Benzodiazole-Based Covalent Organic Frameworks for Enhanced Photocatalytic Dehalogenation of Phenacyl Bromide Derivatives
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
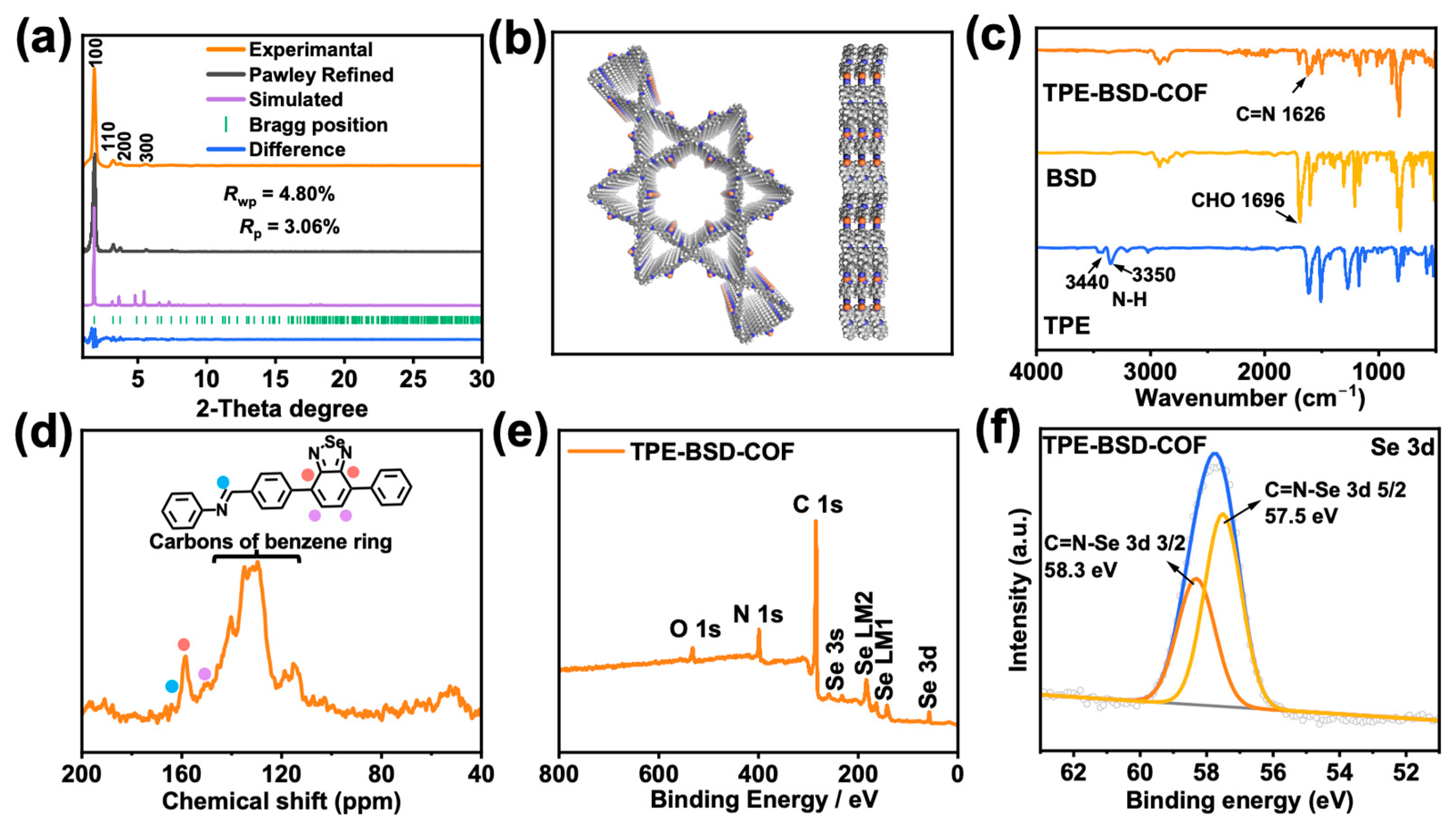
3.1. Synthesis and Characterization of COFs
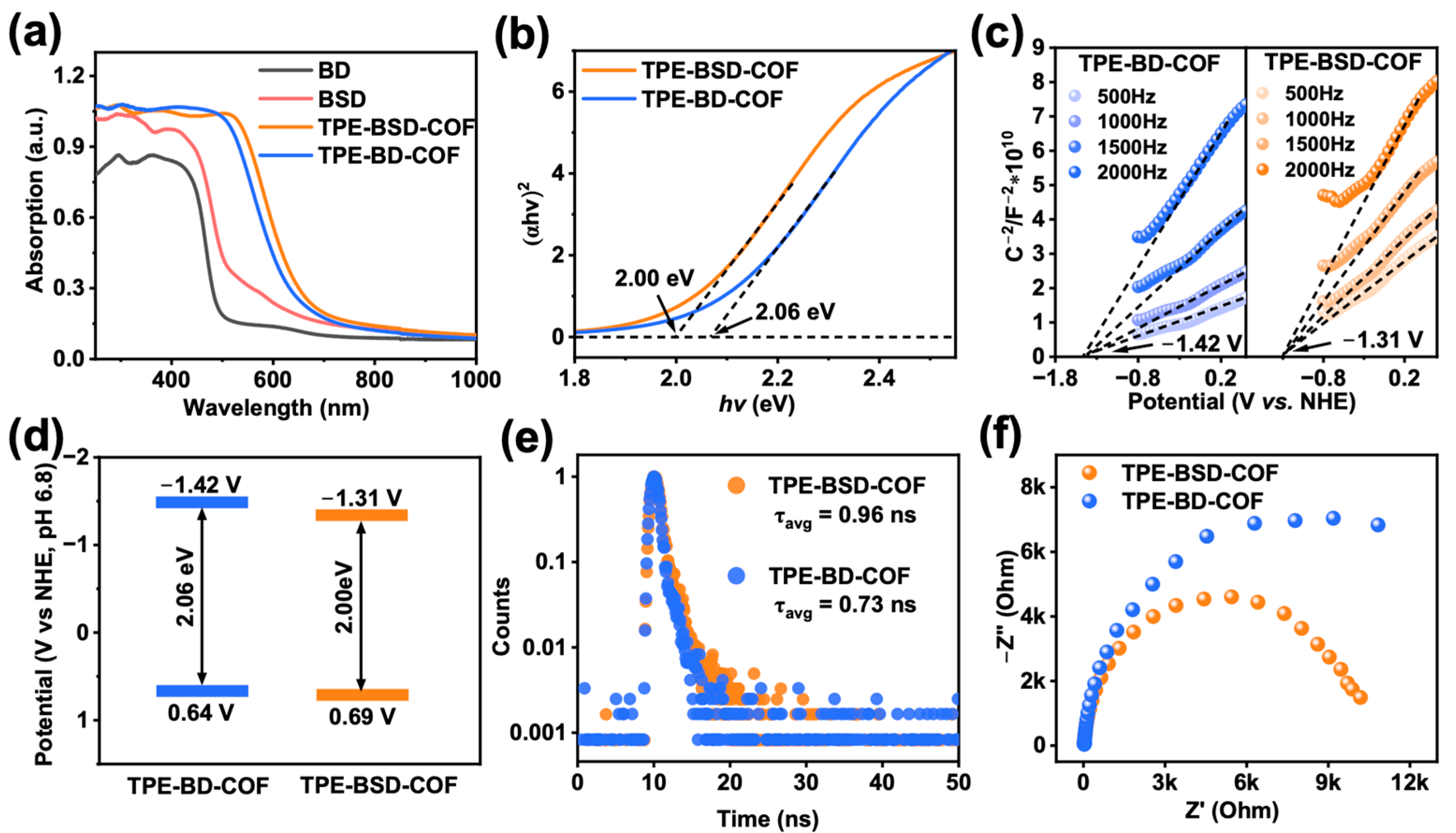
3.2. Photophysical/Electrochemical Properties
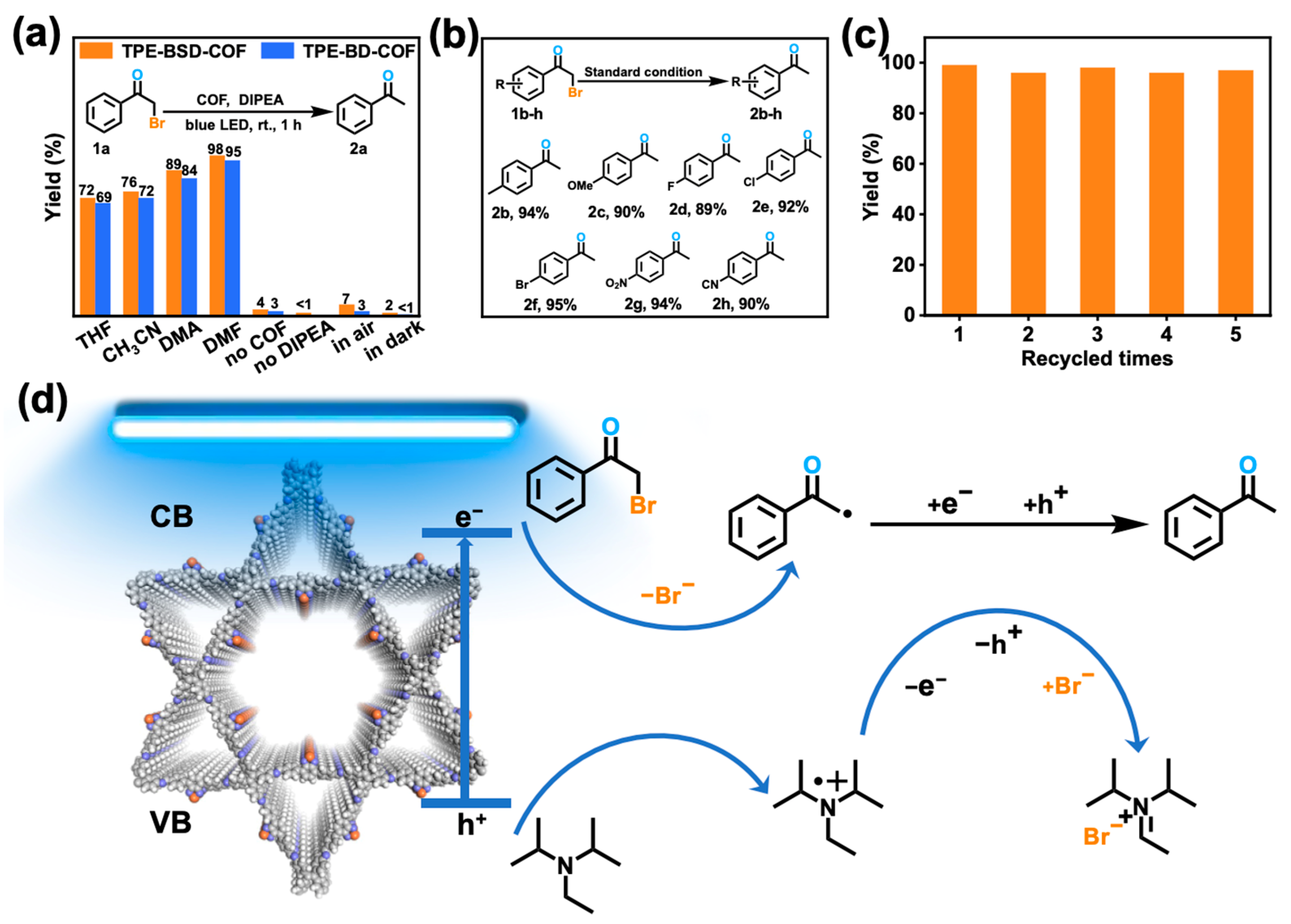
3.3. Photocatalytic Performance and Possible Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Parvatkar, P.T.; Kandambeth, S.; Shaikh, A.C.; Nadinov, I.; Yin, J.; Kale, V.S.; Healing, G.; Emwas, A.-H.; Shekhah, O.; Alshareef, H.N.; et al. A Tailored COF for Visible-Light Photosynthesis of 2,3-Dihydrobenzofurans. J. Am. Chem. Soc. 2023, 145, 5074–5082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Tong, H.; Ji, M.; Chu, W. A β-Ketoenamine-Linked Covalent Organic Framework as a Heterogeneous Photocatalyst for the Synthesis of 2-Arylbenzothiazoles by Cyclization Reaction. Green Chem. 2023, 25, 5195–5205. [Google Scholar] [CrossRef]
- Yuan, L.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Coupling Strategy for CO2 Valorization Integrated with Organic Synthesis by Heterogeneous Photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 21150–21172. [Google Scholar] [CrossRef]
- Ma, J.; Miao, T.J.; Tang, J. Charge Carrier Dynamics and Reaction Intermediates in Heterogeneous Photocatalysis by Time-Resolved Spectroscopies. Chem. Soc. Rev. 2022, 51, 5777–5794. [Google Scholar] [CrossRef]
- Liras, M.; Barawi, M.; O’Shea, V.A.d.l.P. Hybrid Materials Based on Conjugated Polymers and Inorganic Semiconductors as Photocatalysts: From Environmental to Energy Applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef]
- Friedmann, D.; Lee, A.F.; Wilson, K.; Jalili, R.; Caruso, R.A. Printing Approaches to Inorganic Semiconductor Photocatalyst Fabrication. J. Mater. Chem. A 2019, 7, 10858–10878. [Google Scholar] [CrossRef]
