Effect of Different Porous Size of Porous Inorganic Fillers on the Encapsulation of Rosemary Essential Oil for PLA-Based Active Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Essential Oil Encapsulation
2.3. Nanoparticles Sizes Characterization
2.4. Films Processing
2.5. Film Characterization
2.5.1. Scanning Electron Microscopy
2.5.2. Mechanical Test
2.5.3. Thermal Properties
2.5.3.1. Differential Scanning Calorimetry
2.5.3.2. Thermogravimetric Analysis
2.5.4. Antioxidant Activity
2.5.5. Disintegration under Composting Conditions
2.5.6. Statistical Analysis
3. Results
3.1. Particles Characterization
3.1.1. Dynamic Light Scattering
3.1.2. Transmission Electron Microscopy
3.2. Films Characterization
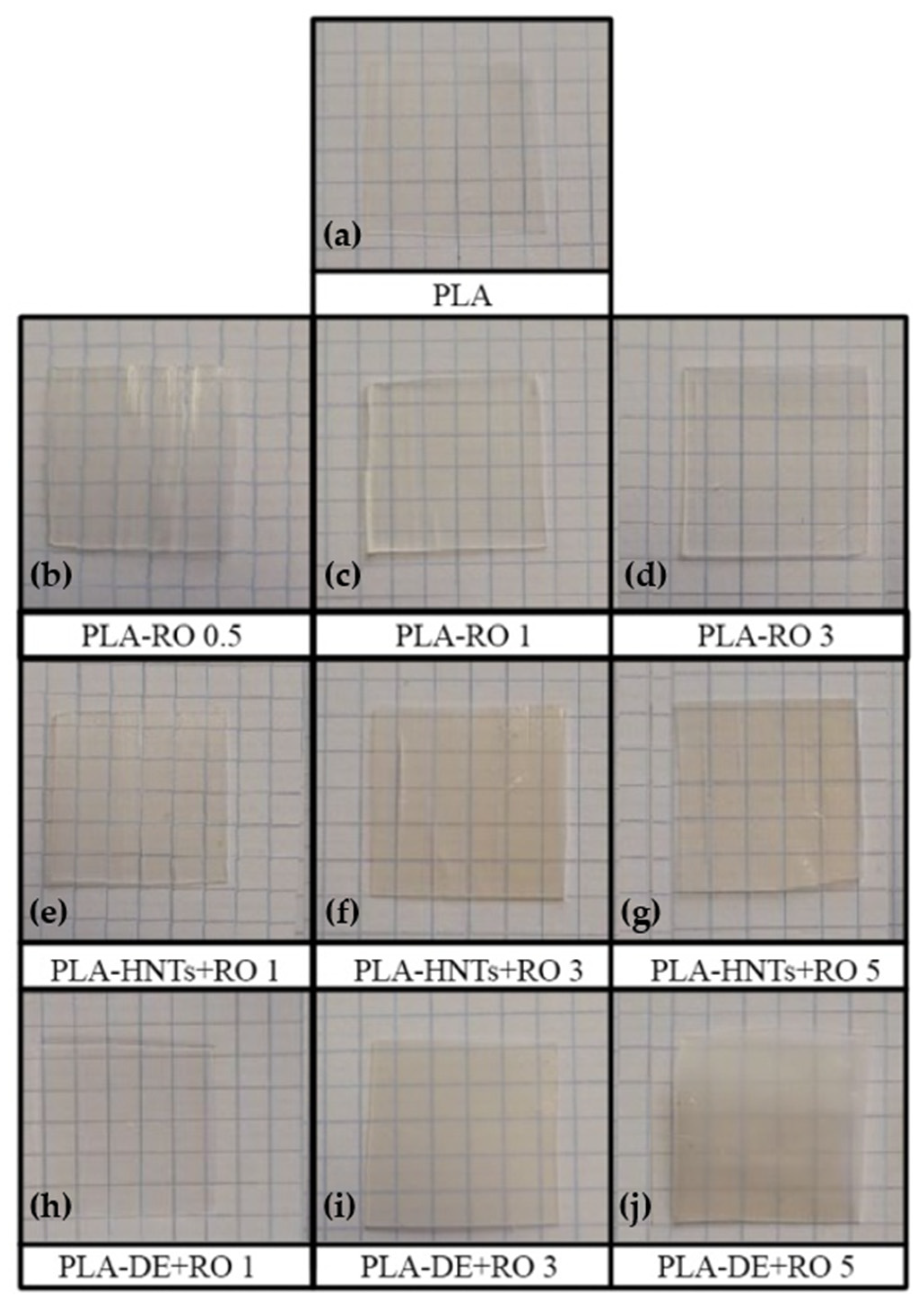
3.2.1. Visual Appearance
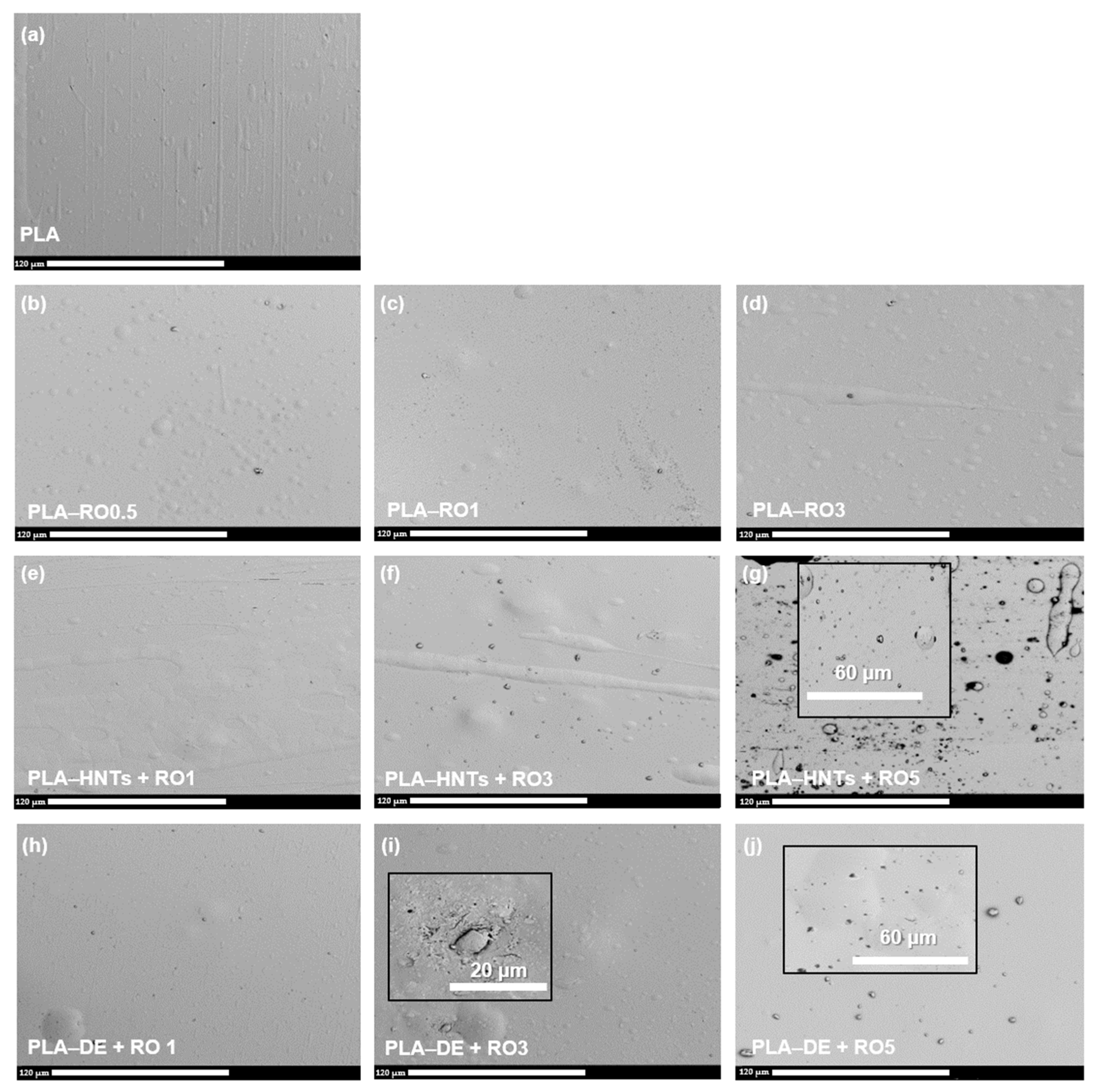
3.2.2. Scanning Electron Microscopy
3.2.3. Mechanical Characterization
3.2.4. Thermal Properties
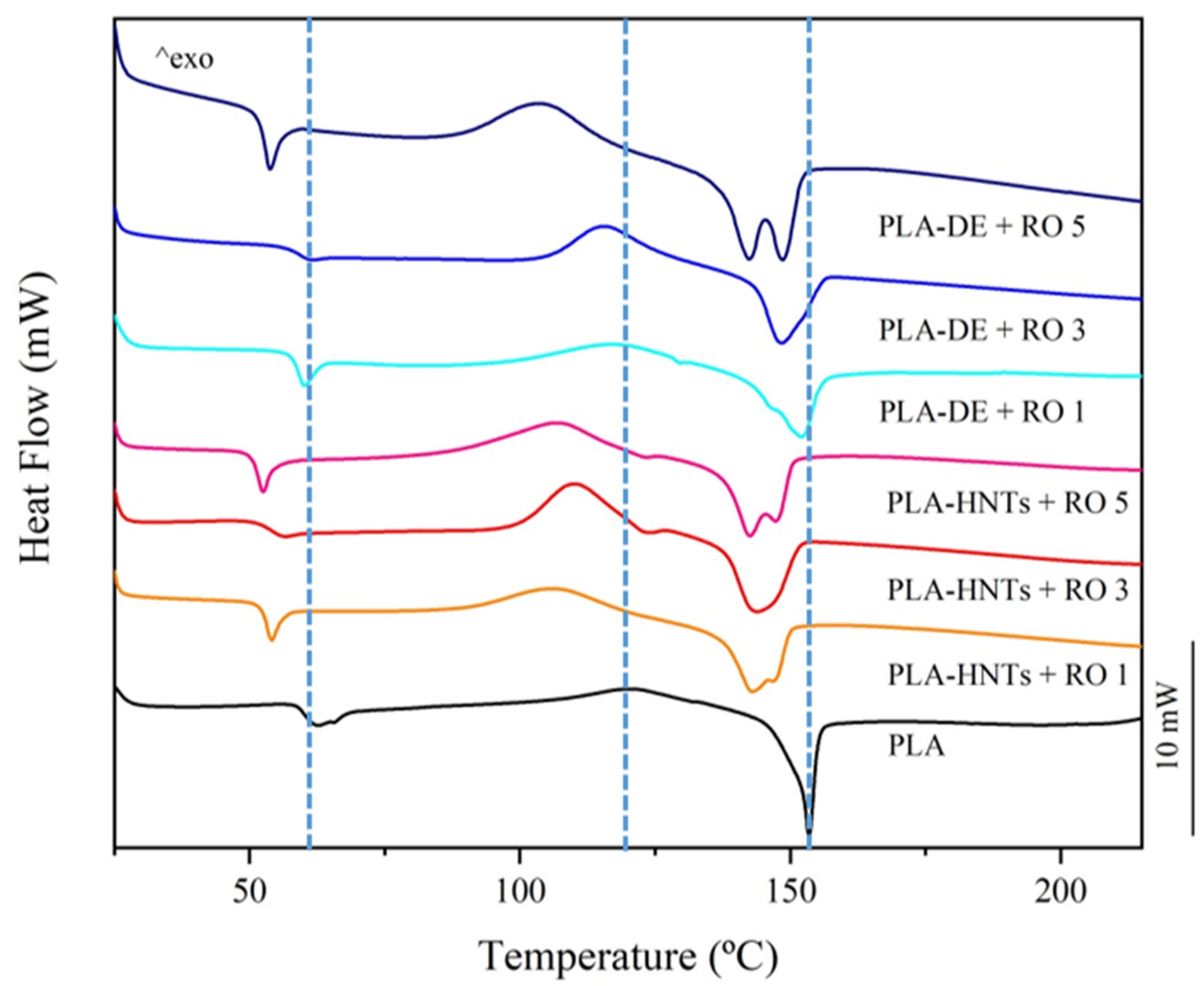
3.2.4.1. Differential Scanning Calorimetric
3.2.4.2. Thermogravimetric Analysis
3.2.5. Antioxidant Activity
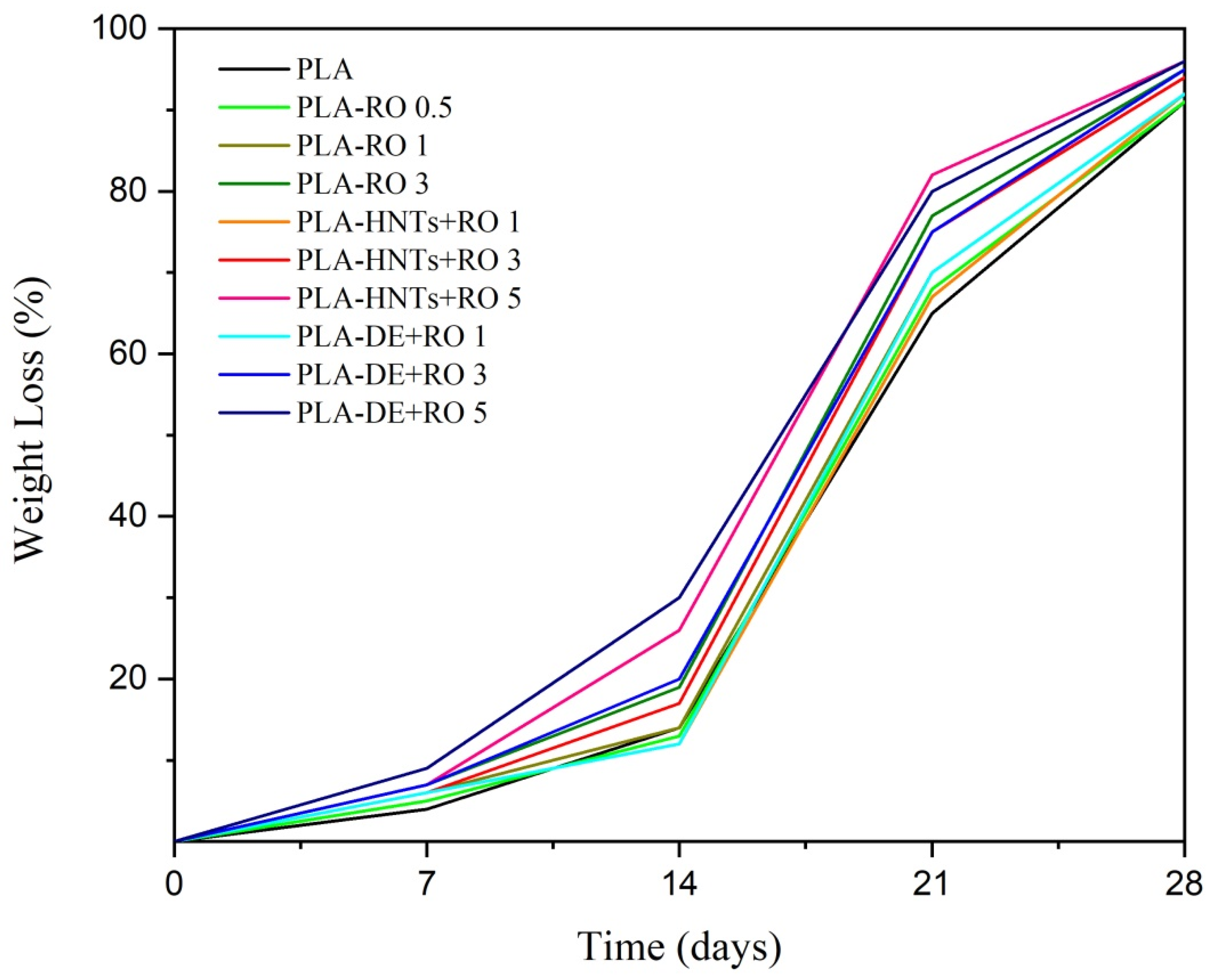
3.3. Disintegration under Composting Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable Oil Based Eco-Friendly Coating Materials: A Review Article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Sarp, A.; Glen, B.; Sithole, B. Nanofiller/Natural Fiber Filled Polymer Hybrid Composite: A Review. J. Eng. Sci. Technol. Rev. 2021, 14, 61–74. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.J. Recent Advances in Mechanical Properties of Biopolymer Composites: A review. Polym. Compos. 2019, 41, 32–59. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable Polymers For Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and Fossil-Based Polymeric Blends and Nanocomposites for Packaging: Structure-property Relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef]
- Han, B.; Chen, P.; Guo, J.; Yu, H.; Zhong, S.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. A Novel Intelligent Indicator Film: Preparation, Characterization, and Application. Molecules 2023, 28, 3384. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of Pla-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, H.J. Preparation of Halloysite Nanotubes Coated with Eudragit for a Controlled Release of Thyme Essential Oil. J. Appl. Polym. Sci. 2015, 132, 42771. [Google Scholar] [CrossRef]
