Bio-Epoxy Resins Based on Lignin and Tannic Acids as Wood Adhesives—Characterization and Bonding Properties
Abstract
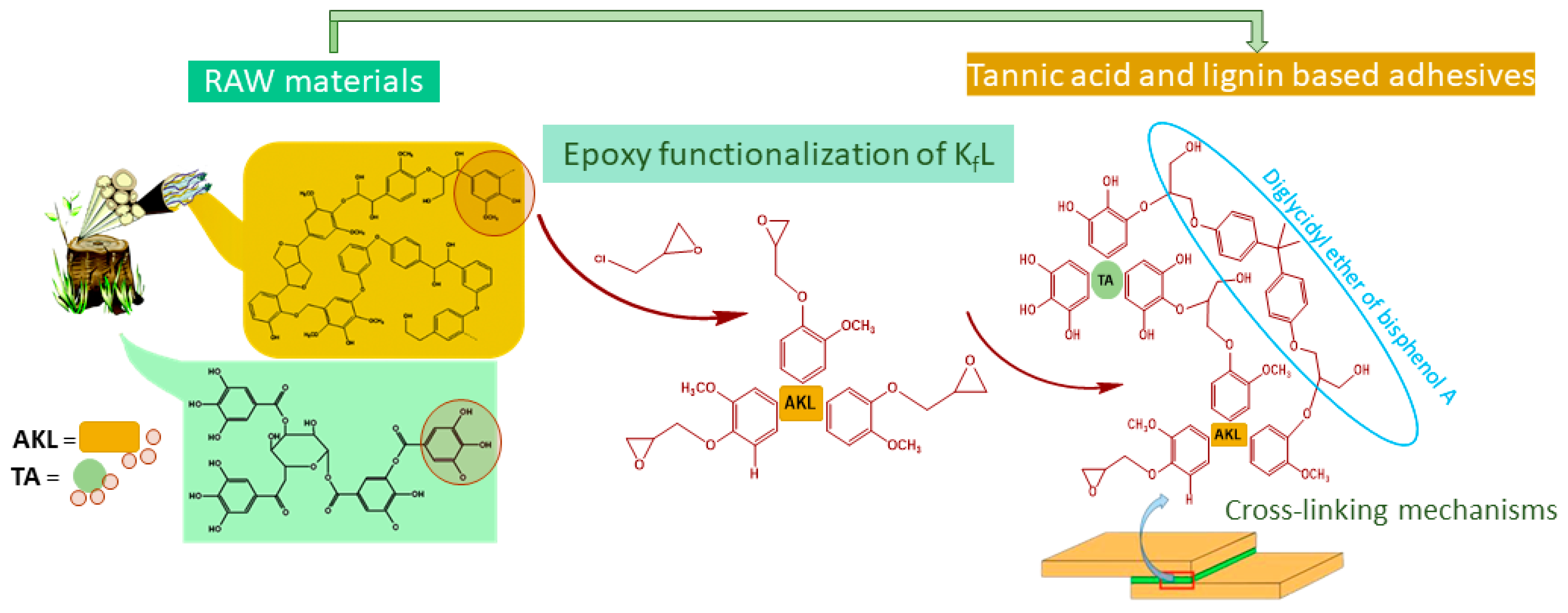
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Epoxy Modification
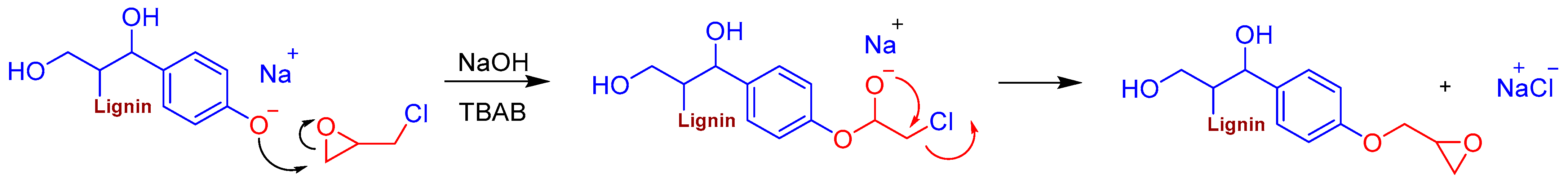
2.2.1. Epoxy Modification of Lignin
2.2.2. Epoxy Equivalent Weight
2.3. Curing (Epoxy Adhesive Preparation)
2.4. FTIR Analysis
2.5. Adhesion Testing Methods
2.5.1. Preparation of Wood Samples
2.5.2. Preparation of Joint Samples for the Determination of Glue-Line Quality
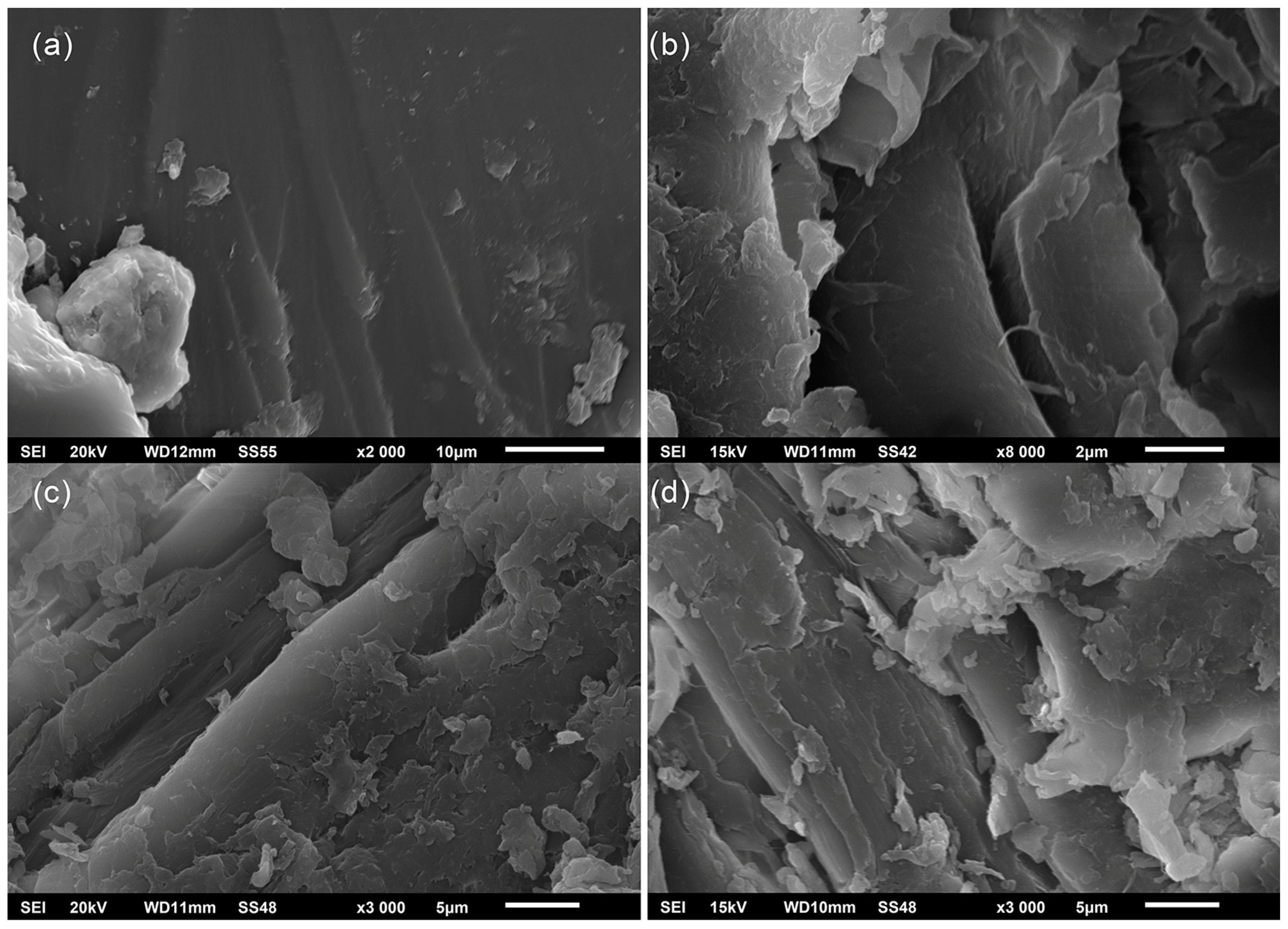
2.6. SEM Analysis
3. Results and Discussion
3.1. Lignin and Epoxy Lignin
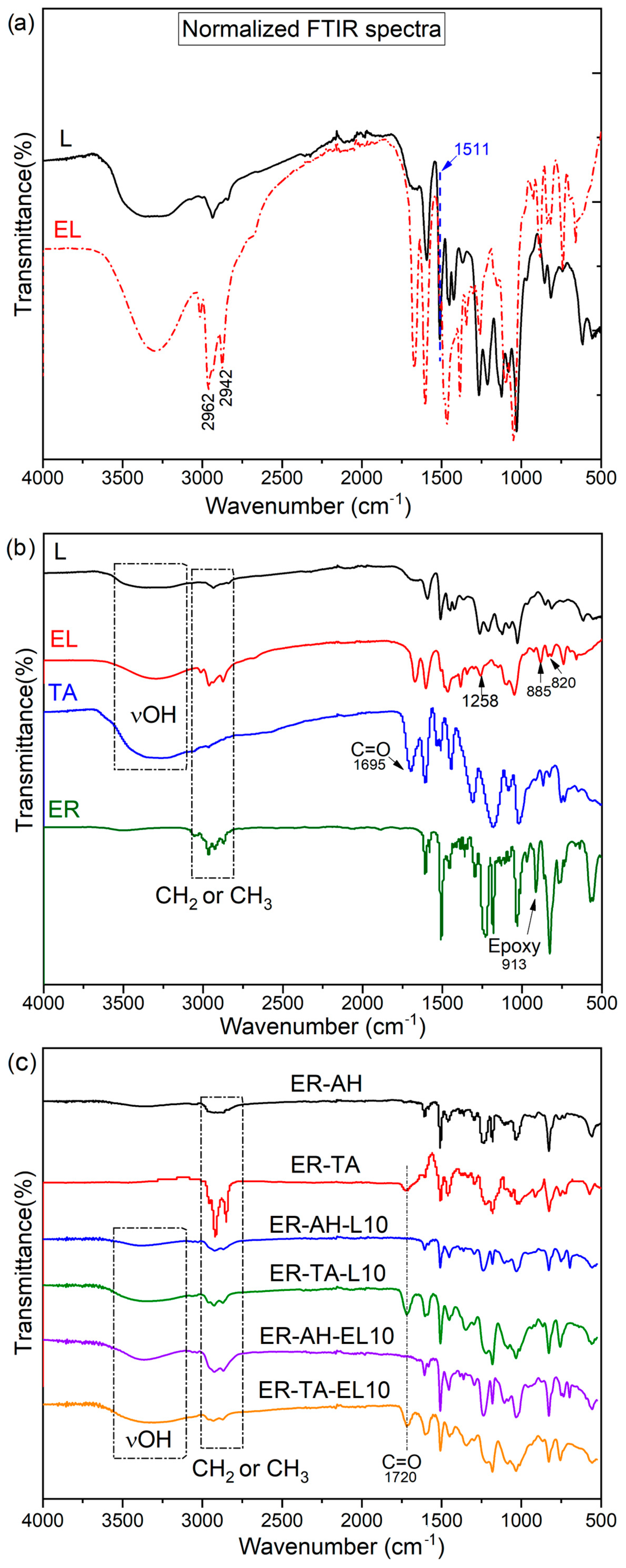
3.2. Structural Characterisation
FTIR Spectra of Epoxy Lignin and Bio-Epoxy Adhesives
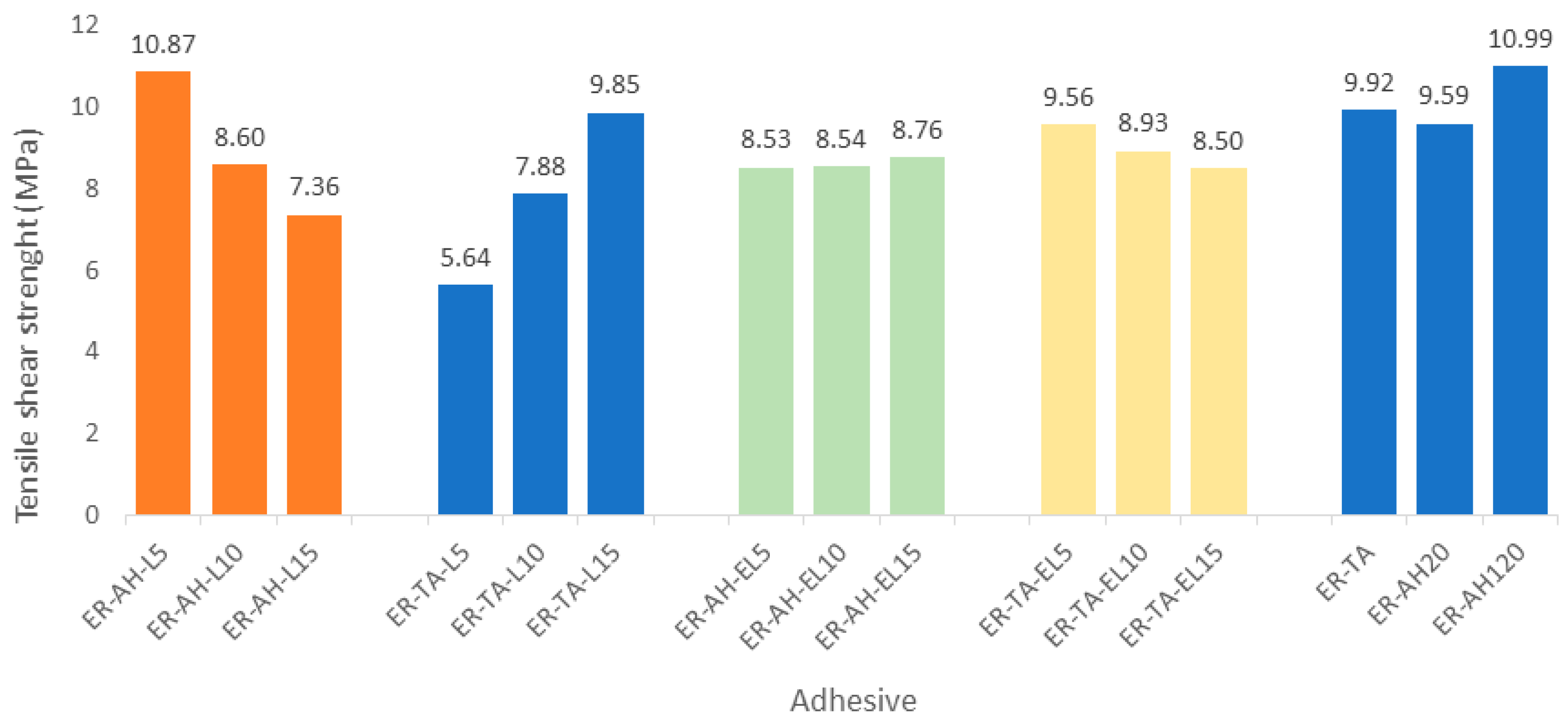
3.3. Tensile Sheer Strength Determination of the Adhesive Joint
3.4. SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Salazar, J.; Meil, J. Prospects for Carbon-Neutral Housing: The Influence of Greater Wood Use on the Carbon Footprint of a Single-Family Residence. J. Clean. Prod. 2009, 17, 1563–1571. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F.; Das, A.K.; Hiziroglu, S. An Overview of Different Types and Potential of Bio-Based Adhesives Used for Wood Products. Int. J. Adhes. Adhes. 2022, 112, 102992. [Google Scholar] [CrossRef]
- Dunky, M. Wood Adhesives Based on Natural Resources: A Critical Review: Part I. Protein-Based Adhesives. Rev. Adhes. Adhes. 2020, 8, 199–332. [Google Scholar]