- Hoffman, E.; Kozakiewicz, K.; Rybczyńska, M.; Mońka, M.; Grzywacz, D.; Liberek, B.; Bojarski, P.; Serdiuk, I.E. Photochemical Transformation of a Perylene Diimide Derivative Beneficial for the in Situ Formation of a Molecular Photocatalyst of the Hydrogen Evolution Reaction. J. Mater. Chem. A 2024, 12, 5233–5243. [Google Scholar] [CrossRef]
- Rana, P.; Singh, N.; Majumdar, P.; Prakash Singh, S. Evolution of BODIPY/Aza-BODIPY Dyes for Organic Photoredox/Energy Transfer Catalysis. Coord. Chem. Rev. 2022, 470, 214698. [Google Scholar] [CrossRef]
- Ekande, O.S.; Kumar, M. Review on Polyaniline as Reductive Photocatalyst for the Construction of the Visible Light Active Heterojunction for the Generation of Reactive Oxygen Species. J. Environ. Chem. Eng. 2021, 9, 105725. [Google Scholar] [CrossRef]
- Shi, X.; Yu, Y.; Yang, Q.; Hong, X. Carboxyl Groups as Active Sites for H2O2 Decomposition in Photodegradation over Graphene Oxide/Polythiophene Composites. Appl. Surf. Sci. 2020, 524, 146397. [Google Scholar] [CrossRef]
- Zong, X.; Miao, X.; Hua, S.; An, L.; Gao, X.; Jiang, W.; Qu, D.; Zhou, Z.; Liu, X.; Sun, Z. Structure Defects Assisted Photocatalytic H2 Production for Polythiophene Nanofibers. Appl. Catal. B Environ. 2017, 211, 98–105. [Google Scholar] [CrossRef]
- Guo, L.; Gao, J.; Huang, Q.; Wang, X.; Li, Z.; Li, M.; Zhou, W. Element Engineering in Graphitic Carbon Nitride Photocatalysts. Renew. Sustain. Energy Rev. 2024, 199, 114482. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Li, Y.; Yan, S.; Jia, X.; Wu, J.; Yang, J.; Zhao, C.; Wang, S.; Song, H.; Yang, X. Uncovering the Origin of Full-Spectrum Visible-Light-Responsive Polypyrrole Supramolecular Photocatalysts. Appl. Catal. B Environ. 2021, 287, 119926. [Google Scholar] [CrossRef]
- Lin, H.; Yang, Y.; Festus, K.W.; Hsu, Y.-C.; Liang, R.-R.; Afolabi, I.; Zhou, H.-C. Integrating Photoactive Ligands into Dimension-Reduced Metal–Organic Frameworks: Harnessing the Power of Organic Photocatalysts. Acc. Mater. Res. 2024, 5, 236–248. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.-R.; Wang, J.; Yu, H.; Zhang, W.; Shen, L.-L.; Mei, D. Enhanced Intermolecular Electron Transfer in Fluorinated Metal–Organic Framework Photocatalysts for Efficient CO2 Reduction. Adv. Funct. Mater. 2024, 34, 2312691. [Google Scholar] [CrossRef]