- Agüero, A.; Lascano, D.; Ivorra-Martinez, J.; Gómez-Caturla, J.; Arrieta, M.P.; Balart, R. Use of Bacterial Cellulose Obtained from Kombucha Fermentation in Spent Coffee Grounds for Active Composites Based on Pla and Maleinized Linseed Oil. Ind. Crops Prod. 2023, 202, 116971. [Google Scholar] [CrossRef]
- García-Arroyo, P.; Arrieta, M.P.; Garcia-Garcia, D.; Cuervo-Rodríguez, R.; Fombuena, V.; Mancheño, M.J.; Segura, J.L. Plasticized Poly(Lactic Acid) Reinforced with Antioxidant Covalent Organic Frameworks (COFs) as Novel Nanofillers Designed for Non-Migrating Active Packaging Applications. Polymer 2020, 196, 122466. [Google Scholar] [CrossRef]
- Faba, S.; Arrieta, M.P.; Romero, J.; Agüero, Á.; Torres, A.; Martínez, S.; Rayón, E.; Galotto, M.J. Biodegradable nanocomposite poly(lactic acid) Foams Containing Carvacrol-Based Cocrystal Prepared by Supercritical CO2 Processing for Controlled Release in Active Food Packaging. Int. J. Biol. Macromol. 2024, 254, 127793. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and Antimicrobial Applications of Biopolymers: A Review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Castro-López, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized Poly(Lactic Acid)-Poly(Hydroxybutyrate) (Pla-PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Krepker, M.; Shemesh, R.; Danin Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active Food Packaging Films with Synergistic Antimicrobial Activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of Essential oils in Food Safety: Antimicrobial and Antioxidant Applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Gachkar, L.; Yadegari, D.; Rezaei, M.; Taghizadeh, M.; Astaneh, S.; Rasooli, I. Chemical and Biological Characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. Pla/Organoclay Bionanocomposites Impregnated with Thymol and Cinnamaldehyde by Supercritical Impregnation for Active and Sustainable Food Packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In Vitro Antibacterial and Antioxidant Properties of Chitosan Edible Films Incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Development of Essential Oil Incorporated Active Film Based on Biodegradable Blends of Poly (Lactide)/Poly (Butylene Adipate-Co-Terephthalate) for Food Packaging Application. J. Packag. Technol. Res. 2020, 4, 235–245. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Z.; Sun, J.; Liu, F.; Du, L.; Wang, D. Preparation and Characterization of Polylactic Acid Nanofiber Films Loading Perilla Essential oil for Antibacterial Packaging of Chilled Chicken. Int. J. Biol. Macromol. 