Functionalized Modified Ti4O7 Polyaniline Coating for 316SS Bipolar Plate in Proton-Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Reagents
2.2. Preparation of PDA-Ti4O7 Composite Powders
2.3. Electrodeposition of PANI and PANI/PDA-Ti4O7 Coatings
2.4. Characterization
3. Results and Discussion
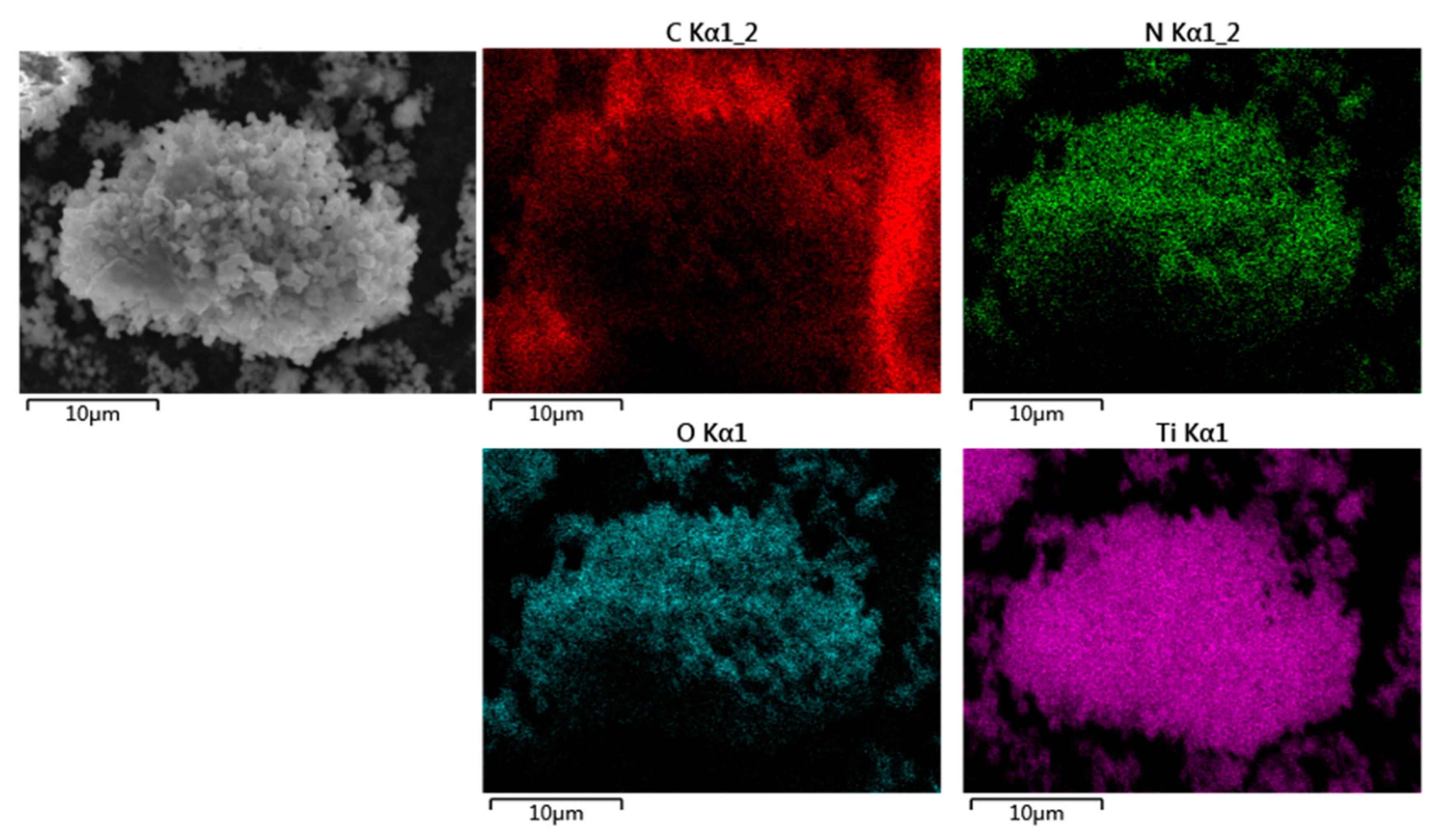
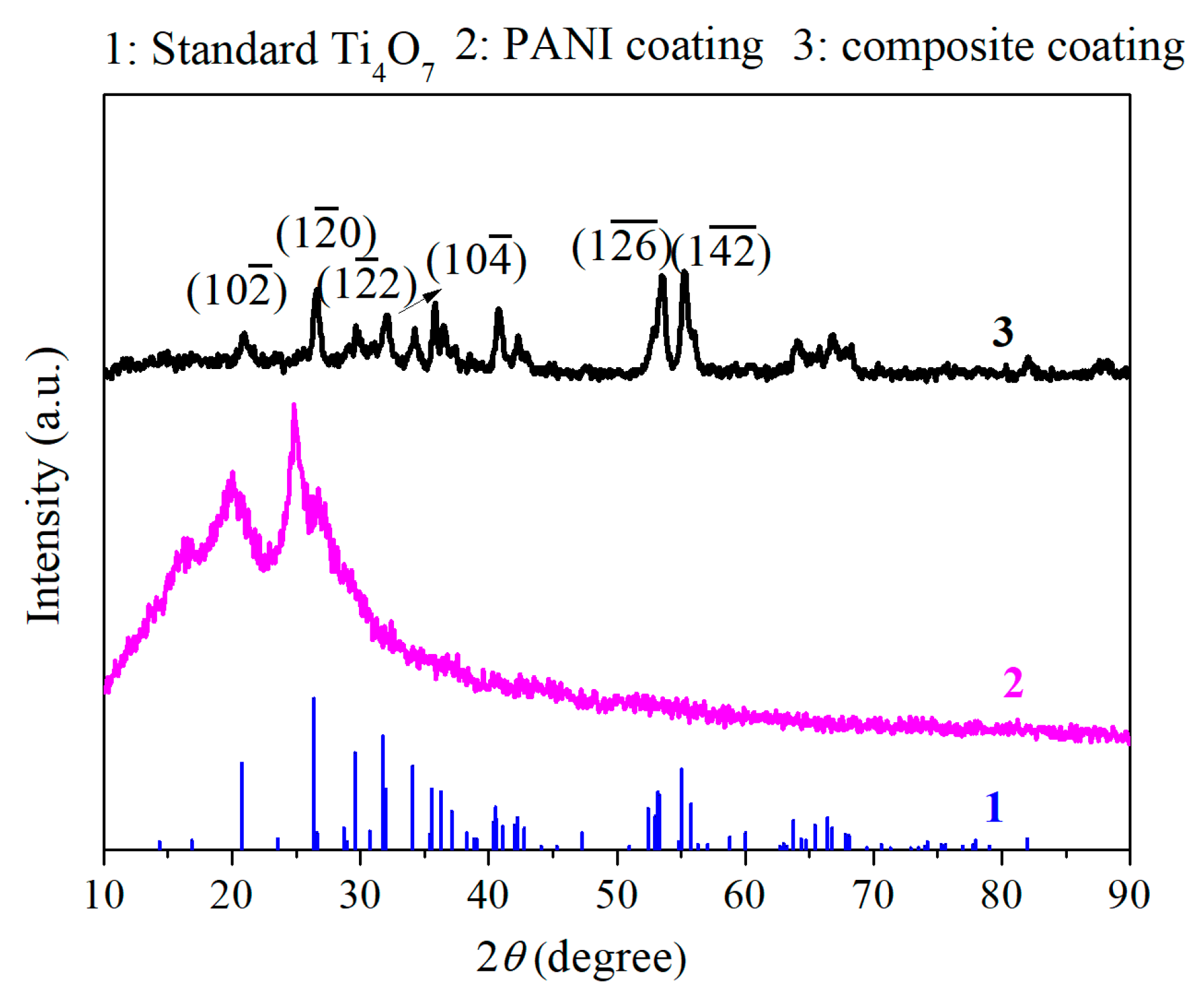
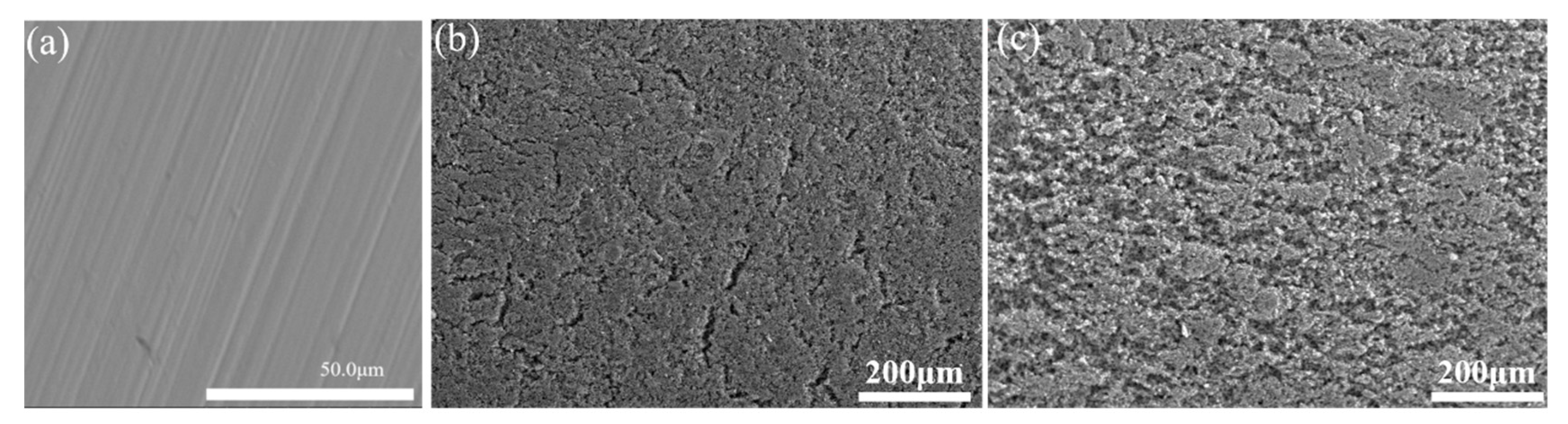
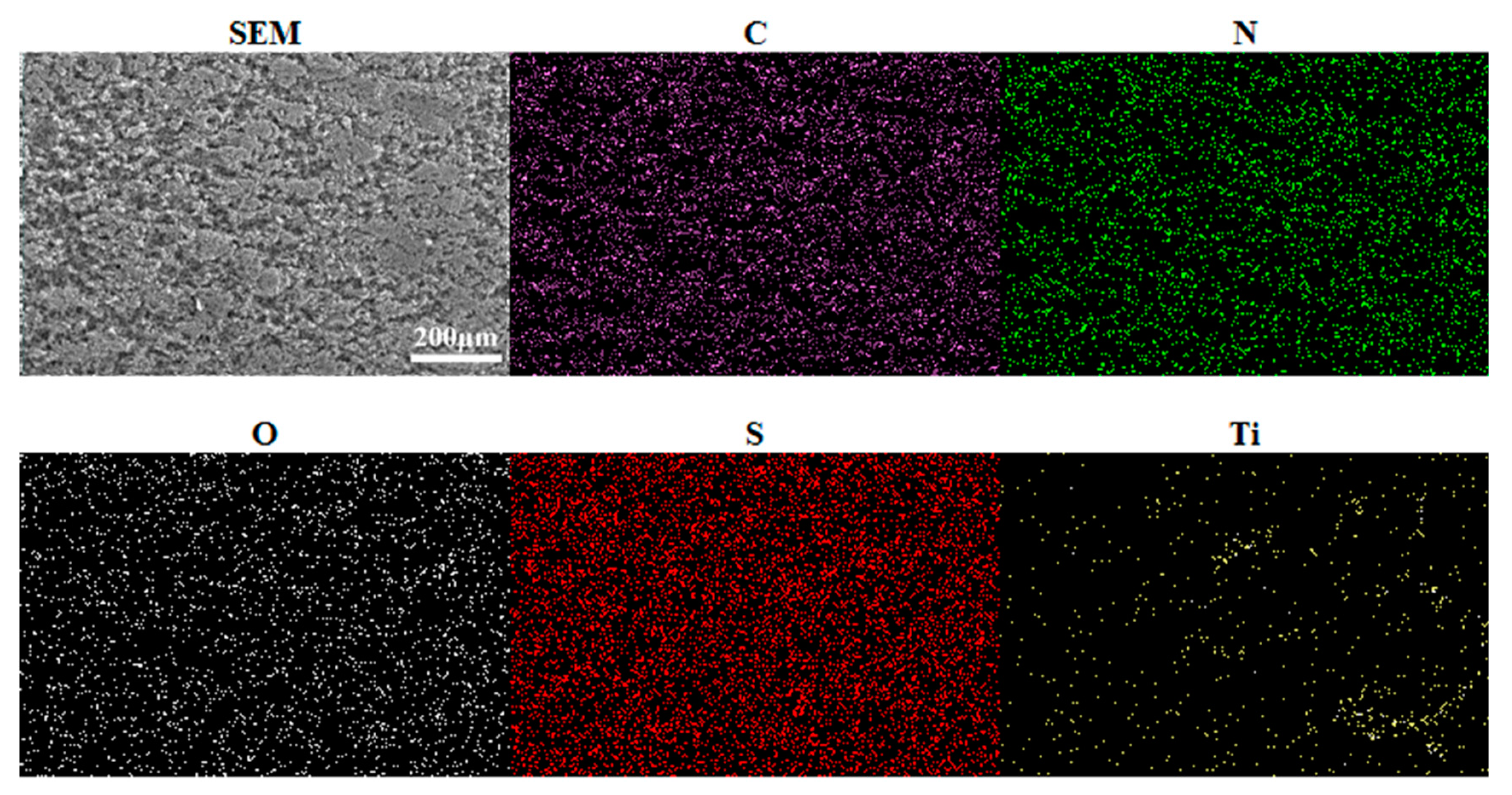
3.1. Characterization of Coatings
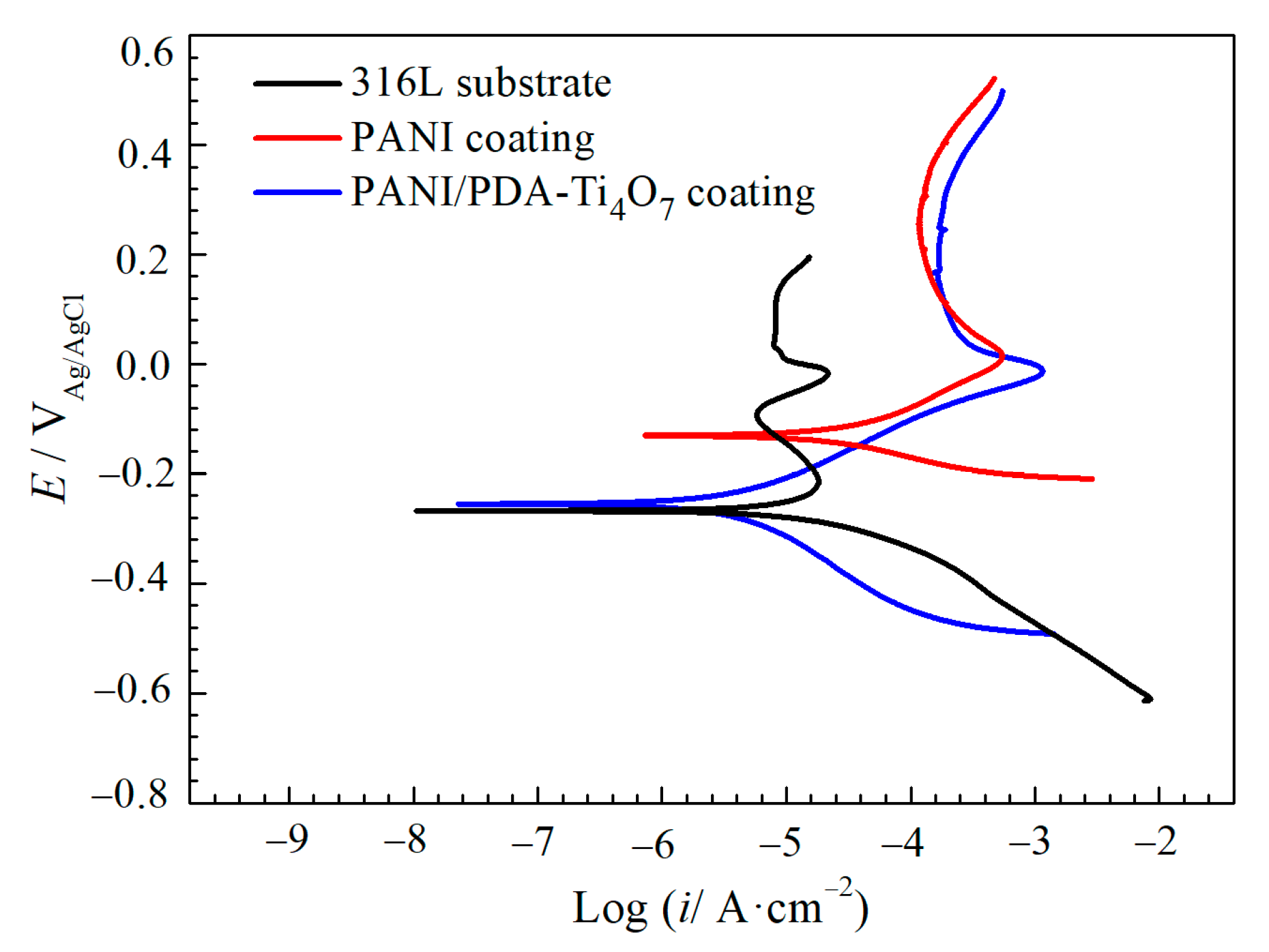
3.2. Potentiodynamic Polarization Curves
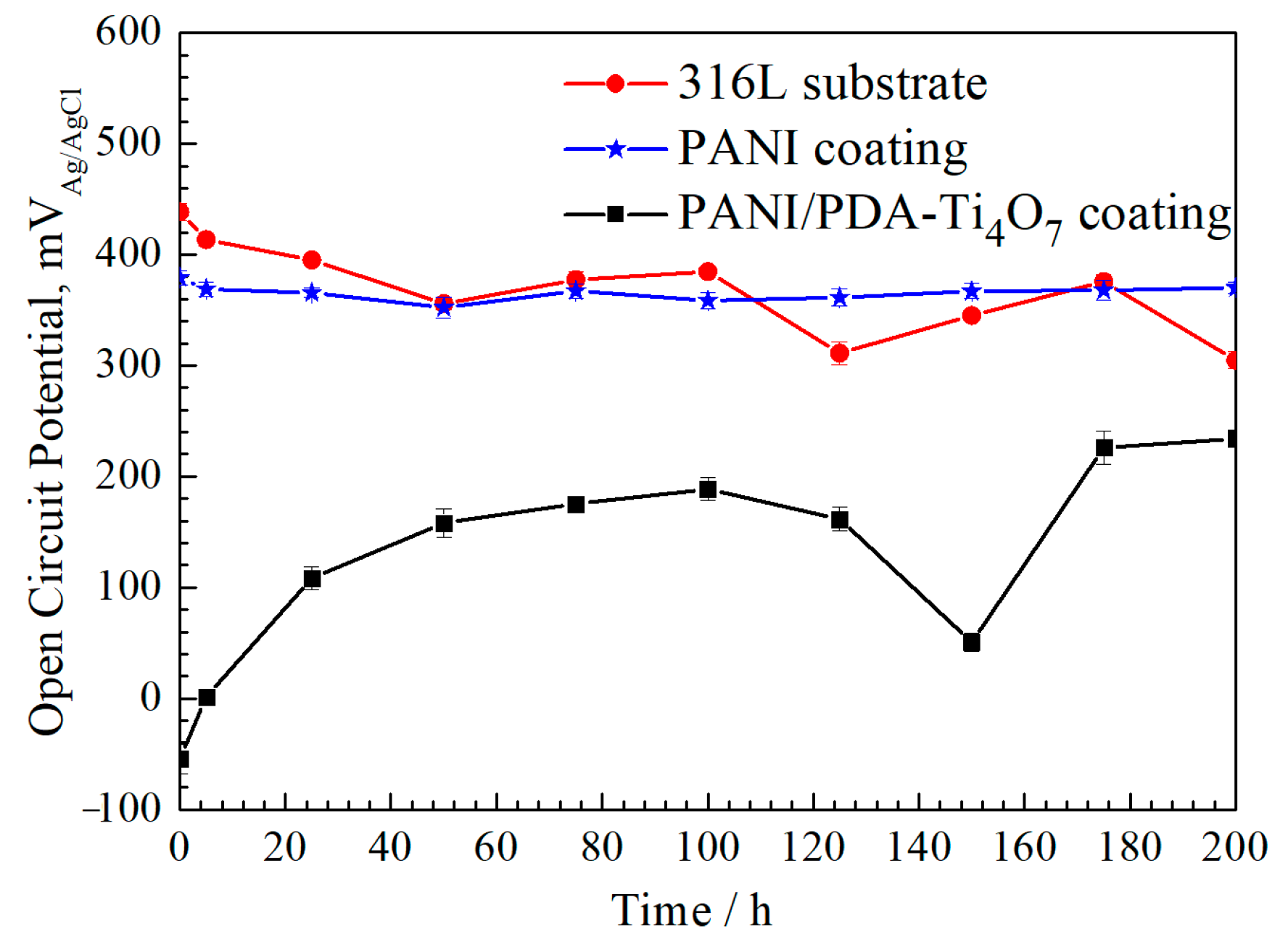
3.3. Open-Circuit Potential Curves
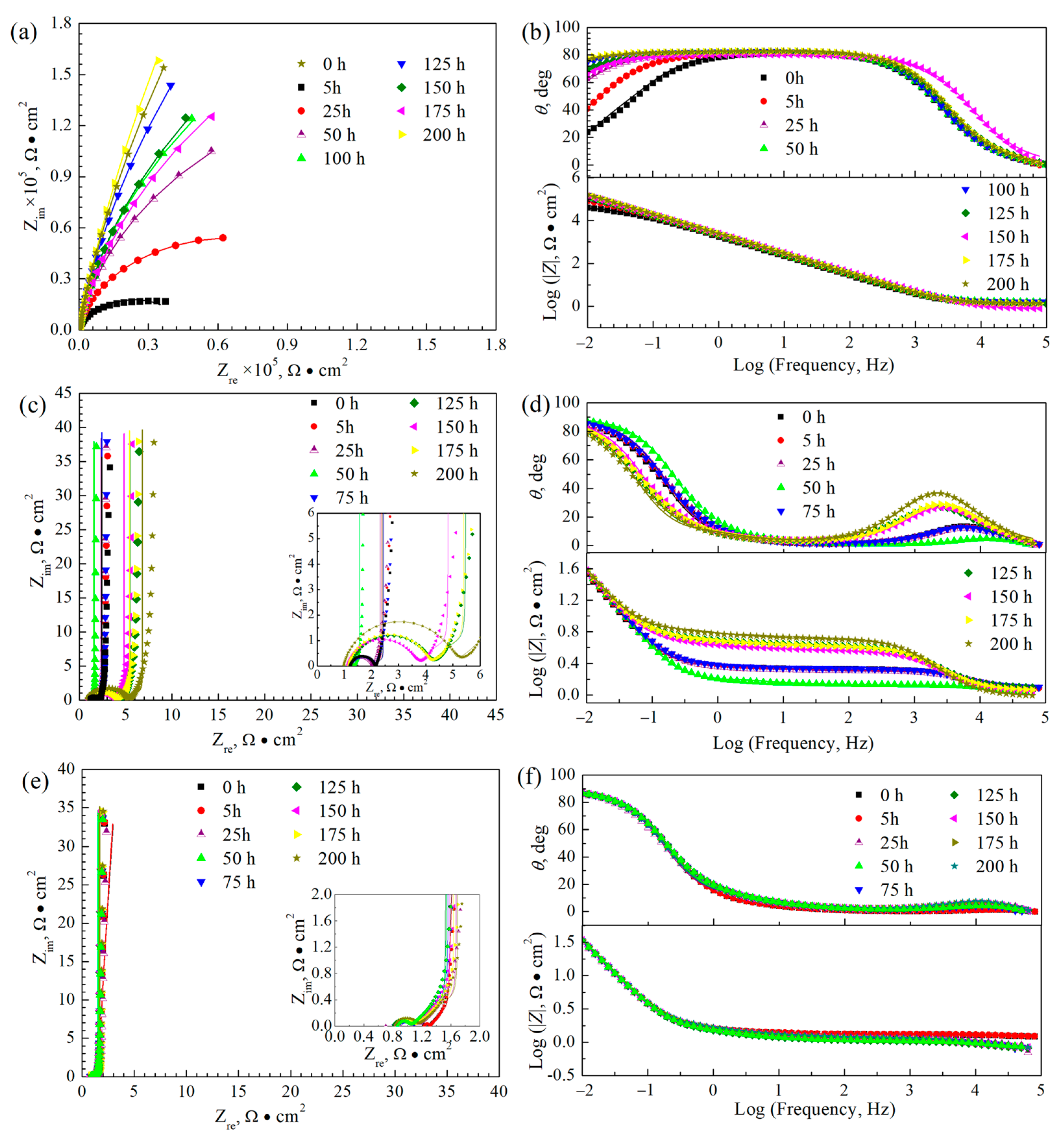
3.4. Electrochemical Impedance Spectra Curves
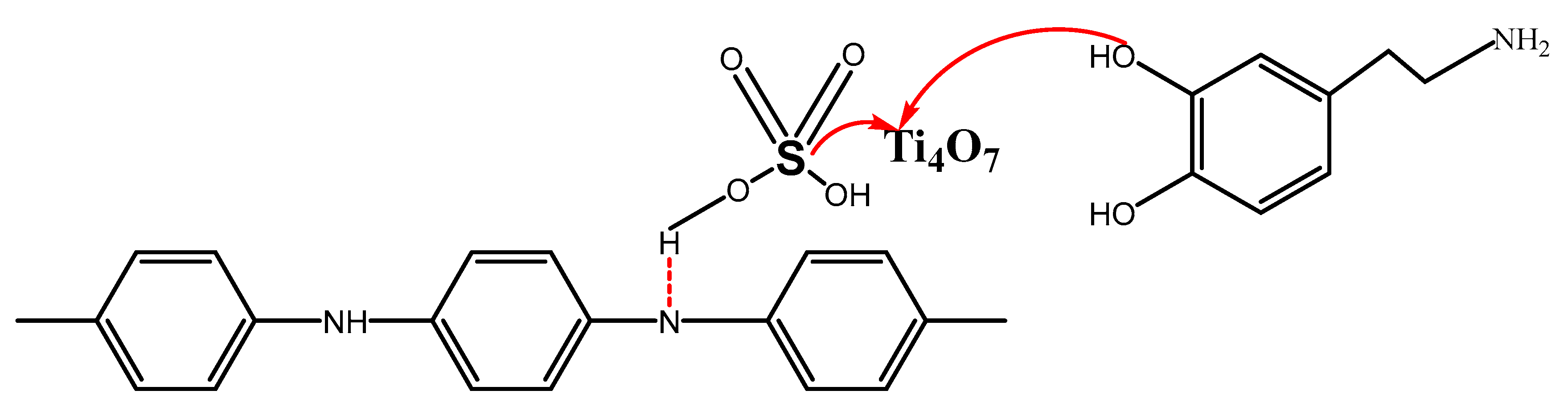
3.5. Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hwang, W.; Sung, Y.E. Recent developments of polymer electrolyte membrane fuel cell design. J. Electrochem. Sci. Technol. 2023, 14, 120–130. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y. Modification and durability of carbon paper gas diffusion layer in proton exchange membrane fuel cell. Ceram. Int. 2023, 49, 9371–9381. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development in design a state-of-art proton exchange membrane fuel cell from stack to system: Theory, integration and prospective. Int. J. Hydrogen Energy 2023, 48, 7828–7865. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Zhao, J.; Wang, J.; Liu, F.; Zhao, P.; Dai, Z.; Zheng, L. Research progress on protective coatings for bipolar plates of proton exchange membrane fuel cells. Surf. Technol. 2023, 52, 26–36. [Google Scholar]
- Wang, Y.; Liao, X.; Liu, G.; Xu, H.; Guan, C.; Wang, H.; Li, H.; He, W.; Qin, Y. Review of flow field designs for polymer electrolyte membrane fuel cells. Energies 2023, 16, 4207. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Y. Preparation and properties of conductive Ti4O7 surface coating for Ti bipolar plates of proton exchange membrane fuel cells. J. Alloys Compd. 2022, 911, 165098. [Google Scholar] [CrossRef]
- Madheswaran, D.K.; Thangavelu, P.; Krishna, R.; Thangamuthu, M.; Chandran, A.J.; Colak, I. Carbon-based materials in proton exchange membrane fuel cells: A critical review on performance and application. Carbon Lett. 2023, 33, 1495–1518. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Y. Research progress on corrosion behavior and surface protection of stainless steel bipolar plate of proton exchange membrane fuel cell. Surf. Technol. 2021, 50, 192–200. [Google Scholar]
- Herrasti, P.; Ocon, P. Polypyrrole layers for steel protection. Appl. Surf. Sci. 2001, 172, 276–284. [Google Scholar] [CrossRef]
- Malik, M.A.; Wlodarczyk, R.; KuleszaBala, P.J.; Miecznikowski, K. Protective properties of hexacyanoferrate containing polypyrrole films on stainless steel. Corros. Sci. 2005, 47, 771–783. [Google Scholar] [CrossRef]
- Tan, C.K.; Blackwood, D.J. Corrosion protection by multilayer conducting polymer coatings. Corros. Sci. 2003, 45, 545–557. [Google Scholar] [CrossRef]
- Ren, Y.J.; Chen, J.; Zeng, C.L. Corrosion protection of type 304 stainless steel bipolar plates of proton-exchange membrane fuel cells by doped polyaniline coating. J. Power Sources 2010, 195, 1914–1919. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, P.; Lu, Z.; Chen, S.; Wang, L. Synthesis and corrosion protection of Nb doped TiO2 nanopowders modified polyaniline coating on 316 stainless steel bipolar plates for proton-exchange membrane fuel cells. Prog. Org. Coat. 2019, 137, 105327. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Q.; Wang, C.; Cao, G.; Liu, Y. Preparation and performance of PANI/CNTs composite coating on 316 stainless steel bipolar plates by pulsed electrodeposition. Prog. Org. Coat. 2023, 182, 107611. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, P.; Chen, S.; Lu, Z.; Li, W. Electropolymerization and corrosion protection performance of the Nb:TiO2 nanofibers/polyaniline composite coating. J. Taiwan Inst. Chem. Eng. 2019, 103, 190–198. [Google Scholar] [CrossRef]
- Krstajić, N.; Grgur, B.; Jovanović, S.; Vojnović, M. Corrosion protection of mild steel by polypyrrole coatings in acid sulfate solutions. Electrochim. Acta 1997, 42, 1685–1691. [Google Scholar] [CrossRef]
- Jafari, Y.; Ghoreishi, S.M.; Shabani-Nooshabadi, M. Polyaniline/Graphene nanocomposite coatings on copper: Electropolymerization, characterization, and evaluation of corrosion protection performance. Synth. Met. 2016, 217, 220–230. [Google Scholar] [CrossRef]
- Lia, X.; Wang, G.; Li, X. Surface modification of nano-SiO2 particles using polyaniline. Surf. Coat. Technol. 2005, 197, 56–60. [Google Scholar] [CrossRef]
