Unlocking the Potential of Food Waste: A Review of Multifunctional Pectins
Abstract
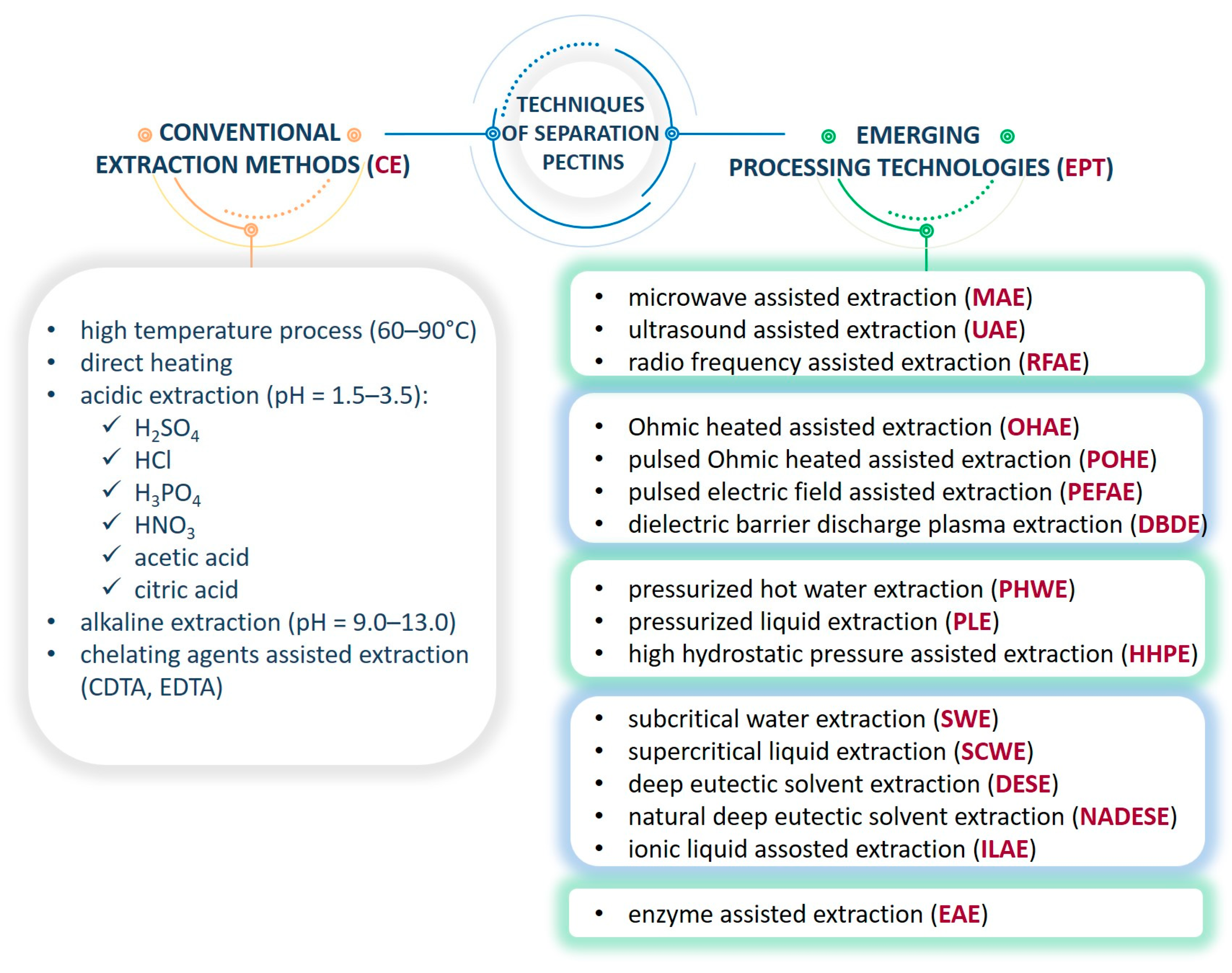
:1. Introduction
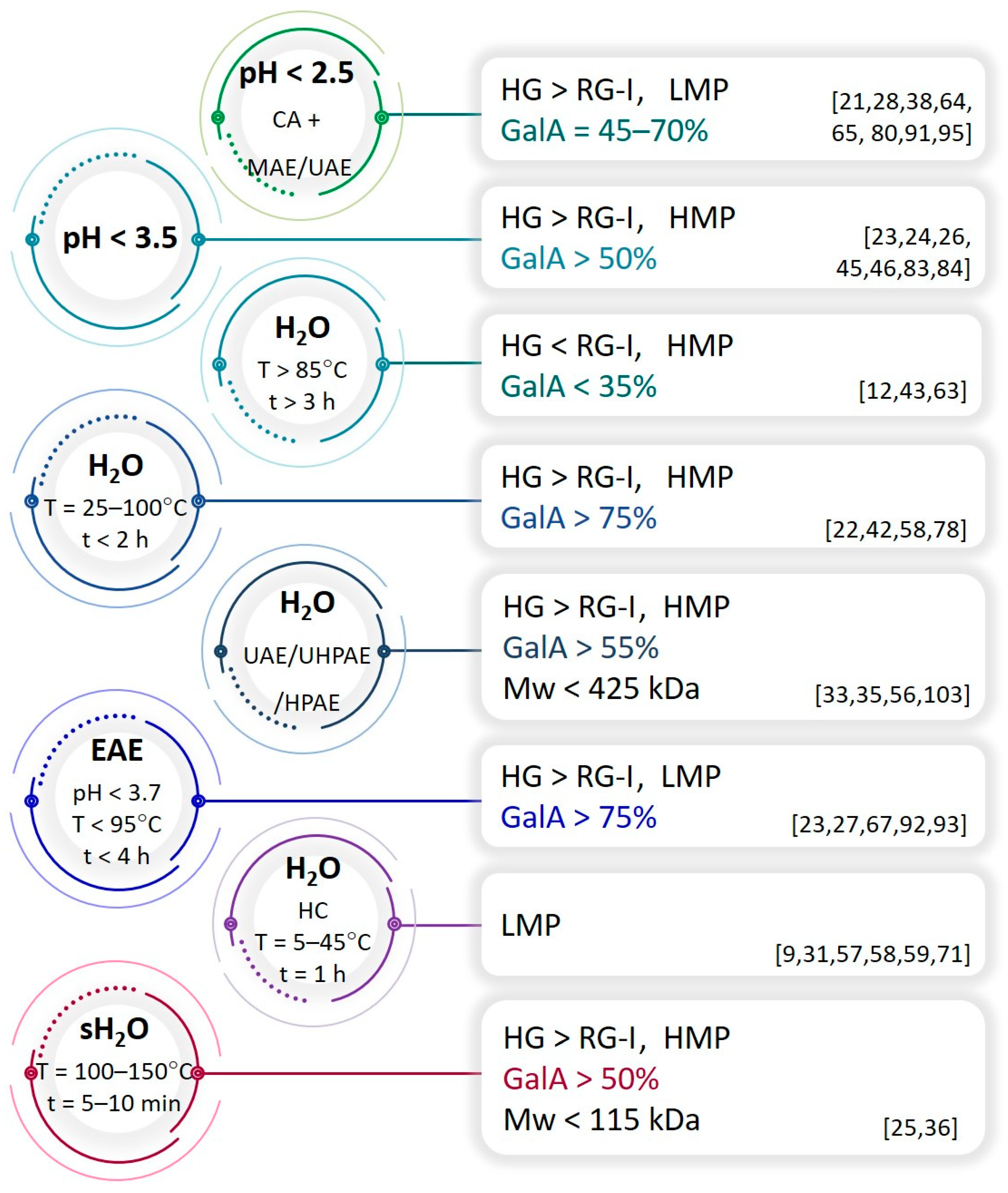
2. Comparative Analysis of Pectin Structure with Recovery Technology
2.1. Overview of the Structural Features of Pectins Extracted from Fruit Biomass
| Plant (Fruit) | Waste By-Product | Extraction Technique | Conditions of Extraction | DE (%) | GalA (%) | Mw (kDa) | Utility | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | pH | T (°C) | t | Factor | |||||||||
| Actinidia chinensis (kiwifruit) | Pomace | CE | C6H8O7 | 2.2 | 50 | 1 h | - | 6.2 | n. a. | 82.4 | 840 | Shear-thinning property | [22] |
| CE | H2O | 3.7 | 25 | 30 min | - | 5.3 | n.a. | 82.7 | 850 | Shear-thinning property | |||
| EAE | H2O | 3.7 | 25 | 30 min | CEL PG EAR | 5.8 | n.a. | 84.6 | 670 | Shear-thinning property | |||
| Akebia Trifoliata (chocolate wine) | Fruit peel | CE | C6H8O7 | 2.2 | 85 | 2 h | - | n.a. | 29.4 | 79.9 | 112 | Biomaterial for sponges; emulsifying activity; gel-forming property; reductant of AgNO3 to Ag nanoparticles | [52] |
| Ananas comosus (pineapple) | Fruit peel | MAE | C6H8O7 | 2.0 | 85 | 10 min | 1400 W | n.a. | 39.4 | 44.8 | 889 | Antioxidant activity; Film-forming property | [64] |
| 30 min | 420 W | ||||||||||||
| Artocarpus heterophyllus (jackfruit) | Fruit peel | CE | C6H8O7 | 2.0 | 90 | 2 h | - | 0.9 | 73.9 | 57.0 | 174 | Gel-forming property; Shear-thinning property | [25] |
| SWE | H2O | - | 138 | 9.15 min | - | 1.0 | 61.1 | 52.3 | 113 | Gel-forming property; Shear-thinning property | |||
| Fruit peel and seeds | CE | (NH4)2C2O4 | 4.6 | 85 | 1 h | 0.7 | 78.3 | 63.3 | 40 | Gel-forming property | [24] | ||
| Fruit peel and seeds | CE | H2SO4 | 1.5 | 90 | 1.5 h | - | 1.1 | 63.0 | 62.7 | 39 | Gel-forming property | ||
| Borassus aethiopum Mart. (palmyra palm) | Fruit pulp | CE | H2O | ~5.3 | 90 | 30 min | - | 24.8 | 73.2 | 84.0 | 338 | Emulsifying activity; Gel-forming property | [42] |
| Campomanesia xanthocarpa (gabiroba) | Fruit pulp | CE | H2O | - | 120 | 4 h | - | 0.5 | 60.0 | 33.5 | ~1000 | Antitumor activity | [43] |
| Carica papaya L. (papaya) | Fruit pulp | CE | H2O | - | 25 | 20 min | - | 3.2 | 59.0 | 74.8 | 102 2 | Antitumor activity | [55] |
| Citrullus lanatus (watermelon) | Fruit rinds | CE | HNO3 | 1.0 | 100 | 1 h | - | 2.8 | 63.0 | 74.2 | 34 | Emulsifying activity; foaming activity; shear-thinning property | [26] |
| UAE | C6H8O7 | 1.8 | - | 43 min | 573 W | 1.9 | 44.1 | 69.0 | 271 | Emulsifying activity | [28] | ||
| CE followed by enzymatic treatment | H2O | 1.4 | 95 | 1.5 h | - | 3.8 | 4.8 | 81.8 | 50 | Biomaterial for hydrogel beads and aerogel beads | [27,72] | ||
| PBS | 4.0 | 40 | 24 h | EAR PME | |||||||||
| Citrus reticulata (mandarin) | Fruit peel | SWE | H2O | - | 100 | 5 min | - | 11.3 | 71.9 | 91.0 | 63 | Antioxidant activity; anti-tumor activity; gel-forming property; shear-thinning property | [35] |
| Citrus junos (yuzu) | Fruit peel | CE followed by enzymatic treatment | C6H8O7 | 1.6 | 80 | 4 h | - | 5.9 | 34.6 | 83.5 | n.a. | Biomaterial for hydrogel beads | [67] |
| H2O | 4.5 | 45 | 3 h | PME | |||||||||
| Citrus aurantium (bigarade) | Fruit peel | CE | HCl | 1.5 | 90 | 2 h | - | 6.8 | 80.3 | 86.0 | 80 | Emulsifying activity; Shear-thinning property | [46] |
| Citrus limon (lemon) | HCAE | H2O | - | 6–45 | 1 h | - | n.a. | 8.0 | n.a. | n.a. | Antibacterial activity; neuroprotective activity | [31,70,71] | |
| MAE | C6H8O7 | 1.5 | - | 3 min | 700 W | 1.3 | 5.8 | 60.0 | 616 | Antioxidant activity; emulsifying activity | [21] | ||
| CE | H2O | 1.8 | 85 | 30 min | - | 5.1 | 48.2 | 52.5 | 82 64 | Prebiotic properties | [30] | ||
| EAE | C6H8O7 | 3.5 | 50 | 4 h | CEL PL | 5.2 | 79.1 | 83.1 | 225 | Gel-forming property; stabilizing ability of food and pharmaceutical products | [29] | ||
| Citrus nobilis x Citrus deliciosa hybrid (kinnow) | Fruit peel | CE | H2O | 5.0 | 90 | 30 min | - | n.a. | 37.2 | 47.7 | 652 | Shear-thinning property; | [49] |
| NADESE | choline chloride: maltose 5:2 | - | 75 | 4 h | - | n.a. | 36.8 | 78.2 | n. a. | Emulsifying activity; | [8] | ||
| Citrus paradisi (grapefruit) | Fruit albedo | HCAE | H2O | - | 7.5–38 | 1 h | 150 W | n.a. | 14.0 | n. a. | n. a. | Anti-apoptotic activity; Antimicrobial activity; antioxidant activity; antitumor activity; cardioprotective effect; immunomodulatory activity; | [9,57,58,59] |
| UAE | H2O | - | 37 | 28 min | 61.5 W | 1.2 | 58.8 | 56.4 | 279 | Antioxidant activity; lipase-inhibitory property; shear-thinning property | [56] | ||
| Citrus sinensis Osbeck (orange) | Fruit peel | CE | C6H8O7 | 3.0 | 100 | 10 min | - | n.a. | >60.0 | 81.2 | 2 | Antimicrobial activity; antioxidant activity; composite film-forming ability | [32] |
| HPAE | H2O | - | - | 15–30 min | 125–500 MPa | 6.2–9.3 | 57.1–63.3 | 87.7–92.6 | 374–422 | Emulsifying activity; gel-forming property | [33] | ||
| UHPAE | H2O | - | 55 | 10 min | 550 MPa | n.a. | 71.0 | 63.0 | 306 | Anti-diabetic property; cholesterol-regulating property | [34,69] | ||
| Citrus unshiu Marc (Satsuma mandarin) | Segment material | CE | processing waste water (HClaq) discharged from citrus canning process | 1.1 | 48.8 | 45.0 | 531 | Obesity-mitigating agent; prebiotic activity | [68,73,74] | ||||
| Cucumis melo L. (melon) | Fruit peel | MAE | H2O | - | - | 13 min | 414 W | 1.0 | 19.3 | 40.7 | 57 | Antioxidant activity; emulsifying activity; foaming capacity | [60] |
| CE | C6H8O7 | 1.0 | 95 | 3.3 h | - | n.a. | 15.0 | 48.0 | 68 | Emulsifying activity; | [51] | ||
| Ficus carica L. (fig) | Peel | UAE followed by MAE | C6H8O7 | 1.4 | 70 | 21.3 min | 70 W | 1.8 | 33.6 | 55.4 | 6890 | Antioxidant activity; antitumor activity | [65] |