2021, 192, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Cheran, E.; Sharmila Rahale, C.; Divyabharathi, P.; Viswanathan, C.; Narayanan, L. Corn Cob Nanocellulose Packaging for Increasing the Shelf Life of Food Products. Int. J. Biol. Macromol. 2024, 268, 131403. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of Pla-Limonene Blends for Food Packaging Applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Darie-Niţa, R.N.; Dumitriu, R.P.; Pamfil, D.; Tarţau, L. Biocompatible Materials Based on Plasticized Poly(Lactic Acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(Lactic Acid) Materials. Polymers 2019, 11, 941. [Google Scholar] [CrossRef]
- Arrieta, M.P.; García, A.D.; López, D.; Fiori, S.; Peponi, L. Antioxidant bilayers based on PHBV and Plasticized Electrospun Pla-PHB Fibers Encapsulating Catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why Does Vacuum Drive to the Loading of Halloysite Nanotubes? The Key Role of Water Confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and Uptake of Halloysite Clay Nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef]
- Cerdá-Gandia, R.; Ivorra Martínez, J.; Agüero, Á.; Quiles-Carrillo, L.; Gomez-Caturla, J.; Fenollar, O.; Arrieta, M.P. Processing of Biopolymers Loaded with Porous Inorganic Fillers Encapsulating Active Substance for Active Food Packaging Applications. In Biopolymers: Synthesis, Properties, and Emerging Applications; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1. [Google Scholar]
- Price, R.R.; Gaber, B.P.; Lvov, Y. In-Vitro Release Characteristics Of Tetracycline HCl, Khellin and Nicotinamide Adenine Dineculeotide from Halloysite; a Cylindrical Mineral. J. Microencapsul. 2001, 18, 713–722. [Google Scholar]
- Gorrasi, G.; Pantani, R.; Murariu, M.; Dubois, P. Pla/Halloysite Nanocomposite Films: Water Vapor Barrier Properties and Specific Key Characteristics. Macromol. Mater. Eng. 2014, 299, 104–115. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/Halloysite Nanotubes Bionanocomposites: Structure, Mechanical Properties and Biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Fombuena, V.; Valles-Lluch, A.; Ellingham, T. Improvement of Mechanical and Biological Properties of Polycaprolactone Loaded with Hydroxyapatite and Halloysite Nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Hao, A.; Wong, I.; Wu, H.; Lisco, B.; Ong, B.; Sallean, A.; Butler, S.; Londa, M.; Koo, J.H. Mechanical, Thermal, and Flame-Retardant Performance of Polyamide 11–Halloysite Nanotube Nanocomposites. J. Mater. Sci. 2014, 50, 157–167. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of Halloysite Nanoclay on the Physical, Mechanical, and Antioxidant Properties of Chitosan Films Incorporated with Clove Essential Oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Lee, M.H.; Seo, H.S.; Park, H.J. Thyme Oil Encapsulated in Halloysite Nanotubes for Antimicrobial Packaging System. J. Food Sci. 2017, 82, 922–932. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of Halloysite Loaded with Natural Antimicrobials into Pectins: Characterization and Controlled Release Analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef]
- Lopez-Cebral, R.; Peng, G.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Chen, J.; Silva, T.H.; Reis, R.L. Dual Delivery of Hydrophilic and Hydrophobic Drugs from Chitosan/Diatomaceous Earth Composite Membranes. J. Mater. Sci. Mater. Med. 2018, 29, 21. [Google Scholar] [CrossRef]
- Delasoie, J.; Zobi, F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef]
- Aguero, A.; Quiles-Carrillo, L.; Jorda-Vilaplana, A.; Fenollar, O.; Montanes, N. Effect of Different Compatibilizers on Environmentally Friendly Composites from Poly(Lactic Acid) and Diatomaceous earth. Polym. Int. 2019, 68, 893–903. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Sriram, G.; Brahmkhatri, V.; Kigga, M.; Jung, H.-Y.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Xerogel Modified Diatomaceous Earth Microparticles for Controlled Drug Release Studies. New J. Chem. 2018, 42, 11964–11971. [Google Scholar] [CrossRef]
- Hakovirta, M.; Aksoy, B.; Hakovirta, J. Self-Assembled Micro-Structured Sensors For Food Safety in Paper Based Food Packaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-Sustainable Systems Based on Poly(Lactic Acid), Diatomite and Coffee Grounds Extract for Food Packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Johnson, S.; Saloni, D.; Hakovirta, M. Novel 3D Printing Filament Composite Using Diatomaceous Earth and Polylactic Acid for Materials Properties And Cost Improvement. Compos. Part B Eng. 2019, 177, 107310. [Google Scholar] [CrossRef]
- Kabouche, Z.; Boutaghane, N.; Laggoune, S.; Kabouche, A.; Aitkaki, Z.; Benlabed, K. Comparative Antibacterial Activity of Five Lamiaceae Essential Oils from Algeria. Int. J. Aromather. 2005, 15, 129–133. [Google Scholar] [CrossRef]