- Su, S.J.; Kuramoto, N. Processable polyaniline-titanium dioxide nanocomposites: Effect of titanium dioxide on the conductivity. Synth. Met. 2000, 114, 147–153. [Google Scholar] [CrossRef]
- Zhu, H.M. Study on Pd, Co Based Anodic Catalysts for Direct Ethanol Fuel Cells Application. Master’s Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Gao, G.; Zhu, J.; Dai, F.; He, W. The application of Ti4O7positive additive to lead-acid battery. Battery 2015, 52, 59–61. [Google Scholar]
- Zhu, J.; Wang, S.; Rao, Y.; Bai, H.; Feng, D.; Zhao, S.; Li, B. Study on physical properties and corrosion kinetics of dopamine modified GO-TiO2 nanocomposites in waterborne epoxy resin. Paint. Coat. Ind. 2019, 49, 1–9+16. [Google Scholar]
- Le, T.M.H.; Wang, R.; Sairiam, S. Self-protecting PVDF-PDA-TiO2 membranes towards highly efficient and prolonged dye wastewater treatment by photocatalytic membranes. J. Membr. Sci. 2023, 683, 121789. [Google Scholar] [CrossRef]
- Dong, S.T.; Xiao, G.Q.; Chen, C.L.; Yang, Z.W.; Chen, C.Y.; Wang, Q.R.; Lin, L.X. Polydopamine enwrapped titanium dioxide-assisted dispersion of graphene to strength fire resistance of intumescent waterborne epoxy coating. Prog. Org. Coat. 2021, 157, 106291. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, Y. Preparation and performances of modified Ti4O7 doped polypyrrole coating for metallic bipolar plates. Corros. Sci. 2021, 190, 109703. [Google Scholar] [CrossRef]




| Samples | Ecorr (mV) | icorr (μA·cm−2) |
|---|---|---|
| 316L | −265 | 23.4 |
| PANI coating | −130 | 35.7 |
| PANI/PDA-Ti4O7 composite coating | −255 | 4.05 |
| Time (h) | Rs (Ω·cm2) | Yf (Ω−1·cm−2·S−n) | nf | Rf (Ω·cm2) | Ydl (Ω−1·cm−2·S−n) | ndl | Rt (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| 0 | 1.38 | 1.03 × 10−4 | 0.91 | 3.35 × 104 | 5.87 × 10−4 | 0.99 | 1.10 × 104 |
| 5 | 1.39 | 9.23 × 10−5 | 0.91 | 9.89 × 104 | 9.62 × 10−5 | 0.60 | 3.78 × 104 |
| 25 | 1.43 | 7.52 × 10−5 | 0.92 | 14.82 | 1.45 × 10−5 | 0.81 | 3.49 × 105 |
| 50 | 1.56 | 7.73 × 10−5 | 0.92 | 15.22 | 1.02 × 10−5 | 0.83 | 5.60 × 105 |
| 100 | 1.57 | 6.91 × 10−5 | 0.93 | 8.66 | 1.26 × 10−5 | 0.84 | 1.13 × 106 |
| 125 | 1.30 | 7.34 × 10−5 | 0.92 | 7.87 | 1.47 × 10−5 | 0.83 | 6.26 × 105 |
| 150 | 0.80 | 6.79 × 10−5 | 0.91 | 0.34 × 103 | 1.18 × 10−5 | 0.79 | 5.55 × 105 |