| 11.7 min | 580 W | ||||||||||||
| Fruit stalks | CE | C6H8O7 | 1.8 | 95 | 1 h | - | 3.6 | 65.9 | 63.0 | n.a. | Film-forming ability | [47] | |
| Fragaria vesca L. wild strawberry | Leaves | CE | NaOH | 13.0 | room | 24 h | - | 0.6 | 18.4 | 24.5 | 14–350 | Anticoagulant activity | [39] |
| CE | NaOH | 13.0 | 100 | 6 h | - | 1.0 | 11.1 | 57.6 | 25–350 | Anticoagulant activity | |||
| UAE | NaOH | 13.0 | 25 | 40 min | 60 W | 1.4 | 18.6 | 40.6 | 2–160 | Anticoagulant activity | |||
| MAE | NaOH | 13.0 | 80 | 20 min | 200 W | 5.5 | 12.5 | 79.5 | 12–160 | Anticoagulant activity | |||
| UF | NaOH | 13.0 | 100 | 6 h | PES 1 Bar | 2.2 | n.a. | 68.1 | 6–180 | Anticoagulant activity | [40] | ||
| Garcinia mangostana (mangosteen) | Fruit rind | CE | H2SO4 | 2.0 | 90 | 2 h | - | n.a. | 2.9 | 76.0 | 6 | Antioxidant activity | [50] |
| Hylocereus polyrhizus (dragon fruit) | Fruit peel | CE | C6H8O7 | 2.0 | 73 | 67 min | - | 0.6 | 63.7 | 39.1 | < 1 | Cholesterol-regulating property | [48,75] |
| Malus domestica (apple) | Pomace | SWE | H2O | - | 150 | 5 min | - | 4.5 | 86.0 | 52.2 | 53 | Antioxidant activity; antitumor activity; gel-forming property; shear-thinning property | [35] |
| MAE followed by heat treatment | HCl | 1.9 | - | 30 min | 945 W | n.a. | 64.8 | 68.5 | 1158 | Antioxidant activity; foaming activity; | [36] | ||
| 100 | 1 h | - | |||||||||||
| UAE followed by heat treatment | HCl | 1.9 | - | 30 min | 700 W | n.a. | 64.2 | 64.9 | 1158 | Antioxidant activity; emulsifying activity | |||
| 100 | 1 h | - | |||||||||||
| MAE | C6H8O7 | 2.0 | - | 10 min | 450 W | n.a. | 47.7 | 65.7 | < 1 | Antioxidant activity; binding and coating agent for food and pharmaceutical products | [38] | ||
| CE | Na2CO3 + NaBH4 | 7.0 | room | 24 h | - | 1.4 | 4.9 | 55.1 | n.a. | Gel-forming property; shear-thinning property; | [37,76] | ||
| Mangifera indica (mango) | Fruit peel | UAE | C6H8O7 | 2.5 | 80 | 15 min | 500 W | n.a. | 88.6 | 53.3 | 2320 | Emulsifying activity; shear-thinning property; | [62] |
| Musa paradisiaca (banana) | Fruit peel | MAE | HCl | 3.0 | 195 | 60 s | 1000 W | n.a. | 2.0 | 26.0 | 1 | Prebiotic properties; shear-thinning property; viscosity modifier for food and pharmaceutical products; | [63,77] |
| UAE | H2SO4 | 2.5 | 10–15 | 20 min | 300 W | n.a. | 3.2 | 69.2 | < 1 | Emulsifying activity; shear-thinning property; viscosity modifier for food and pharmaceutical products | [61] | ||
| Opuntia albicarpa (pricky pear cactus) | Fruit peel | CE | EDTA | 4.0 | 70 | 2 h | - | 2.6 | 30.7 | 65.4 | 1016 | Gel-forming property; shear-thinning property; | [53] |
| Passiflora edulis f. flavicarpa L. (passion fruit) | Fruit peel | UAE followed by MAE | (NH4)2SO4 | 5.0 | 25 | 30 min | 400 W | 0.9 | 65.0 | 68.2 | 363 | Shear-thinning property; | [66] |
| 9 min | 600 W | ||||||||||||
| Passiflora tripartita var. mollissima (banana passion fruit) | Fruit epicarp | CE | HCl | 1.0 | 90 | 1 h | - | n.a. | 52.0 | 82.2 | 14 | Emulsifying activity | [45] |
| Rubus chingii Hu (blackberry) | Leaves | CE | H2O | - | 87.9 | 3.1 h | - | <0.1 | n.a. | 16.4 | 17 | Antitumor activity; immunomodulatory activity; antioxidant activity | [44] |
| Solanum betaceum (red chilto) | Fruit peel | CE | H2O | - | 100 | 2 h | - | 1.4 | 60.8 | 77.6 | n.a. | Antioxidant activity; emulsifying activity; film-forming ability; foaming activity; hypoglycemic potential; inhibitory activity to α-amylase; | [41,78] |
2.2. Overview of the Structural Features of Pectins Extracted from Vegetable Biomass
| Plant (Vegetable) | Waste By-Product | Extraction Technique | Conditions of Extraction | DE (%) | GalA (%) | Mw (kDa) | Utility | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | pH | T (°C) | t | Factor | |||||||||
| Arctium lappa L. (edible burdock) | Root | CE | H2O | - | 85 | 3 h | - | 0.6 | 67.5 | 68.8 | 1840 | Anti-constipation activity; Shear-thinning property | [12] |
| Beta vulgaris (sugar beet) | Root pulp | CE | H2O | 1.8 | 85 | 30 min | - | 2.9 | 60.6 | 40.8 | 6–82 | Prebiotic properties | [30] |
| Root | CE followed by alkali treatment | HNO3 | 1.7 | 70 | 4 h | - | 0.6 | 18.0 | 52.5 | 419 | Antitumor activity | [84] | |
| Brassica oleracea var. Italica (broccoli) | Stalk | CE | HNO3 | 2.0 | 100 | 30 min | - | 2.7 | 56.2 | 74.7 | 72 | Immunomodulatory activity; emulsifying activity; foaming ability; shear-thinning property; | [23,83] |
| Cucurbita maxima (pumpkin) | Fruit pulp | HCAE | H2O | 3.7–4.2 | 65–70 | 15–30 min | - | 1.6 | 71.9 | 62.0 | 26–96 | Antioxidant activity; cytoprotective effect | [87] |
| Cynara scolymus L. (artichoke) | Bracts, leaves, and stems | EAE | H2O | 5.0 | 50 | 48 h | CEL | 3.7 | 19.5 | 75.7 | 5–660 | Immunomodulatory activity | [92,93,94] |
| Daucus carota L. Ssp. Sativus var. Atrorubens Alef. (black carrot) | Pomace | MAE | CH3COOH | 2.5 | 110 | 5 min | 180 W | n.a. | 38.3 | 32.8 | 1170 * | Antioxidant activity | [85] |
| Root powder | UAE & EAE | Na3C6H5O7 | 5.2 | - | 20 min | 600 W + hCEL | 1.2 | 42.0 | 50.0 | 35–787 | Antioxidant activity; emulsifying activity; film-forming property; gel-forming property | [80,95] | |
| Nelumbo nucifera Gaertn (lotus) | Leaves | EAE | H2O | 4.5 | 50 | 48 h | AMS | 0.6 | n. a. | 32.0 | 79 16 | Immunomodulatory activity | [90] |
| EAE | H2O | 4.5 | 50 | 48 h | CEL | 0.3 | n.a. | 31.0 | 16 | Immunomodulatory activity | |||
| EAE | H2O | 4.5 | 50 | 48 h | PE | 0.1 | n.a. | 28.7 | 15 | Immunomodulatory activity | |||
| Solanum lycopersicum (tomato) | Fruit skin, seed, and pulp | CE | HCl | 2.0 | 85 | 1 h | - | n.a. | 76.3 | 80.0 | n.a. | Gel-forming property; Shear-thinning property; | [81] |
| Solanum melongena (eggplant) | Fruit peel | UAE | C6H8O7 | 1.5 | - | 30 min | 50 W | n.a. | 61.2 | 66.1 | n.a. | Antioxidant activity; Emulsifying activity; foaming capacity | [86] |