- Yinrong Lu, L.; Foo, Y. Antioxidant Activities of Polyphenols from Sage (Salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar]
- Othman, S.H.; Hassan, N.; Talib, R.A.; Kadir Basha, R.; Risyon, N.P. Mechanical and Thermal Properties of Pla/Halloysite Bio-Nanocomposite Films: Effect of Halloysite Nanoclay Concentration and Addition Of Glycerol. J. Polym. Eng. 2017, 37, 381–389. [Google Scholar] [CrossRef]
- Gonzalez, L.; Aguero, A.; Quiles-Carrillo, L.; Lascano, D.; Montanes, N. Optimization of the Loading of an Environmentally Friendly Compatibilizer Derived from Linseed Oil in Poly(Lactic Acid)/Diatomaceous Earth Composites. Mater. 2019, 12, 1627. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.; Balart, R.; Torres-Giner, S. Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers. Appl. Sci. 2019, 9, 533. [Google Scholar] [CrossRef]
- ISO 527-2:2012; Plastics-Determination of Tensile Properties Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: Geneva, Switzerland, 2012.
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(Lactic Acid) Crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- ISO 20200:2023; Plastics–Determination of the Degree of Disintegration of Plastic Materials under Composting Conditions in a Laboratory-Scale Test. International Organization for Standardization: Geneva, Switzerland, 2023.
- Montava-Jorda, S.; Chacon, V.; Lascano, D.; Sanchez-Nacher, L.; Montanes, N. Manufacturing and Characterization of Functionalized Aliphatic Polyester from Poly(Lactic Acid) with Halloysite Nanotubes. Polymers 2019, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of Thermo-Mechanical, Chemical and Degradative Properties of Pla-Limonene Films Reinforced with Cellulose Nanocrystals Extracted from Phormium Tenax leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Bio-Based Pla_PHB Plasticized Blend Films: Processing and Structural Characterization. LWT Food Sci. Technol. 2015, 64, 980–988. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Quiles-Carrillo, L.; Garcia-Garcia, D.; Melendez-Rodriguez, B.; Balart, R.; Torres-Giner, S. Tailoring the Properties of Thermo-Compressed Polylactide Films for Food Packaging Applications by Individual and Combined Additions of Lactic Acid Oligomer and Halloysite Nanotubes. Molecules 2020, 25, 1976. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.T.; Pasbakhsh, P.; Goh, K.L.; Chai, S.-P.; Chen, J. Synthesis and Characterisation of Poly (Lactic Acid)/Halloysite Bionanocomposite Films. J. Compos. Mater. 2014, 48, 3705–3717. [Google Scholar] [CrossRef]
- Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L.; Balart, R.; Garcia-Garcia, D.; Dominici, F.; Puglia, D.; Torre, L. Development of Biodegradable Pla Composites And Tangerine Peel Flour with Improved Toughness Containing a Natural-Based Terpenoid. Express Polym. Lett. 2023, 17, 789–805. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jimenez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and Characterization of plasticized Pla/PHB Blends for Biodegradable Multiphase Systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, Rheological, and Thermal Properties of Plasticized Polylactide Films Incorporated with Essential Oils to Inhibit Staphylococcus aureus and Campylobacter jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef]
- Pirani, S.I.; Krishnamachari, P.; Hashaikeh, R. Optimum Loading Level of Nanoclay in Pla Nanocomposites. J. Thermoplast. Compos. Mater. 2013, 27, 1461–1478. [Google Scholar] [CrossRef]
- Othman, S.H.; Ling, H.N.; Talib, R.A.; Naim, M.N.; Risyon, N.P.; Saifullah, M. Pla/MMT and Pla/Halloysite Bio-Nanocomposite Films: Mechanical, Barrier, and Transparency. J. Nano Res. 2019, 59, 77–93. [Google Scholar] [CrossRef]
- Silverajah, V.S.; Ibrahim, N.A.; Zainuddin, N.; Yunus, W.M.; Hassan, H.A. Mechanical, Thermal and Morphological Properties of Poly(Lactic Acid)/Epoxidized Palm Olein Blend. Molecules 2012, 17, 11729–11747. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, M.; Hu, H. Study on Graphene Oxide Modified Inorganic Phase Change Materials and Their Packaging Behavior. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2018, 33, 788–792. [Google Scholar] [CrossRef]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Halloysite Nanotube Reinforced Polylactic Acid Composite. Polym. Compos. 2017, 38, 2166–2173. [Google Scholar] [CrossRef]
- Carrasco, F.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(Lactic Acid)/Organomontmorillonite Nanocomposites: Microstructure, Thermal Stability And Kinetics of the Thermal Decomposition. Chem. Eng. J. 2011, 178, 451–460. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative Activity of Rosmarinus officinalis L. Essential Oil Compared to Its Main Components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Zeid, A.; Karabagias, I.K.; Nassif, M.; Kontominas, M.G. Preparation and Evaluation of Antioxidant Packaging Films Made of Polylactic Acid Containing Thyme, Rosemary, and Oregano Essential Oils. J. Food Process. Preserv. 