossRef]
- Zenga, Q.H.; Yua, A.B.; Lub, G.Q. Multiscale modeling and simulation of polymer nanocomposites. Prog. Polym. Sci. 2008, 33, 191–269. [Google Scholar] [CrossRef]
- Gahleitner, M. Melt rheology of Polyolefins. Prog. Polym. Sci. 2001, 26, 895–944. [Google Scholar] [CrossRef]
- Ginzburg, V.V.; Peng, G.; Qiu, F.; Jasnow, D.; Balazs, A.C. Kinetic model of phase separation in binary mixtures with hard mobile impurities. Phys. Rev. E 1999, 60, 4352–4359. [Google Scholar] [CrossRef]
- Hietaoja, P.T.; Holsti-Miettinen, R.M.; Seppälä, J.V.; Ikkala, O.T. The effect of viscosity ratio on the phase inversion of polyamide 66/polypropylene blends. J. Appl. Polym. Sci. 1994, 54, 1613–1623. [Google Scholar] [CrossRef]
- Gabriele, M.; Pasquino, R.; Grizzuti, N. Effects of viscosity-controlled interfacial mobility on the coalescence of immiscible polymer blends. Macromol. Mater. Eng. 2011, 296, 263–269. [Google Scholar] [CrossRef]
- González-Montiel, A.; Keskkula, H.; Paul, D.R. Morphology of nylon 6/ polypropylene blends compatibilized with maleated polypropylene, Journal of Polymer Science. Polym. Phys. 1995, 33, 1751–1767. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Collar, E.P. The Role of a Succinyl Fluorescein-Succinic Anhydride Grafted Atactic Polypropylene on the Dynamic Mechanical Properties of Polypropylene/Polyamide-6 Blends at the Polypropylene Glass Transition. Polymers 2020, 12, 1216. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Collar, E.P. Industrial Waste Origin Succinic anhydride grafted atactic polypropylene as compatibilizer of full range Polypropylene/Polyamide 6 Blends as Revealed by Dynamic Mechanical Analysis at the Polypropylene Glass Transition. Polym. Eng. Sci. 2019, 59, 2458–2466. [Google Scholar] [CrossRef]
- Verdier, C.; Vinagre HT, M.; Piau, M.; Joseph, D.D. High temperature interfacial tension measurements of PA6/PP interfaces compatibilized with copolymers using a spinning drop tensiometer. Polymer 2000, 41, 6683–6689. [Google Scholar] [CrossRef]
- García Martínez, J.M.; Areso, S.; Collar, E.P. Structure Development on PA6/PP Blends. Interfacial modifications and preliminary modellization starting on macroscopic properties. J. Macromol. Sci. Part B Phys. 2001, B40, 387–404. [Google Scholar] [CrossRef]
- García Martínez, J.M.; Marco, C.; Areso, S.; Collar, E.P. Thermal behavior of PA6/PP blends under dynamic conditions. The effect of different interfacial agents based on chemically modified atactic polypropylene. J. Macromol. Science. Part B Phys. 2001, B40, 369–385. [Google Scholar]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters; Wiley: New York, NY, USA, 1978. [Google Scholar]
- García Martínez, J.M.; Areso, S.; Gómez, M.A.; Marco, C.; Collar, E.P. Role of Succinic Anhydride Grafted Atactic Polypropylene (a-PP-SA) on the Melting and Crystallization Behaviour under Dynamic Conditions of the PP Phase in Interfacial Modified Polypropylene/Polyamide 6 Blends. A Synchrotron WAXS and SAXS Study. HASYLAB Annual Report 2002 Part I. pp. 845–846. Available online: http://hasyweb.desy.de/science/annual_reports/2002_report/index.html (accessed on 15 January 2022).
- García Martínez, J.M.; Areso, S.; Gómez, M.A.; Marco, C.; Collar, E.P. On the Changes of the PP Phase in Interfacial Modified PP/PA6 by Means of Succinic Anhydride or Succinyl-Fluoresceine/Succinic Anhydride Grafted Atactic Polypropylenes. A Synchrotron WAXS and SAXS Comparative Study. HASYLAB Annual Report 2003 Part I. pp. 957–958. Available online: http://hasyweb.desy.de/science/annual_reports/2003_report/index.html (accessed on 15 January 2022).