| Fruit calyx | MAE | C6H8O7 | 1.5 | - | 2 min | 700 W | 1.3 | 60.7 | 60.2 | n.a. | Antioxidant activity; emulsifying activity; foaming capacity | [89] | |
| Solanum tuberosum L. (potato) | Tuber peel | CE | C2H2O4 | 4.6 | 85 | 2 h | - | 1.3 | 35.9 | 58.4 | 1819 | Emulsifying activity; shear-thinning property | [54] |
| CE followed by HPH | C2H2O4 | 4.6 | 85 | 2 h | - | 1.1 | 18.0 | 72.3 | 597 | Emulsifying activity | |||
| C2H2O4 | 4.6 | - | 5 min | 200 MPa | |||||||||
| Tuber pulp | CE | HCl | 2.0 | 90 | 1 h | - | 0.5 | 28.6 | 29.8 | 280 | Emulsifying activity | [79] | |
| Zea mays (maize) | Husks | UA pretreatment followed by NaOH and EAE | H2O | - | - | 20 min | 750 W | 5.2 | 8.8 | 67.0 | 109 | Gel-forming property; texture modifiers for food and pharmaceutical products | [91] |
| NaOH | 13.0 | - | - | - | |||||||||
| Na3C6H5O7 | 5.2 | 40 | 4 h | CEL | |||||||||
| Badami cultivar (sunflower) | Heads and stems | UAE | H2O | - | 33 | 30 min | 400 W | 3.0 | 34.1 | 72.9 | 175 | Antioxidant activity; emulsifying activity; foaming activity | [88] |
| CE | (NH4)2C2O4 | - | 85 | 45 min | - | 2.8 | 27.3 | 82.1 | 606 | Shear-thinning property | [82] | ||
2.3. Overview of the Structural Features of Pectins Extracted from Miscellaneous Plant Biomass
| Plant (Nuts) | Waste By-Product | Extraction Technique | Extraction Parameters | DE (%) | GalA (%) | Mw (kDa) | Utility | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | pH | T (°C) | t | P (W) | |||||||||
| Conocarpus erectus (buttonwood) | Leaves | CE | H2O | - | 60 | 4 h | - | 0.4 | 37.5 | 36.0 | 24 | Antioxidant activity; immunomodulatory activity; prebiotic effect; | [97] |
| Juglans regia L. (walnut) | Green husks | UAE | HCl | 1.5 | - | 10 min | 200 W | n. a. | 59.2 | 69.4 | 93 | Antioxidant activity; emulsifying activity; | [103] |
| MAE | HCl | 1.5 | - | 3 min | 500 W | n.a. | 54.1 | 68.4 | 260 | Antioxidant activity; emulsifying activity; | [102] | ||
| Nicotiana tabacum L. (tabbaco) | Root | UAE followed by EAE | (NH4)2SO4 | - | - | 9 min | 180 W | 0.1 | n. a. | 2.3 | n.a. | Antioxidant activity | [107] |
| H2O | - | 50 °C | 1.5 h | CEL PE | |||||||||
| Pistacia vera L. (pistachio) | Hull | UAE | H2SO4 | 1.5 | - | 24 min | 150 W | n.a. | 41.3 | 59.3 | n.a. | Antioxidant activity; emulsifying activity; foaming activity; | [104] |
| MAE | H2SO4 | 1.5 | - | 165 s | 700 W | 1.8 | 12.1 | 66.0 | 2 | Antioxidant activity; emulsifying activity; | [105] | ||
| Suaeda fruticose (shrubby seablite) | Leaves | CE | C6H8O7 | 2.9 | 90 | 37 min | - | 0.9 | 33.0 | 47.5 | 229 | Analgesic properties; immunomodulatory activity; antioxidant activity; | [99] |
| Theobroma cacao L. (cocoa tree) | Pod husk | CE | HNO3 | 3.5 | 100 | 30 min | 1.1 | 41.0 | 59.2 | 1989 229 | Gel-forming property; shear-thinning property; | [98] | |
| CE followed by saponification | C6H8O7 | 3.0 | 95 | 1.5 h | - | 0.7 | 20.8 | 56.0 | 259 | Antimicrobial activity; immunomodulatory activity; | [101] | ||
| NaOH + NaBH4 | - | 4 | 16 h | - | |||||||||
| CE | C6H8C6 | 2.5 | 95 | 45 min | - | n.a. | 8.1 | 74.5 | n.a. | Shear-thinning property; | [100] | ||
| EAE | Na3C6H5O7 | 4.6 | 50 | 18.6 h | CEL | n.a. | 24.0 | 52.1 | n.a. | Gel-forming property; shear-thinning property | [106] | ||
3. Techno-Functional Usefulness of Pectins
3.1. Structure–Function Relationship of Pectin at Interfaces
3.2. Rheology of Pectin Solutions and Gels
3.3. Pectins as Functional Biomaterials
3.4. Pectins for Biological Application
3.4.1. Antioxidant Activity of Pectins
3.4.2. Immunomodulatory Properties of Pectins
3.4.3. Antitumor Activity of Pectins
3.4.4. Antimicrobial Activity of Pectins
3.4.5. Pectins as Prebiotics
3.4.6. Pectins as Metabolic Modulators
3.4.7. Pectins as Protective Agents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of Citrus Processing Waste: A Review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging Concepts in the Nutraceutical and Functional Properties of Pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Jha, A.; Kumar, A. Biobased Technologies for the Efficient Extraction of Biopolymers from Waste Biomass. Bioprocess. Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Banožić, M.; Babić, J.; Jokić, S. Recent Advances in Extraction of Bioactive Compounds from Tobacco Industrial Waste-a Review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of Citrus Peel Waste for the Sustainable Production of Value-Added Products. Bioresour. Technol. 2022, 351. [Google Scholar] [CrossRef]
- Santra, S.; Das, M.; Karmakar, S.; Banerjee, R. NADES Assisted Integrated Biorefinery Concept for Pectin Recovery from Kinnow (Citrus Reticulate) Peel and Strategic Conversion of Residual Biomass to L(+) Lactic Acid. Int. J. Biol. Macromol. 2023, 250, 126169. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Martino, D.C.; Alduina, R.; et al. A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics 2020, 9, 586. [Google Scholar] [CrossRef]
- Jin, M.Y.; Li, M.Y.; Huang, R.M.; Wu, X.Y.; Sun, Y.M.; Xu, Z.L. Structural Features and Anti-Inflammatory Properties of Pectic Polysaccharides: A Review. Trends Food Sci. Technol. 2021, 107, 284–298. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Verkempinck, S.H.E.; Kyomugasho, C.; Moldenaers, P.; Van Loey, A.M.; Hendrickx, M.E. Modified Rhamnogalacturonan-Rich Apple Pectin-Derived Structures: The Relation between Their Structural Characteristics and Emulsifying and Emulsion-Stabilizing Properties. Foods 2021, 10, 1586. [Google Scholar] [CrossRef]