| 175 | 1.37 | 7.37 × 10−5 | 0.92 | 1.42 × 105 | 2.75 × 10−6 | 0.66 | 2.56 × 106 |
| 200 | 1.36 | 7.50 × 10−5 | 0.92 | 1.04 × 105 | 2.90 × 10−6 | 0.66 | 1.75 × 106 |
| Time (h) | R (Ω·cm2) | Yf (Ω−1·cm−2·sn) | nf | Rf (Ω·cm2) | T (Ω−1·cm−2·s0.5) | t (s0.5) |
|---|---|---|---|---|---|---|
| 0 | 1.22 | 5.79 × 10−5 | 0.97 | 0.84 | 0.64 | 0.70 |
| 5 | 1.24 | 6.54 × 10−5 | 0.96 | 0.78 | 0.64 | 0.67 |
| 25 | 1.24 | 6.24 × 10−5 | 0.97 | 0.74 | 0.62 | 0.66 |
| 50 | 1.11 | 6.27 × 10−5 | 0.99 | 0.23 | 0.76 | 0.55 |
| 75 | 1.26 | 7.43×10−5 | 0.95 | 0.81 | 0.60 | 0.67 |
| 125 | 1.24 | 1.25 × 10−4 | 0.89 | 2.85 | 0.32 | 1.32 |
| 150 | 1.13 | 1.16 × 10−4 | 0.90 | 2.47 | 0.33 | 1.22 |
| 175 | 1.16 | 1.08 × 10−4 | 0.90 | 2.90 | 0.32 | 1.28 |
| 200 | 0.98 | 1.00 × 10−4 | 0.90 | 4.05 | 0.27 | 1.46 |
| Time (h) | R (Ω·cm2) | Yf (Ω−1·cm−2·sn) | nf | Rf (Ω·cm2) | T (Ω−1·cm−2·s0.5) | t (s0.5) |
|---|---|---|---|---|---|---|
| 25 | 0.87 | 1.03 × 10−4 | 0.94 | 0.26 | 0.54 | 0.90 |
| 50 | 0.88 | 8.98 × 10−5 | 0.99 | 0.15 | 0.55 | 0.85 |
| 75 | 0.89 | 7.50 × 10−5 | 0.99 | 0.17 | 0.55 | 0.84 |
| 100 | 0.82 | 9.00 × 10−5 | 0.99 | 0.17 | 0.55 | 0.84 |
| 125 | 0.83 | 8.52 × 10−5 | 0.98 | 0.20 | 0.55 | 0.84 |