2019, 43, e14102. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Stoleru, E.; Pamfil, D.; Zaharescu, T.; Tarţău, L.; Tudorachi, N.; Brebu, M.A.; Pricope, G.M.; Dumitriu, R.P.; et al. Evaluation of the Rosemary Extract Effect on the Properties of Polylactic Acid-Based Materials. Materials 2018, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary Pla–PHB–Limonene Blends Intended for Biodegradable Food Packaging Applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Alakrach, A.M.; Noriman, N.Z.; Dahham, O.S.; Hamzah, R.; Alsaadi, M.A.; Shayfull, Z.; Syed Idrus, S.Z. Chemical and Hydrophobic Properties of Pla/HNTs-ZrO2Bionanocomposites. J. Phys. Conf. Ser. 2018, 1019, 012065. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and Characterization of Pla_PBS Biodegradable Blends Reinforced with Cellulose Nanocrystals Extracted From Hemp Fibres. Ind. Crops Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]





| Film | E (MPa) | σy (MPa) | εb (%) |
|---|---|---|---|
| PLA | 3700 ± 80 a,b,c | 38.5 ± 0.5 a | 2.3 ± 0.1 a,b |
| PLA-RO 0.5 | 3880 ± 80 d,e | 39.8 ± 0.4 a | 1.7 ± 0.2 c |
| PLA-RO 1 | 3980 ± 40 d | 38.4 ± 0.5 a | 1.2 ± 0.2 a |
| PLA-RO 3 | 3120 ± 30 f | 26.0 ± 0.9 b | 3.6 ± 0.7 d |
| PLA-HNTs + RO 1 | 3770 ± 40 a,e | 28.8 ± 0.2 b | 3.1 ± 0.3 b,d |
| PLA-HNTs + RO 3 | 3590 ± 80 b | 36.6 ± 0.4 a | 2.7 ± 0.4 b |
| PLA-HNTs + RO 5 | 2940 ± 60 g | 25.9 ± 0.2 b | 2.9 ± 0.3 b,d |
| PLA-DE + RO 1 | 4110 ± 70 h | 46.9 ± 0.7 c | 4.8 ± 0.4 e |
| PLA-DE + RO 3 | 4550 ± 60 i | 39.2 ± 0.4 a | 1.6 ± 0.2 c,d |
| PLA-DE + RO 5 | 3670 ± 40 c | 29.7 ± 0.6 d | 4.3 ± 0.7 e |
| Film | Tg (°C) | Tcc (°C) | ΔHcc (J g−1) | Tm (°C) | ΔHm (J g−1) | χc (%) |
|---|---|---|---|---|---|---|
| PLA | 60.3 ± 0.8 a | 123.5 ± 1.3 a | 9.5 ± 0.8 | 153.5 ± 0.9 a | 20.7± 0.7 | 11.9 ± 0.1 |
| PLA-RO 0.5 | 58.1 ± 1.4 a | 114.0 ± 0.9 b,c | 18.7 ± 0.7 | 153.6 ± 1.1 a | 27.3 ± 1.1 | 9.2 ± 0.2 |
| PLA-RO 1 | 59.1 ± 1.2 a | 114.0 ± 1.1 b,c | 21.2 ± 0.9 | 147.8 ± 0.8 b,c | 29.4 ± 0.9 | 8.9 ± 0.2 |
| PLA-RO 3 | 58.4 ± 1.4 a | 111.6 ± 1.4 b,c | 20.7 ± 0.6 | 148.2 ± 1.3 b,c | 26.0 ± 0.8 | 5.9 ± 0.7 |
| PLA-HNTs + RO 1 | 58.1 ± 1.1 a | 111.3 ± 0.8 b,d | 16.6 ± 0.8 | 147.7 ± 1.1 c | 27.8 ± 0.7 | 12.1 ± 0.3 |
| PLA-HNTs + RO 3 | 59.2 ± 1.1 a | 115.1 ± 1.2 b,c | 21.5 ± 0.8 | 148.7 ± 1.2 b,c | 22.8 ± 0.9 | 1.4 ± 0.4 |
| PLA-HNT + RO 5 | 56.6 ± 1.3 a | 112.0 ± 1.1 b,e | 26.7 ± 0.9 | 147.7 ± 0.9 b,c | 27.7 ± 1.1 | 1.2 ± 0.3 |