- Li, K.; Zhu, L.; Li, H.; Zhu, Y.; Pan, C.; Gao, X.; Liu, W. Structural Characterization and Rheological Properties of a Pectin with Anti-Constipation Activity from the Roots of Arctium Lappa L. Carbohydr. Polym. 2019, 215, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Abodinar, A.; Smith, A.M.; Morris, G.A. A Novel Method to Estimate the Stiffness of Carbohydrate Polyelectrolyte Polymers Based on the Ionic Strength Dependence of Zeta Potential. Carbohydr. Polym. 2014, 112, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-L.; Liao, J.-S.; Qi, J.-R.; Jiang, W.-X.; Yang, X.-Q. Physicochemical Characteristics and Functional Properties of High Methoxyl Pectin with Different Degree of Esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Mathad, G.N.; Pandiselvam, R.; Lin, J.; Sun, D.W. Emerging Technologies to Obtain Pectin from Food Processing By-Products: A Strategy for Enhancing Resource Efficiency. Trends Food Sci. Technol. 2021, 115, 42–54. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Wicker, L.; Kim, Y.; Kim, M.J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a Bioactive Polysaccharide—Extracting Tailored Function from Less. Food Hydrocoll. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Singhal, S.; Swami Hulle, N.R. Citrus Pectins: Structural Properties, Extraction Methods, Modifications and Applications in Food Systems—A Review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Georgiev, Y.; Ognyanov, M.; Yanakieva, I.; Kussovski, V.; Kratchanova, M. Isolation, Characterization and Modification of Citrus Pectins. J. BioSci. Biotech. 2012, 1, 223–233. [Google Scholar]
- Kumar, S.; Konwar, J.; Purkayastha, M.D.; Kalita, S.; Mukherjee, A.; Dutta, J. Current Progress in Valorization of Food Processing Waste and By-Products for Pectin Extraction. Int. J. Biol. Macromol. 2023, 239, 124332. [Google Scholar] [CrossRef]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of Microwave-Assisted Extraction and Structural Characterization of Pectin from Sweet Lemon Peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef]
- Yuliarti, O.; Goh, K.K.T.; Matia-Merino, L.; Mawson, J.; Brennan, C. Extraction and Characterisation of Pomace Pectin from Gold Kiwifruit (Actinidia Chinensis). Food Chem. 2015, 187, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Petkowicz, C.L.O.; Williams, P.A. Pectins from Food Waste: Characterization and Functional Properties of a Pectin Extracted from Broccoli Stalk. Food Hydrocoll. 2020, 107, 105930. [Google Scholar] [CrossRef]
- Begum, R.; Aziz, M.G.; Yusof, Y.A.; Saifullah, M.; Uddin, M.B. Evaluation of Gelation Properties of Jackfruit (Artocarpus Heterophyllus) Waste Pectin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100160. [Google Scholar] [CrossRef]
- Li, W.J.; Fan, Z.G.; Wu, Y.Y.; Jiang, Z.G.; Shi, R.C. Eco-Friendly Extraction and Physicochemical Properties of Pectin from Jackfruit Peel Waste with Subcritical Water. J. Sci. Food Agric. 2019, 99, 5283–5292. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Vriesmann, L.C.; Williams, P.A. Pectins from Food Waste: Extraction, Characterization and Properties of Watermelon Rind Pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Méndez, D.A.; Schroeter, B.; Martínez-Abad, A.; Fabra, M.J.; Gurikov, P.; López-Rubio, A. Pectin-Based Aerogel Particles for Drug Delivery: Effect of Pectin Composition on Aerogel Structure and Release Properties. Carbohydr. Polym. 2023, 306, 120604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, S.; Zuo, H.; Lin, J.; Zheng, H.; Lei, H.; Yu, Q.; Wu, X.; Guo, Z. Freeze-Drying Pretreatment of Watermelon Peel to Improve the Efficiency of Pectin Extraction: RSM Optimization, Extraction Mechanism, and Characterization. Int. J. Biol. Macromol. 2023, 249, 125944. [Google Scholar] [CrossRef]
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.T.; Meyer, A.S.; Mikkelsen, J.D. Application of Enzymes for Efficient Extraction, Modification, and Development of Functional Properties of Lime Pectin. Food Hydrocoll. 2014, 40, 273–282. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic Potential of Pectins and Pectic Oligosaccharides Derived from Lemon Peel Wastes and Sugar Beet Pulp: A Comparative Evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional Antioxidant, Non-Cytotoxic Activity of Integral Lemon Pectin from Hydrodynamic Cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, Antioxidant and Antibacterial Properties of Fish Gelatin-Based Edible Films Enriched with Orange Peel Pectin: Wrapping Application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Dorado, C.; Chau, H.K.; Hotchkiss, A.T.; Cameron, R.G. Modification of Pectin with High-Pressure Processing Treatment of Fresh Orange Peel before Pectin Extraction: Part I. The Effects on Pectin Extraction and Structural Properties. Food Hydrocoll. 2024, 149, 109516. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-Diabetic Effect of Citrus Pectin in Diabetic Rats and Potential Mechanism via PI3K/Akt Signaling Pathway. Int. J. Biol. Macromol. 2016, 89, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Lü, X. Pectin Extracted from Apple Pomace and Citrus Peel by Subcritical Water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Abu-Salem, F.M.; Azab, D.E.S.H. A Comparative Study of Pectin Green Extraction Methods from Apple Waste: Characterization and Functional Properties. Int. J. Food Sci. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Cross-Linking of Sodium Carbonate-Soluble Pectins from Apple by Zinc Ions. Carbohydr. Polym. 2018, 196, 1–7. [Google Scholar] [CrossRef]