- Han, X.; Wu, H.; Chen, S.; Deng, S.; Wang, J. Novel Layer-to-Layer Charge Transfer in an Anion-Pillared Metal-Organic Framework for Efficient CO2 Photoreduction to CH4. Chem. Eng. J. 2024, 479, 147694. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, G. Porous Aromatic Frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef]
- Cao, L.; Wang, C.; Wang, H.; Xu, X.; Tao, X.; Tan, H.; Zhu, G. Rationally Designed Cyclooctatetrathiophene-Based Porous Aromatic Frameworks (COTh-PAFs) for Efficient Photocatalytic Hydrogen Peroxide Production. Angew. Chem. Int. Ed. 2024, 63, e202402095. [Google Scholar] [CrossRef]
- Segura, J.L.; Royuela, S.; Ramos, M.M. Post-Synthetic Modification of Covalent Organic Frameworks. Chem. Soc. Rev. 2019, 48, 3903–3945. [Google Scholar] [CrossRef]
- Qian, C.; Teo, W.L.; Gao, Q.; Wu, H.; Liao, Y.; Zhao, Y. Polycrystalline Covalent Organic Frameworks. Mater. Today 2023, 71, 91–107. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, M.; Liu, K.; Cao, H.; Yan, H. Covalent Organic Frameworks for Photocatalytic. Appl. Catal. B Environ. 2020, 276, 119174. [Google Scholar] [CrossRef]
- Medina, D.D.; Sick, T.; Bein, T. Photoactive and Conducting Covalent Organic Frameworks. Adv. Energy Mater. 2017, 7, 1700387. [Google Scholar] [CrossRef]
- Li, R.; Tang, X.; Wu, J.; Zhang, K.; Zhang, Q.; Wang, J.; Zheng, J.; Zheng, S.; Fan, J.; Zhang, W.; et al. A Sulfonate-Functionalized Covalent Organic Framework for Record-High Adsorption and Effective Separation of Organic Dyes. Chem. Eng. J. 2023, 464, 142706. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Q.; Zeng, G. Ionic Covalent Organic Frameworks in Adsorption and Catalysis. Angew. Chem. Int. Ed. 2024, 63, e202404886. [Google Scholar] [CrossRef]