- Dunky, M. Wood Adhesives Based on Natural Resources: A Critical Review Part III. Tannin- and Lignin-Based Adhesives. Prog. Adhes. Adhes. 2021, 6, 383–530. [Google Scholar] [CrossRef]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology; Dekker, M., Ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0824709861. [Google Scholar]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-Based Adhesives and Evaluation for Wood Composites Application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-O.; Cho, J.; Yeo, H.; Lee, B.W.; Moon, B.J.; Ha, Y.-M.; Jo, Y.R.; Jung, Y.C. Flame Retardant Epoxy Derived from Tannic Acid as Biobased Hardener. ACS Sustain. Chem. Eng. 2019, 7, 3858–3865. [Google Scholar] [CrossRef]
- Tomuta, A.; Ferrando, F.; Serra, À.; Ramis, X. New Aromatic–Aliphatic Hyperbranched Polyesters with Vinylic End Groups of Different Length as Modifiers of Epoxy/Anhydride Thermosets. React. Funct. Polym. 2012, 72, 556–563. [Google Scholar] [CrossRef]
- Frias, C.F.; Serra, A.C.; Ramalho, A.; Coelho, J.F.J.; Fonseca, A.C. Preparation of Fully Biobased Epoxy Resins from Soybean Oil Based Amine Hardeners. Ind. Crop. Prod. 2017, 109, 434–444. [Google Scholar] [CrossRef]
- Van de Velde, N.; Javornik, S.; Sever, T.; Štular, D.; Šobak, M.; Štirn, Ž.; Likozar, B.; Jerman, I. Bio-Based Epoxy Adhesives with Lignin-Based Aromatic Monophenols Replacing Bisphenol a. Polymers 2021, 13, 3879. [Google Scholar] [CrossRef]
- Ebrahimnezhad-Khaljiri, H.; Ghadi, A. Recent Advancement in Synthesizing Bio-Epoxy Nanocomposites Using Lignin, Plant Oils, Saccharides, Polyphenols, and Natural Rubbers: A Review. Int. J. Biol. Macromol. 2024, 256, 128041. [Google Scholar] [CrossRef]
- Mustapha, R.; Rahmat, A.R.; Abdul Majid, R.; Mustapha, S.N.H. Vegetable Oil-Based Epoxy Resins and Their Composites with Bio-Based Hardener: A Short Review. Polym.-Plast. Technol. Mater. 2019, 58, 1311–1326. [Google Scholar] [CrossRef]