- Gurev, A.; Cesko, T.; Dragancea, V.; Ghendov-Mosanu, A.; Pintea, A.; Sturza, R. Ultrasound-and Microwave-Assisted Extraction of Pectin from Apple Pomace and Its Effect on the Quality of Fruit Bars. Foods 2023, 12, 2773. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I.; Balicki, S.; Wilk, K.A. Effect of Various Extraction Methods on the Structure of Polyphenolic-Polysaccharide Conjugates from Fragaria Vesca L. Leaf. Int. J. Biol. Macromol. 2019, 130, 664–674. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I.; Balicki, S.; Ziewiecki, R.; Capek, P.; Matulová, M.; Wilk, K.A. New Isolation Process for Bioactive Food Fiber from Wild Strawberry Leaf. Biochem. Eng. J. 2020, 161, 107639. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Méndez, D.A.; Martínez-Abad, A.; Zampini, C.; Torres, S.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Feasibility of Active Biobased Films Produced Using Red Chilto Wastes to Improve the Protection of Fresh Salmon Fillets via a Circular Economy Approach. Food Hydrocoll. 2022, 133, 107888. [Google Scholar] [CrossRef]
- Assoi, S.; Konan, K.; Walker, L.T.; Holser, R.; Agbo, G.N.; Dodo, H.; Wicker, L. Functionality and Yield of Pectin Extracted from Palmyra Palm (Borassus Aethiopum Mart) Fruit. LWT 2014, 58, 214–221. [Google Scholar] [CrossRef]
- da Costa Amaral, S.; Barbieri, S.F.; Ruthes, A.C.; Bark, J.M.; Brochado Winnischofer, S.M.; Silveira, J.L.M. Cytotoxic Effect of Crude and Purified Pectins from Campomanesia xanthocarpa Berg on Human Glioblastoma Cells. Carbohydr. Polym. 2019, 224, 115140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Lu, C.L.; Jiang, J.G.; Wang, M.; Wang, D.M.; Zhu, W. Bioactivities and Extraction Optimization of Crude Polysaccharides from the Fruits and Leaves of Rubus chingii Hu. Carbohydr. Polym. 2015, 130, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of Pectin Properties on Lipid Digestion under Simulated Gastrointestinal Conditions: Comparison of Citrus and Banana Passion Fruit (Passiflora tripartita Var. mollissima) Pectins. Food Hydrocoll. 2016, 52, 329–342. [Google Scholar] [CrossRef]
- El Fihry, N.; El Mabrouk, K.; Eeckhout, M.; Schols, H.A.; Filali-Zegzouti, Y.; Hajjaj, H. Physicochemical and Functional Characterization of Pectin Extracted from Moroccan Citrus Peels. LWT 2022, 162, 113508. [Google Scholar] [CrossRef]
- Çavdaroğlu, E.; Büyüktaş, D.; Farris, S.; Yemenicioğlu, A. Novel Edible Films of Pectins Extracted from Low-Grade Fruits and Stalk Wastes of Sun-Dried Figs: Effects of Pectin Composition and Molecular Properties on Film Characteristics. Food Hydrocoll. 2023, 135, 108136. [Google Scholar] [CrossRef]
- Zaid, R.M.; Mishra, P.; Wahid, Z.A.; Sakinah, A.M.M. Hylocereus Polyrhizus Peel’s High-Methoxyl Pectin: A Potential Source of Hypolipidemic Agent. Int. J. Biol. Macromol. 2019, 134, 361–367. [Google Scholar] [CrossRef]
- Ghoshal, G.; Negi, P. Isolation of Pectin from Kinnow Peels and Its Characterization. Food Bioprod. Process. 2020, 124, 342–353. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuan Shan, C.; Yi Shan, W.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and Antioxidant Activity of Pectin from Indonesian Mangosteen (Garcinia mangostana L.) Rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef]
- Raji, Z.; Khodaiyan, F.; Rezaei, K.; Kiani, H.; Hosseini, S.S. Extraction Optimization and Physicochemical Properties of Pectin from Melon Peel. Int. J. Biol. Macromol. 2017, 98, 709–716. [Google Scholar] [CrossRef]
- Yu, N.; Wang, X.; Ning, F.; Jiang, C.; Li, Y.; Peng, H.; Xiong, H. Development of Antibacterial Pectin from Akebia trifoliata Var. Australis Waste for Accelerated Wound Healing. Carbohydr. Polym. 2019, 217, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Lira-Ortiz, A.L.; Reséndiz-Vega, F.; Ríos-Leal, E.; Contreras-Esquivel, J.C.; Chavarría-Hernández, N.; Vargas-Torres, A.; Rodríguez-Hernández, A.I. Pectins from Waste of Prickly Pear Fruits (Opuntia albicarpa Scheinvar ‘Reyna’): Chemical and Rheological Properties. Food Hydrocoll. 2014, 37, 93–99. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of High Hydrostatic Pressure and High Pressure Homogenization Processing on Characteristics of Potato Peel Waste Pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Prado, S.B.R.D.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-Induced Chemical Modifications of Papaya Pectin Inhibit Cancer Cell Proliferation. Sci. Rep. 2017, 7, 16564. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Flori, L.; Albanese, L.; Calderone, V.; Meneguzzo, F.; Pagliaro, M.; Ciriminna, R.; Zabini, F.; Testai, L. Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels. Foods 2022, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Urone, G.; Frinchi, M.; Valenza, C.; Bonura, A.; Cipollina, C.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M.; Mudò, G.; et al. Anti-Apoptotic and Anti-Inflammatory Properties of Grapefruit IntegroPectin on Human Microglial HMC3 Cell Line. Cells 2024, 13, 355. [Google Scholar] [CrossRef]
- Nuzzo, D.; Scordino, M.; Scurria, A.; Giardina, C.; Giordano, F.; Meneguzzo, F.; Mudò, G.; Pagliaro, M.; Picone, P.; Attanzio, A.; et al. Protective, Antioxidant and Antiproliferative Activity of Grapefruit Integropectin on Sh-Sy5y Cells. Int. J. Mol. Sci. 2021, 22, 9368. [Google Scholar] [CrossRef]
- Golbargi, F.; Gharibzahedi, S.M.T.; Zoghi, A.; Mohammadi, M.; Hashemifesharaki, R. Microwave-Assisted Extraction of Arabinan-Rich Pectic Polysaccharides from Melon Peels: Optimization, Purification, Bioactivity, and Techno-Functionality. Carbohydr. Polym. 2021, 256, 117522. [Google Scholar] [CrossRef]