- Gong, C.; Yan, C.; Liu, J.; Li, J.; Fu, J.; Chen, C.; Huang, Y.; Yuan, G.; Peng, Y. Insights into Sensing Applications of Fluorescent Covalent Organic Frameworks. TrAC Trends Anal. Chem. 2024, 173, 117625. [Google Scholar] [CrossRef]
- Wu, X.; Han, X.; Xu, Q.; Liu, Y.; Yuan, C.; Yang, S.; Liu, Y.; Jiang, J.; Cui, Y. Chiral BINOL-Based Covalent Organic Frameworks for Enantioselective Sensing. J. Am. Chem. Soc. 2019, 141, 7081–7089. [Google Scholar] [CrossRef]
- Hegazy, H.H.; Sana, S.S.; Ramachandran, T.; Kumar, Y.A.; Kulurumotlakatla, D.K.; Abd-Rabboh, H.S.M.; Kim, S.C. Covalent Organic Frameworks in Supercapacitors: Unraveling the Pros and Cons for Energy Storage. J. Energy Storage 2023, 74, 109405. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Campbell, A.; Ambus, A.; Brown, B.; Smith-Petty, K.; Li, X. Covalent Organic Frameworks in Heterogeneous Catalysis: Recent Advances and Future Perspective. Mater. Chem. Front. 2023, 7, 3298–3331. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, D. Covalent Organic Frameworks for Heterogeneous Catalysis: Principle, Current Status, and Challenges. ACS Cent. Sci. 2020, 6, 869–879. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Zhang, J.; Wu, X.; Liu, Y.; Cui, Y. Homochiral 2D Porous Covalent Organic Frameworks for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2016, 138, 12332–12335. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Bein, T. Optoelectronic Processes in Covalent Organic Frameworks. Chem. Soc. Rev. 2021, 50, 1813–1845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, X.; Yang, Z.; Su, X.; Xie, Z.; Chen, W.; Zhang, W.; Chen, L. Quinacridone Based 2D Covalent Organic Frameworks as Efficient Photocatalysts for Aerobic Oxidative Povarov Reaction. Appl. Catal. B Environ. 2022, 312, 121406. [Google Scholar] [CrossRef]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A Hydrazone-Based Covalent Organic Framework for Photocatalytic Hydrogen Production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef]