- Ramon, E.; Sguazzo, C.; Moreira, P.M.G.P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Domínguez, G.; Eugenio, M.E.; Franco, J.M. Rheology and Adhesion Performance of Adhesives Formulated with Lignins from Agricultural Waste Straws Subjected to Solid-State Fermentation. Ind. Crops Prod. 2021, 171, 113876. [Google Scholar] [CrossRef]
- Gioia, C.; Colonna, M.; Tagami, A.; Medina, L.; Sevastyanova, O.; Berglund, L.A.; Lawoko, M. Lignin-Based Epoxy Resins: Unravelling the Relationship between Structure and Material Properties. Biomacromolecules 2020, 21, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Mennani, M.; Ait Benhamou, A.; Kasbaji, M.; Boussetta, A.; Ablouh, E.H.; Kassab, Z.; El Achaby, M.; Boussetta, N.; Grimi, N.; Moubarik, A. Insights on the Physico-Chemical Properties of Alkali Lignins from Different Agro-Industrial Residues and Their Use in Phenol-Formaldehyde Wood Adhesive Formulation. Int. J. Biol. Macromol. 2022, 221, 149–162. [Google Scholar] [CrossRef]
- Gendron, J.; Stambouli, I.; Bruel, C.; Boumghar, Y.; Montplaisir, D. Characterization of Different Types of Lignin and Their Potential Use in Green Adhesives. Ind. Crop. Prod. 2022, 182, 114893. [Google Scholar] [CrossRef]
- Ndiwe, B.; Pizzi, A.; Tibi, B.; Danwe, R.; Konai, N.; Amirou, S. African Tree Bark Exudate Extracts as Biohardeners of Fully Biosourced Thermoset Tannin Adhesives for Wood Panels. Ind. Crop. Prod. 2019, 132, 253–268. [Google Scholar] [CrossRef]
- González-Rodríguez, S.; Lu-Chau, T.A.; Chen, X.; Eibes, G.; Pizzi, A.; Feijoo, G.; Moreira, M.T. Functionalisation of Organosolv Lignin by Enzymatic Demethylation for Bioadhesive Formulation. Ind. Crop. Prod. 2022, 186, 115253. [Google Scholar] [CrossRef]