- Rivadeneira, J.P.; Castillo-Israel, K.A.T.; Wu, T. Physicochemical Characteristics, Rheology, and Emulsifying Properties of Ultrasound-Extracted Pectin from “saba” Banana Peel. Food Res. 2023, 7, 211–233. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and Functional Properties of Mango Peel Pectin Extracted by Ultrasound Assisted Citric Acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneira, J.P.; Wu, T.; Ybanez, Q.; Dorado, A.A.; Migo, V.P.; Nayve, F.R.P.; Castillo-Israel, K.A.T. Microwave-Assisted Extraction of Pectin from “Saba” Banana Peel Waste: Optimization, Characterization, and Rheology Study. Int. J. Food Sci. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Preparation and Characterization of Pectin Fraction from Pineapple Peel as a Natural Plasticizer and Material for Biopolymer Film. Food Bioprod. Process. 2019, 118, 198–206. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-Microwave Assisted Extraction of Pectin from Fig (Ficus carica L.) Skin: Optimization, Characterization and Bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, L.; Li, L.; Zhu, J.; Chen, X.; Zhang, S.; Li, L.; Yan, J.K. Physicochemical, Structural, and Rheological Characteristics of Pectic Polysaccharides from Fresh Passion Fruit (Passiflora edulis f. Flavicarpa L.) Peel. Food Hydrocoll. 2023, 136, 108301. [Google Scholar] [CrossRef]
- Lee, T.; Chang, Y.H. Structural, Physicochemical, and in-Vitro Release Properties of Hydrogel Beads Produced by Oligochitosan and de-Esterified Pectin from Yuzu (Citrus junos) Peel as a Quercetin Delivery System for Colon Target. Food Hydrocoll. 2020, 108, 106086. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Wei, C.; Ye, X.; Shi, J.; Chen, S. Pectin from Citrus Canning Wastewater as Potential Fat Replacer in Ice Cream. Molecules 2018, 23, 925. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of Pectin from Navel Orange Peel Assisted by Ultra-High Pressure, Microwave or Traditional Heating: A Comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A.; et al. New Neuroprotective Effect of Lemon Integropectin on Neuronal Cellular Model. Antioxidants 2021, 10, 669. [Google Scholar] [CrossRef]
- Méndez, D.A.; Martínez-Abad, A.; Martínez-Sanz, M.; López-Rubio, A.; Fabra, M.J. Tailoring Structural, Rheological and Gelling Properties of Watermelon Rind Pectin by Enzymatic Treatments. Food Hydrocoll. 2023, 135, 108119. [Google Scholar] [CrossRef]
- Zhu, K.; Mao, G.; Wu, D.; Yu, C.; Cheng, H.; Xiao, H.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Highly Branched RG-I Domain Enrichment Is Indispensable for Pectin Mitigating against High-Fat Diet-Induced Obesity. J. Agric. Food Chem. 2020, 68, 8688–8701. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.J.; Ye, X.; Chen, S. Depolymerized RG-I-Enriched Pectin from Citrus Segment Membranes Modulates Gut Microbiota, Increases SCFA Production, and Promotes the Growth of Bifidobacterium Spp., Lactobacillus Spp. and Faecalibaculum Spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, K.; Nur, N.I.; Gannasin, S.P.; Adzahan, N.M.; Bakar, J. High Methoxyl Pectin from Dragon Fruit (Hylocereus Polyrhizus) Peel. Food Hydrocoll. 2014, 42, 289–297. [Google Scholar] [CrossRef]
- Gawkowska, D.; Ciesla, J.; Zdunek, A.; Cybulska, J. The Effect of Concentration on the Cross-Linking and Gelling of Sodium Carbonate-Soluble Apple Pectins. Molecules 2019, 24, 1635. [Google Scholar] [CrossRef]
- Mada, T.; Duraisamy, R.; Abera, A.; Guesh, F. Effect of Mixed Banana and Papaya Peel Pectin on Chemical Compositions and Storage Stability of Ethiopian Traditional Yoghurt (Ergo). Int. Dairy. J. 2022, 131, 105396. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Zampini, I.C.; Torres, S.; Isla, M.I. Functional Characterization and Toxicity of Pectin from Red Chilto Fruit Waste (Peels). Plants 2023, 12, 2603. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, Structure, and Emulsifying Properties of Pectin from Potato Pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Idrovo Encalada, A.M.; Pérez, C.D.; Gerschenson, L.N.; Rojas, A.M.; Fissore, E.N. Gelling Pectins from Carrot Leftovers Extracted by Industrial-Enzymes with Ultrasound Pretreatment. LWT 2019, 111, 640–646. [Google Scholar] [CrossRef]
- Alancay, M.M.; Lobo, M.O.; Quinzio, C.M.; Iturriaga, L.B. Extraction and Physicochemical Characterization of Pectin from Tomato Processing Waste. J. Food Meas. Charact. 2017, 11, 2119–2130. [Google Scholar] [CrossRef]
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Zhang, J. Rheological Properties of Natural Low-Methoxyl Pectin Extracted from Sunflower Head. Food Hydrocoll. 2015, 44, 122–128. [Google Scholar] [CrossRef]
- Busato, B.; de Almeida Abreu, E.C.; de Oliveira Petkowicz, C.L.; Martinez, G.R.; Rodrigues Noleto, G. Pectin from Brassica Oleracea Var. Italica Triggers Immunomodulating Effects in Vivo. Int. J. Biol. Macromol. 2020, 161, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified Sugar Beet Pectin Induces Apoptosis of Colon Cancer Cells via an Interaction with the Neutral Sugar Side-Chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Sucheta; Misra, N.N.; Yadav, S.K. Extraction of Pectin from Black Carrot Pomace Using Intermittent Microwave, Ultrasound and Conventional Heating: Kinetics, Characterization and Process Economics. Food Hydrocoll. 2020, 102, 105592. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant Peel as a High Potential Source of High Methylated Pectin: Ultrasonic Extraction Optimization and Characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Torkova, A.A.; Lisitskaya, K.V.; Filimonov, I.S.; Glazunova, O.A.; Kachalova, G.S.; Golubev, V.N.; Fedorova, T.V. Physicochemical and Functional Properties of Cucurbita maxima Pumpkin Pectin and Commercial Citrus and Apple Pectins: A Comparative Evaluation. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Ezzati, S.; Ayaseh, A.; Ghanbarzadeh, B.; Heshmati, M.K. Pectin from Sunflower By-Product: Optimization of Ultrasound-Assisted Extraction, Characterization, and Functional Analysis. Int. J. Biol. Macromol. 2020, 165, 776–786. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of Food Processing Wastes of Eggplant as a High Potential Pectin Source and Characterization of Extracted Pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef]