- Shan, H.; Cai, D.; Zhang, X.; Zhu, Q.; Qin, P.; Baeyens, J. Donor-Acceptor Type Two-Dimensional Porphyrin-Based Covalent Organic Framework for Visible-Light-Driven Heterogeneous Photocatalysis. Chem. Eng. J. 2022, 432, 134288. [Google Scholar] [CrossRef]
- Wang, W.; Huang, D.; Zheng, W.; Zhao, X.; He, K.; Pang, H.; Xiang, Y. Construction of Amide-Linked Covalent Organic Frameworks by N-Heterocyclic Carbene-Mediated Selective Oxidation for Photocatalytic Dehalogenation. Chem. Mater. 2023, 35, 7154–7163. [Google Scholar] [CrossRef]
- Dash, B.P.; Hamilton, I.; Tate, D.J.; Crossley, D.L.; Kim, J.-S.; Ingleson, M.J.; Turner, M.L. Benzoselenadiazole and Benzotriazole Directed Electrophilic C–H Borylation of Conjugated Donor-Acceptor Materials. J. Mater. Chem. C 2019, 7, 718–724. [Google Scholar] [CrossRef]
- Yue, J.-Y.; Wang, Y.-T.; Ding, X.-L.; Fan, Y.-F.; Song, L.-P.; Yang, P.; Ma, Y.; Tang, B. Single-Atom Substitution in Donor-Acceptor Covalent Organic Frameworks for Tunable Visible Light Photocatalytic Cr(VI) Reduction. Mater. Chem. Front. 2022, 6, 3748–3754. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Qu, H.-Y.; Kan, J.-L.; Guo, Y.; Dong, Y.-B. A Selenium Atom Involved Covalent Organic Framework for Window Ledge Photocatalytic Oxidation of Sulfides. Chin. J. Chem. 2024, 42, 1960–1966. [Google Scholar] [CrossRef]
- Liang, R.-R.; Jiang, S.-Y.; A, R.-H.; Zhao, X. Two-Dimensional Covalent Organic Frameworks with Hierarchical Porosity. Chem. Soc. Rev. 2020, 49, 3920–3951. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Q.; Chen, W.; Tang, Y.; Aguila, B.; Pan, Y.; Zheng, A.; Yang, Z.; Wojtas, L.; Ma, S.; et al. Programming Covalent Organic Frameworks for Photocatalysis: Investigation of Chemical and Structural Variations. Matter 2020, 2, 416–427. [Google Scholar] [CrossRef]