- Chupin, L.; Charrier, B.; Pizzi, A.; Perdomo, A.; Charrier-El Bouhtoury, F. Study of Thermal Durability Properties of Tannin–Lignosulfonate Adhesives. J. Therm. Anal. Calorim. 2015, 119, 1577–1585. [Google Scholar] [CrossRef]
- Milošević, M.; Daničić, D.; Kovačina, J.; Bugarčić, M.; Rusmirović, J.; Kovačević, T.; Marinković, A. Modified Tannins for Alkyd Based Anticorrosive Coatings. Zast. Mater. 2019, 60, 81–95. [Google Scholar] [CrossRef]
- Peres, R.S.; Cassel, E.; Ferreira, C.A.; Azambuja, D.S. Black Wattle Tannin as a Zinc Phosphating Coating Sealer. Surf. Interface Anal. 2014, 46, 1–6. [Google Scholar] [CrossRef]
- Laoutid, F.; Karaseva, V.; Costes, L.; Brohez, S.; Mincheva, R.; Dubois, P. Novel Bio-Based Flame Retardant Systems Derived from Tannic Acid. J. Renew. Mater. 2018, 6, 559–572. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Gou, B.; Zhou, J.; Xu, H.; Cai, H.; Zhong, A.; Zhang, D.; Li, L.; Wang, R.; Xie, C. Epoxy Polymer Using Tannic Acid as the Green Crosslinker, Exhibiting Globally Enhanced Mechanical, Insulating and Thermally Conductive Properties. React. Funct. Polym. 2023, 191, 105646. [Google Scholar] [CrossRef]
- Feng, X.; Fan, J.; Li, A.; Li, G. Biobased Tannic Acid Cross-Linked Epoxy Thermosets with Hierarchical Molecular Structure and Tunable Properties: Damping, Shape Memory, and Recyclability. ACS Sustain. Chem. Eng. 2019, 8, 874–883. [Google Scholar] [CrossRef]
- Kumar, C.; Leggate, W. An Overview of Bio-Adhesives for Engineered Wood Products. Int. J. Adhes. Adhes. 2022, 118, 103187. [Google Scholar] [CrossRef]