- Zhao, Z.; Yu, W.; Liu, Y.; Zhang, J.; Shao, Z. Isothermal crystallization behaviors of nylon 6/montmorillonite nanocomposite. Mater. Lett. 2004, 58, 802–806. [Google Scholar] [CrossRef]
- Phillips, P.J. Polymer crystals. Rep. Prog. Phys. 1990, 53, 549–604. [Google Scholar] [CrossRef]
- Wu, M.-H.; Wang, C.-C.; Chen, C.-Y. Chemical modification of atactic polypropylene and its applications as a crystallinity additive and compatibility agent. Polymer 2020, 194, 122386. [Google Scholar] [CrossRef]
- McCrum, N.G.; Read, B.E.; Williams, G. Anelastic and Dielectric Effects in Polymer Solids; Wiley: London, UK, 1967. [Google Scholar]
- Ong, E.S.; Kim, Y.; Williams, L. Dynamic Mechanical Properties of some nylon and their Blends. J. Appl. Polym. Sci. 1986, 31, 367–383. [Google Scholar] [CrossRef]
- Liang, Z.; Williams, H.L. Dynamic Mechanical properties of Polypropylene-Polyamide Blends: Effect of Compatibilization. J. Appl. Polym. Sci. 1992, 44, 699–717. [Google Scholar] [CrossRef]
- Kohan, M. Nylon Plastics Handbook; Hanser: Wilmington, NC, USA, 1995. [Google Scholar]
- García-Martínez, J.M.; Areso, S.; Taranco, J.; Collar, E.P. Dynamic Mechanical Analysis of the interfacial changes in Polypropylene/Talc composites induced by Different Interfacial Modifications from the Reinforcement side. J. Appl. Polym. Sci. 2009, 114, 551–561. [Google Scholar] [CrossRef]
- Pepin, J.; Miri, V.; Lefebvre, J.M. New insights into the Brill Transition in Polyamide 11 and Polyamide 6. Macromolecules 2016, 49, 564–573. [Google Scholar] [CrossRef]
- Holmes, D.R.; Bunn, C.W.; Smith, D.J. The Crystal Structure of Polycaproamide: Nylon 6. J. Polym. Sci. 1955, 55, 159–177. [Google Scholar] [CrossRef]
- McCrum, N.G.; Buckley, C.P.; Bucknall, C.B. Principles of Polymer Engineering; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Passaglia, E.; Martin, G.M.J. Dependence of mechanical relaxation on morphology in isotactic polypropylene. Res. Natl. Bur. Stand. 1964, 68, 519. [Google Scholar] [CrossRef] [PubMed]