- Li, P.; Ge, F.; Yang, Y.; Wang, T.; Zhang, X.; Zhang, K.; Shen, J. 1D Covalent Organic Frameworks Triggering Highly Efficient Photosynthesis of H2O2 via Controllable Modular Design. Angew. Chem. Int. Ed. 2024, 63, e202319885. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Wang, L.; Wen, Z.; Chakraborty, J.; Sun, J.; Wang, G.; Voort, P.V.D. Donor-Acceptor sp2 Covalent Organic Frameworks for Photocatalytic H2O2 Production and Tandem Bisphenol-A Degradation. Green Chem. 2024, 26, 3239–3248. [Google Scholar] [CrossRef]
- Bredas, J.L. Mind the Gap! Mater. Horiz. 2013, 1, 17–19. [Google Scholar] [CrossRef]
- Wang, T.; Li, M.; Chen, Y.; Che, X.; Bi, F.; Yang, Y.; Yang, R.; Li, C. Regioisomeric Benzotriazole-Based Covalent Organic Frameworks for High Photocatalytic Activity. ACS Catal. 2023, 13, 15439–15447. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Li, J.; Hou, Y.; Zhang, Q.; Li, Z.; Yue, H.; Liu, X. Benzotrifuran-Based Donor-Acceptor Covalent Organic Frameworks for Enhanced Photocatalytic Hydrogen Generation. Green Chem. 2024, 26, 2605–2612. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Li, H.; Ren, Y.; Chen, J.; Tang, J.; Yang, Q. Structural Engineering of Two-Dimensional Covalent Organic Frameworks for Visible-Light-Driven Organic Transformations. ACS Appl. Mater. Interfaces 2020, 12, 20354–20365. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Qian, J.; Wang, S.; Wen, Z.; Xiao, S.; Hu, H.; Gao, Y. Benzodiazole-Based Covalent Organic Frameworks for Enhanced Photocatalytic Dehalogenation of Phenacyl Bromide Derivatives. Polymers 2024, 16, 2578. https://doi.org/10.3390/polym16182578
Wang M, Qian J, Wang S, Wen Z, Xiao S, Hu H, Gao Y. Benzodiazole-Based Covalent Organic Frameworks for Enhanced Photocatalytic Dehalogenation of Phenacyl Bromide Derivatives. Polymers. 2024; 16(18):2578. https://doi.org/10.3390/polym16182578
Chicago/Turabian StyleWang, Ming, Jiaying Qian, Shenglin Wang, Zhongliang Wen, Songtao Xiao, Hui Hu, and Yanan Gao. 2024. "Benzodiazole-Based Covalent Organic Frameworks for Enhanced Photocatalytic Dehalogenation of Phenacyl Bromide Derivatives" Polymers 16, no. 18: 2578. https://doi.org/10.3390/polym16182578