- Nikafshar, S.; Wang, J.; Dunne, K.; Sangthonganotai, P.; Nejad, M. Choosing the Right Lignin to Fully Replace Bisphenol A in Epoxy Resin Formulation. ChemSusChem 2021, 14, 1184–1195. [Google Scholar] [CrossRef]
- Truncali, A.; Laxminarayan, T.; Rajagopalan, N.; Weinell, C.E.; Kiil, S.; Johansson, M. Epoxidized Technical Kraft Lignin as a Particulate Resin Component for High-Performance Anticorrosive Coatings. J. Coat. Technol. Res. 2024, 1–17. [Google Scholar] [CrossRef]
- Gioia, C.; Lo Re, G.; Lawoko, M.; Berglund, L. Tunable Thermosetting Epoxies Based on Fractionated and Well-Characterized Lignins. J. Am. Chem. Soc. 2018, 140, 4054–4061. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Vevere, L.; Telysheva, G.; Dizhbite, T.; Gosselink, R.J.A.; Bikovens, O.; Jablonski, A. Functionality and Physico-Chemical Characteristics of Wheat Straw Lignin, BioligninTM, Derivatives Formed in the Oxypropylation Process. Holzforschung 2015, 69, 785–793. [Google Scholar] [CrossRef]
- Aouf, C.; Nouailhas, H.; Fache, M.; Caillol, S.; Boutevin, B.; Fulcrand, H. Multi-Functionalization of Gallic Acid. Synthesis of a Novel Bio-Based Epoxy Resin. Eur. Polym. J. 2013, 49, 1185–1195. [Google Scholar] [CrossRef]
- Yin, Q.; Yang, W.; Sun, C.; Di, M. Preparation and Properties of Lignin-Epoxy Resin Composite. BioResources 2012, 7, 5737–5748. [Google Scholar] [CrossRef]
- Huang, C.; Peng, Z.; Li, J.; Li, X.; Jiang, X.; Dong, Y. Unlocking the Role of Lignin for Preparing the Lignin-Based Wood Adhesive: A Review. Ind. Crops Prod. 2022, 187, 115388. [Google Scholar] [CrossRef]