| PLA-DE + RO 1 | 58.9 ± 0.9 a | 118.1 ± 0.9 c | 11.0 ± 1.2 | 152.1 ± 0.8 a,b,d | 22.7 ± 0.8 | 12.6 ± 0.4 |
| PLA-DE + RO 3 | 59.3 ± 1.2 a | 115.3 ± 1.3 b,c | 17.4± 1.1 | 148.2 ± 1.4 c,d | 22.9 ± 0.9 | 6.1 ± 0.2 |
| PLA-DE + RO 5 | 57.7 ± 1.1 a | 108.6 ± 1.1 d,e | 12.8 ± 1.2 | 153.4 ± 1.2 a | 28.4 ± 0.7 | 17.6 ± 0.7 |
| Film | (°C) | Residual Mass (%) | |
|---|---|---|---|
| PLA | 322 ± 1.2 a | 366.94 ± 1.1 a,b,c | 1.6 ± 0.2 |
| PLA-RO 0.5 | 328. ± 1.2 b | 354.09 ± 0.9 a | 0.4 ± 0.3 |
| PLA-RO 1 | 328.7 ± 0.9 b | 361.27 ± 1.1 a,b,c | 0.4 ± 0.2 |
| PLA-RO 3 | 343.6 ± 1.1 c | 359.64 ± 0.7 a,b | 0.3 ± 0.2 |
| PLA-HNTs + RO 1 | 322.1 ± 0.9 a | 353.29 ± 1.1 a | 0.6 ± 0.3 |
| PLA-HNTs + RO 3 | 316.6 ± 1.5 d | 362.55 ± 1.4 a,b,c | 3.0 ± 0.1 |
| PLA-HNTs + RO 5 | 313.8 ± 1.4 d | 372.61 ± 1.2 b,c | 5.0 ± 0.2 |
| PLA-DE + RO 1 | 350.0 ± 1.1 e | 378.49 ± 1.2 c | 1.2 ± 0.3 |
| PLA-DE + RO 3 | 306.2 ± 1.2 f | 360.81 ± 0.9 a,b,c | 2.3 ± 0.2 |
| PLA-DE + RO 5 | 328.3 ± 0.9 b | 358.76 ± 1.1 a,b | 2.1 ± 0.1 |
| Film | RSA (%) |
|---|---|
| PLA | - |
| PLA-RO 0.5 | 12.7 ± 2.5 a |
| PLA-RO 1 | 22.1 ± 3.1 b |
| PLA-RO 3 | 32.9 ± 1.9 c,d |
| PLA-HNTs + RO 1 | 37.3 ± 2.1 e |
| PLA-HNTs + RO 3 | 22.4 ± 3.2 b,f |
| PLA-HNTs + RO 5 | 43.8 ± 1.6 e |
| PLA-DE + RO 1 | 43.3 ± 1.4 e |
| PLA-DE + RO 3 | 28.8 ± 1.3 d,f |
| PLA-DE + RO 5 | 27.6 ± 0.9 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerdá-Gandia, R.; Agüero, Á.; Arrieta, M.P.; Fenollar, O. Effect of Different Porous Size of Porous Inorganic Fillers on the Encapsulation of Rosemary Essential Oil for PLA-Based Active Packaging. Polymers 2024, 16, 2632. https://doi.org/10.3390/polym16182632
Cerdá-Gandia R, Agüero Á, Arrieta MP, Fenollar O. Effect of Different Porous Size of Porous Inorganic Fillers on the Encapsulation of Rosemary Essential Oil for PLA-Based Active Packaging. Polymers. 2024; 16(18):2632. https://doi.org/10.3390/polym16182632
Chicago/Turabian StyleCerdá-Gandia, Raúl, Ángel Agüero, Marina Patricia Arrieta, and Octavio Fenollar. 2024. "Effect of Different Porous Size of Porous Inorganic Fillers on the Encapsulation of Rosemary Essential Oil for PLA-Based Active Packaging" Polymers 16, no. 18: 2632. https://doi.org/10.3390/polym16182632
APA StyleCerdá-Gandia, R., Agüero, Á., Arrieta, M. P., & Fenollar, O. (2024). Effect of Different Porous Size of Porous Inorganic Fillers on the Encapsulation of Rosemary Essential Oil for PLA-Based Active Packaging. Polymers, 16(18), 2632. https://doi.org/10.3390/polym16182632