- Struick, L.C.E. The mechanical and physical ageing of semicrystalline polymers: 1. Polymer 1987, 28, 1521. [Google Scholar] [CrossRef]
- Struick, L.C.E. The mechanical behaviour and physical ageing of semicrystalline polymers: 2. Polymer 1987, 28, 1534. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]


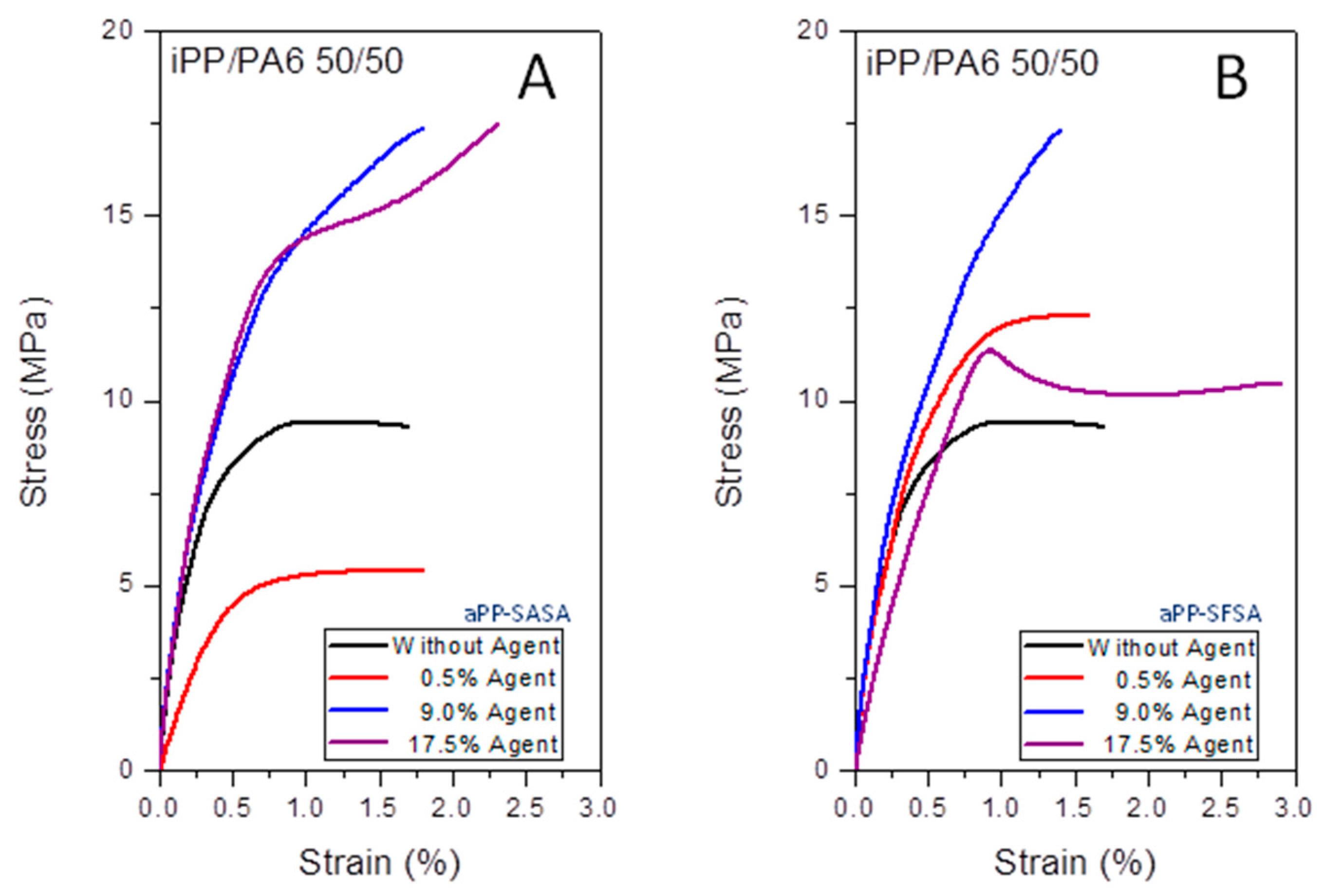
| Interfacial Agent | iPP/Interfacial Agent (w/w %) | Strength at Yield (MPa) | Strain at Yield (%) | Strength at Break (MPa) | Strain at Break (%) |
|---|---|---|---|---|---|
| None | 50/0 (fresh iPP/PA6) | 9.4 ± 0.1 | 0.70 ± 0.16 | 9.3 ± 0.1 | 1.51 ± 0.10 |
| aPP-SASA aPP-SFSA | 50/0.1 | 5.4 ± 0.1 12.3 ± 0.2 | 0.70 ± 0.12 1.31 ± 0.10 | 5.4 ± 0.1 12.3 ± 0.3 | 1.82 ± 0.18 1.61 ± 0.20 |
| aPP-SASA aPP-SFSA | 50/9.0 | 15.0 ± 0.1 17.0 ± 0.1 | 0.91± 0.12 1.33 ± 0.16 | 17.2 ± 0.1 17.3 ± 0.1 | 1.93 ± 0.21 1.42 ± 0.18 |
| aPP-SASA aPP-SFSA | 50/17.5 | 14.5 ± 0.2 11.9 ± 0.1 | 0.81 ± 0.08 0.92 ± 0.09 | 17.5 ± 0.2 10.5 ± 0.1 | 2.31 ± 0.21 2.92 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collar, E.P.; García-Martínez, J.-M. A Dynamic Mechanical Analysis on the Compatibilization Effect of Two Different Polymer Waste-Based Compatibilizers in the Fifty/Fifty Polypropylene/Polyamide 6 Blend. Polymers 2024, 16, 2523. https://doi.org/10.3390/polym16172523
Collar EP, García-Martínez J-M. A Dynamic Mechanical Analysis on the Compatibilization Effect of Two Different Polymer Waste-Based Compatibilizers in the Fifty/Fifty Polypropylene/Polyamide 6 Blend. Polymers. 2024; 16(17):2523. https://doi.org/10.3390/polym16172523
Chicago/Turabian StyleCollar, Emilia. P., and Jesús-María García-Martínez. 2024. "A Dynamic Mechanical Analysis on the Compatibilization Effect of Two Different Polymer Waste-Based Compatibilizers in the Fifty/Fifty Polypropylene/Polyamide 6 Blend" Polymers 16, no. 17: 2523. https://doi.org/10.3390/polym16172523