- Li, R.J.; Gutierrez, J.; Chung, Y.L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A Lignin-Epoxy Resin Derived from Biomass as an Alternative to Formaldehyde-Based Wood Adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Salanti, A.; Zoia, L.; Simonutti, R.; Orlandi, M. Epoxidized lignin derivatives as bio-based cross-linkers used in the preparation of epoxy resins. BioResources 2018, 13, 2374–2396. [Google Scholar] [CrossRef]
- Dos Santos, D.J.; Tavares, L.B.; Gouveia, J.R.; Batalha, G.F. Lignin-Based Polyurethane and Epoxy Adhesives: A Short Review. Arch. Mater. Sci. Eng. 2021, 107, 56–63. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Synthesis of Lignin-Based Epoxy Resins: Optimization of Reaction Parameters Using Response Surface Methodology. RSC Adv. 2014, 4, 31745–31753. [Google Scholar] [CrossRef]
- Ramachandrareddy, B.; Solt-Rindler, P.; van Herwijnen, H.W.; Pramreiter, M.; Konnerth, J. Sensitivity of Lap-Shear Test to Errors in Groove Cutting and Influence of Wood Type/Treatment. Int. J. Adhes. Adhes. 2024, 130. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Yeo, J.C.C.; Yuan, D.; Li, H.; Stubbs, L.P.; He, C. Lignin Epoxy Composites: Preparation, Morphology, and Mechanical Properties. Macromol. Mater. Eng. 2016, 301, 328–336. [Google Scholar] [CrossRef]
- Wang, X.; Leng, W.; Nayanathara, R.M.O.; Caldona, E.B.; Liu, L.; Chen, L.; Advincula, R.C.; Zhang, Z.; Zhang, X. Anticorrosive Epoxy Coatings from Direct Epoxidation of Bioethanol Fractionated Lignin. Int. J. Biol. Macromol. 2022, 221, 268–277. [Google Scholar] [CrossRef]
- Gouveia, J.R.; Garcia, G.E.S.; Antonino, L.D.; Tavares, L.B.; Dos Santos, D.J. Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive. Molecules 2020, 25, 2513. [Google Scholar] [CrossRef] [PubMed]