- Song, Y.R.; Han, A.R.; Park, S.G.; Cho, C.W.; Rhee, Y.K.; Hong, H. Do Effect of Enzyme-Assisted Extraction on the Physicochemical Properties and Bioactive Potential of Lotus Leaf Polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef]
- Higuera-Coelho, R.A.; Lizarraga, L.; Ponce, N.M.A.; Stortz, C.A.; Rojas, A.M.; Bernhardt, D.C.; Fissore, E.N. Novel Gelling Pectins from Zea mays Husks’ Agro-Industrial Residue and Their Interaction with Calcium and Iron (II). Bioact. Carbohydr. Diet. Fibre 2021, 26, 100273. [Google Scholar] [CrossRef]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal Anti-Inflammatory Effects of Artichoke Pectin and Modified Pectin Fractions in the Dextran Sulfate Sodium Model of Mice Colitis. Artificial Neural Network Modelling of Inflammatory Markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Corzo, N.; Olano, A.; Montilla, A. Enzymatic Extraction of Pectin from Artichoke (Cynara scolymus L.) by-Products Using Celluclast®1.5L. Carbohydr. Polym. 2018, 190, 43–49. [Google Scholar] [CrossRef]
- Sabater, C.; Abad-García, C.; Delgado-Fernández, P.; Corzo, N.; Montilla, A. Carbohydrate Fraction Characterisation of Functional Yogurts Containing Pectin and Pectic Oligosaccharides through Convolutional Networks. J. Food Compos. Anal. 2020, 90, 103484. [Google Scholar] [CrossRef]
- Idrovo Encalada, A.M.; De’Nobili, M.D.; Ponce, A.N.M.; Stortz, C.A.; Fissore, E.N.; Rojas, A.M. Antioxidant Edible Film Based on a Carrot Pectin-Enriched Fraction as an Active Packaging of a Vegan Cashew Ripened Cheese. Int. J. Food Sci. Technol. 2021, 56, 3691–3702. [Google Scholar] [CrossRef]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef]
- Dias do Nascimento Santos, D.K.; da Silva Barros, B.R.; da Cruz Filho, I.J.; da Silva Bezerra, N., Jr.; da Silva, P.R.; do Bomfim Nascimento, P.H.; do Carmo Alves de Lima, M.; Napoleão, T.H.; de Melo, C.M.L. Pectin-like Polysaccharide Extracted from the Leaves of Conocarpus Erectus Linnaeus Promotes Antioxidant, Immunomodulatory and Prebiotic Effects. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100263. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Cacao Pod Husks as a Source of Low-Methoxyl, Highly Acetylated Pectins Able to Gel in Acidic Media. Int. J. Biol. Macromol. 2017, 101, 146–152. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized Extraction of Pectin-like Polysaccharide from Suaeda fruticosa Leaves: Characterization, Antioxidant, Anti-Inflammatory and Analgesic Activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Priyangini, F.; Walde, S.G.; Chidambaram, R. Extraction Optimization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) with Ascorbic Acid Using Response Surface Methodology. Carbohydr. Polym. 2018, 202, 497–503. [Google Scholar] [CrossRef]
- Amorim, J.C.; Vriesmann, L.C.; Petkowicz, C.L.O.; Martinez, G.R.; Noleto, G.R. Modified Pectin from Theobroma Cacao Induces Potent Pro-Inflammatory Activity in Murine Peritoneal Macrophage. Int. J. Biol. Macromol. 2016, 92, 1040–1048. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. Valorization of Walnut Processing Waste as a Novel Resource: Production and Characterization of Pectin. J. Food Process Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-Methylated Pectin from Walnut Processing Wastes as a Potential Resource: Ultrasound Assisted Extraction and Physicochemical, Structural and Functional Analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Hosseini, S.S. Ultrasonic and Heating Extraction of Pistachio By-Product Pectin: Physicochemical, Structural Characterization and Functional Measurement. J. Food Meas. Charact. 2020, 14, 679–693. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Saeid Hosseini, S.; Hojjati, M. Pistachio Green Hull Pectin: Optimization of Microwave-Assisted Extraction and Evaluation of Its Physicochemical, Structural and Functional Properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Vasco-Correa, J.; Astudillo, I.C.P. Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L. Molecules 2021, 26, 1473. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Long, X.; Wang, Y.; Chen, K.; Zhao, M.; Zhu, L.; Chen, Q. Synergized Enzyme-Ultrasound-Assisted Aqueous Two-Phase Extraction and Antioxidant Activity Validation of Polysaccharides from Tobacco Waste. Microchem. J. 2024, 202, 110799. [Google Scholar] [CrossRef]
- Posé, S.; Kirby, A.R.; Paniagua, C.; Waldron, K.W.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. The Nanostructural Characterization of Strawberry Pectins in Pectate Lyase or Polygalacturonase Silenced Fruits Elucidates Their Role in Softening. Carbohydr. Polym. 2015, 132, 134–145. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at Interfaces and the Influence on the Properties of Dispersed Systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Kazemi, M.; Amiri Samani, S.; Ezzati, S.; Khodaiyan, F.; Hosseini, S.S.; Jafari, M. High-Quality Pectin from Cantaloupe Waste: Eco-Friendly Extraction Process, Optimization, Characterization and Bioactivity Measurements. J. Sci. Food Agric. 2021, 101, 6552–6562. [Google Scholar] [CrossRef]
- Min, B.; Lim, J.; Ko, S.; Lee, K.G.; Lee, S.H.; Lee, S. Environmentally Friendly Preparation of Pectins from Agricultural Byproducts and Their Structural/Rheological Characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef]
- Hwang, J.K.; Kim, C.J.; Kim, C.T. Extrusion of Apple Pomace Facilitates Pectin Extraction. J. Food Sci. 1998, 63, 841–844. [Google Scholar] [CrossRef]