| 150 | 0.85 | 9.79 × 10−5 | 0.97 | 0.22 | 0.54 | 0.84 |
| 175 | 0.86 | 1.06 × 10−4 | 0.97 | 0.24 | 0.53 | 0.86 |
| 200 | 0.85 | 1.17 × 10−4 | 0.95 | 0.28 | 0.51 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Chen, Z.; Yi, X.; Huang, E.; Wang, Y. Functionalized Modified Ti4O7 Polyaniline Coating for 316SS Bipolar Plate in Proton-Exchange Membrane Fuel Cells. Polymers 2024, 16, 2592. https://doi.org/10.3390/polym16182592
Zhao T, Chen Z, Yi X, Huang E, Wang Y. Functionalized Modified Ti4O7 Polyaniline Coating for 316SS Bipolar Plate in Proton-Exchange Membrane Fuel Cells. Polymers. 2024; 16(18):2592. https://doi.org/10.3390/polym16182592
Chicago/Turabian StyleZhao, Ting, Zibin Chen, Xiaoqi Yi, Enfeng Huang, and Yanli Wang. 2024. "Functionalized Modified Ti4O7 Polyaniline Coating for 316SS Bipolar Plate in Proton-Exchange Membrane Fuel Cells" Polymers 16, no. 18: 2592. https://doi.org/10.3390/polym16182592
APA StyleZhao, T., Chen, Z., Yi, X., Huang, E., & Wang, Y. (2024). Functionalized Modified Ti4O7 Polyaniline Coating for 316SS Bipolar Plate in Proton-Exchange Membrane Fuel Cells. Polymers, 16(18), 2592. https://doi.org/10.3390/polym16182592