- Sesia, R.; Cardone, A.G.; Ferraris, S.; Spriano, S.; Sangermano, M. Exploitation of Tannic Acid as Additive for the Adhesion Enhancement of UV-Curable Bio-Based Coating. Prog. Org. Coat. 2024, 189, 108311. [Google Scholar] [CrossRef]
- Kong, X.; Xu, Z.; Guan, L.; Di, M. Study on Polyblending Epoxy Resin Adhesive with Lignin I-Curing Temperature. Int. J. Adhes. Adhes. 2014, 48, 75–79. [Google Scholar] [CrossRef]
- Zou, S.L.; Xiao, L.P.; Li, X.Y.; Yin, W.Z.; Sun, R.C. Lignin-Based Composites with Enhanced Mechanical Properties by Acetone Fractionation and Epoxidation Modification. iScience 2023, 26, 106187. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Sustainable Lignin-Based Epoxy Resins Cured with Aromatic and Aliphatic Amine Curing Agents: Curing Kinetics and Thermal Properties. Thermochim. Acta 2015, 618, 48–55. [Google Scholar] [CrossRef]
- Qi, M.; Xu, Y.J.; Rao, W.H.; Luo, X.; Chen, L.; Wang, Y.Z. Epoxidized Soybean Oil Cured with Tannic Acid for Fully Bio-Based Epoxy Resin. RSC Adv. 2018, 8, 26948–26958. [Google Scholar] [CrossRef]
- Fei, X.; Zhao, F.; Wei, W.; Luo, J.; Chen, M.; Liu, X. Tannic Acid as a Bio-Based Modifier of Epoxy/Anhydride Thermosets. Polymers 2016, 8, 314. [Google Scholar] [CrossRef]



| No. | Adhesive Formulation | Label | ER (g) | AH/TA (g) | L/EL (g) |
|---|---|---|---|---|---|
| 1 | CompA + CompB + L (5%) | ER-AH-L5 | 4 | 2 | 0.3 |
| 2 | CompA + CompB + L (10%) | ER-AH-L10 | 4 | 2 | 0.6 |
| 3 | CompA + CompB + L (15%) | ER-AH-L15 | 4 | 2 | 0.9 |
| 4 | CompA + TA + L (5%) | ER-TA-L5 | 4 | 2 | 0.3 |
| 5 | CompA + TA + L (10%) | ER-TA-L10 | 4 | 2 | 0.6 |
| 6 | CompA + TA + L (15%) | ER-TA-L15 | 4 | 2 | 0.9 |
| 7 | CompA + CompB + EL (5%) | ER-AH-EL5 | 4 | 2 | 0.3 |
| 8 | CompA + CompB + EL (10%) | ER-AH-EL10 | 4 | 2 | 0.6 |
| 9 | CompA + CompB + EL (15%) | ER-AH-EL15 | 4 | 2 | 0.9 |
| 10 | CompA + TA + EL (5%) | ER-TA-EL5 | 4 | 2 | 0.3 |
| 11 | CompA + TA + EL (10%) | ER-TA-EL10 | 4 | 2 | 0.6 |
| 12 | CompA + TA + EL (15%) | ER-TA-EL15 | 4 | 2 | 0.9 |
| 13 | CompA + CompB | ER-AH20 | 4 | 2 | - |
| 14 | CompA + CompB | ER-AH120 | 4 | 2 | - |
| 15 | CompA + TA | ER-TA | 4 | 2 | - |
| No | Label | MPa |
| 1 | ER-AH-L5 | 10.87 ± 0.37 |
| 2 | ER-AH-L10 | 8.60 ± 0.25 |
| 3 | ER-AH-L15 | 7.36 ± 0.22 |
| 4 | ER-TA-L5 | 5.64 ± 0.17 |
| 5 | ER-TA-L10 | 7.88 ± 0.27 |
| 6 | ER-TA-L15 | 9.85 ± 0.33 |
| 7 | ER-AH-EL5 | 8.53 ± 0.18 |
| 8 | ER-AH-EL10 | 8.54 ± 0.22 |
| 9 | ER-AH-EL15 | 8.76 ± 0.16 |
| 10 | ER-TA-EL5 | 9.56 ± 0.32 |
| 11 | ER-TA-EL10 | 8.93 ± 0.25 |
| 12 | ER-TA-EL15 | 8.50 ± 0.14 |
| 13 | ER-AH20 | 9.59 ± 0.15 |
| 14 | ER-AH120 | 10.99 ± 0.30 |
| 15 | ER-TA | 9.92 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilović-Grmuša, I.; Rančić, M.; Tešić, T.; Stupar, S.; Milošević, M.; Gržetić, J. Bio-Epoxy Resins Based on Lignin and Tannic Acids as Wood Adhesives—Characterization and Bonding Properties. Polymers 2024, 16, 2602. https://doi.org/10.3390/polym16182602
Gavrilović-Grmuša I, Rančić M, Tešić T, Stupar S, Milošević M, Gržetić J. Bio-Epoxy Resins Based on Lignin and Tannic Acids as Wood Adhesives—Characterization and Bonding Properties. Polymers. 2024; 16(18):2602. https://doi.org/10.3390/polym16182602
Chicago/Turabian StyleGavrilović-Grmuša, Ivana, Milica Rančić, Tamara Tešić, Stevan Stupar, Milena Milošević, and Jelena Gržetić. 2024. "Bio-Epoxy Resins Based on Lignin and Tannic Acids as Wood Adhesives—Characterization and Bonding Properties" Polymers 16, no. 18: 2602. https://doi.org/10.3390/polym16182602