- Maneerat, N.; Tangsuphoom, N.; Anadi, N. Effect of Extraction Condition on Properties of Pectin from Banana Peels and Its Function as Fat Replacer in Salad Cream. J Food Sci Technol 2017, 54, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, Y.; Zhu, X.; Xiong, H.; Woo, M.W.; Hu, J. Physicochemical and Comparative Properties of Pectins Extracted from Akebia trifoliata Var. Australis Peel. Carbohydr. Polym. 2012, 87, 1663–1669. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound Effects on the Degradation Kinetics, Structure and Rheological Properties of Apple Pectin. Ultrason. Sonochem 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Salimi, A.; Khodaiyan, F.; Askari, G.; Hosseini, S.S. A Zero-Waste Approach towards a Sustainable Waste Management of Apple: Extraction of Value-Added Products and Their Application as Edible Coating. Food Hydrocoll. 2024, 147, 109304. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Lamch, Ł.; Weżgowiec, J.; Warszyński, P.; Wilk, K.A. Benefits of PH-Responsive Polyelectrolyte Coatings for Carboxymethyl Cellulose-Based Microparticles in the Controlled Release of Esculin. Mater. Sci. Eng. C 2021, 118, 111397. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Maniecki, Ł.; Szczęsna, W.; Warszyński, P.; Wilk, K.A. Tailoring the Composition of Hydrogel Particles for the Controlled Delivery of Phytopharmaceuticals. Eur. Polym. J. 2021, 151, 110429. [Google Scholar] [CrossRef]
- Hussain, M.; Bakalis, S.; Gouseti, O.; Zahoor, T.; Anjum, F.M.; Shahid, M. Dynamic and Shear Stress Rheological Properties of Guar Galactomannans and Its Hydrolyzed Derivatives. Int. J. Biol. Macromol. 2015, 72, 687–691. [Google Scholar] [CrossRef]
- Salehi, F.; Inanloodoghouz, M.; Karami, M. Rheological Properties of Carboxymethyl Cellulose (CMC) Solution: Impact of High Intensity Ultrasound. Ultrason. Sonochem 2023, 101, 106655. [Google Scholar] [CrossRef]
- Ro, J.; Kim, Y.; Kim, H.; Jang, S.B.; Lee, H.J.; Chakma, S.; Jeong, J.H.; Lee, J. Anti-Oxidative Activity of Pectin and Its Stabilizing Effect on Retinyl Palmitate. Korean J. Physiol. Pharmacol. 2013, 17, 197–201. [Google Scholar] [CrossRef]
- Rodrigues, J.A.G.; Vanderlei, E.D.S.O.; Silva, L.M.C.M.; De Araújo, I.W.F.; De Queiroz, I.N.L.; De Paula, G.A.; Abreu, T.M.; Ribeiro, N.A.; Bezerra, M.M.; Chaves, H.V.; et al. Antinociceptive and Anti-Inflammatory Activities of a Sulfated Polysaccharide Isolated from the Green Seaweed Caulerpa Cupressoides. Pharmacol. Rep. 2012, 64, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Han, N.; Teng, F.; Wang, X.; Xue, R.; Yin, J. Extraction Optimization of Polysaccharides of Schisandrae Fructus and Evaluation of Their Analgesic Activity. Int. J. Biol. Macromol. 2013, 57, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical Polysaccharides: Macrophage Immunomodulation and Therapeutic Potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sakuragi, Y.; Pasternak, T.P.; Showalter, A.M.; Basu, D. Extensin and Arabinogalactan-Protein Biosynthesis: Glycosyltransferases, Research Challenges, and Biosensors. Front. Plant Sci. 2016, 7, 814. [Google Scholar] [CrossRef]
- de Oliveira, A.F.; do Nascimento, G.E.; Iacomini, M.; Cordeiro, L.M.C.; Cipriani, T.R. Chemical Structure and Anti-Inflammatory Effect of Polysaccharides Obtained from Infusion of Sedum Dendroideum Leaves. Int. J. Biol. Macromol. 2017, 105, 940–946. [Google Scholar] [CrossRef]
- Heinzel, S.; Marchingo, J.M.; Horton, M.B.; Hodgkin, P.D. The Regulation of Lymphocyte Activation and Proliferation. Curr. Opin. Immunol. 2018, 51, 32–38. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in Cancer Therapy: A Review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Du Toit, A. FtsZ and FtsA Find the Right Place. Nat. Rev. Microbiol. 2014, 13, 67. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Ho, Y.Y.; Lin, C.M.; Wu, M.C. Evaluation of the Prebiotic Effects of Citrus Pectin Hydrolysate. J. Food Drug Anal. 2017, 25, 550–558. [Google Scholar] [CrossRef]
- WHO. Human Health Risk Assessment Toolkit; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Brownlee, I.A. The Physiological Roles of Dietary Fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Cao, J.J.; Liu, R.; Chen, H.Q. Structural Characterization, α-Amylase and α-Glucosidase Inhibitory Activities of Polysaccharides from Wheat Bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef]
- Cudrey, C.; van Tilbeurgh, H.; Gargouri, Y.; Verger, R. Inactivation of Pancreatic Lipases by Amphiphilic Reagents 5-(Dodecyldithio)-2-Nitrobenzoic Acid and Tetrahydrolipstatin. Dependence upon Partitioning between Micellar and Oil Phases. Biochemistry 1993, 32, 13800–13808. [Google Scholar] [CrossRef]
- Stephens, R.W.; Arhire, L.; Covasa, M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity 2018, 26, 801–809. [Google Scholar] [CrossRef]
- Bianchi, F.; Larsen, N.; de Mello Tieghi, T.; Adorno, M.A.T.; Kot, W.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. Modulation of Gut Microbiota from Obese Individuals by in vitro Fermentation of Citrus Pectin in Combination with Bifidobacterium Longum BB-46. Appl. Microbiol. Biotechnol. 2018, 102, 8827–8840. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Holtug, K.; Rasmussen, H.S. Short-Chain Fatty Acid Production from Mono- and Disaccharides in a Fecal Incubation System: Implications for Colonic Fermentation of Dietary Fiber in Humans. J. Nutr. 1988, 118, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Ross, A.W.; Walker, A.W.; Morgan, P.J. Dietary Uncoupling of Gut Microbiota and Energy Harvesting from Obesity and Glucose Tolerance in Mice. Cell Rep. 2017, 21, 1521–1533. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and Naringin in Cardiovascular Disease Prevention: A Preclinical Review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsirigotis-Maniecka, M.; Górska, E.; Mazurek-Hołys, A.; Pawlaczyk-Graja, I. Unlocking the Potential of Food Waste: A Review of Multifunctional Pectins. Polymers 2024, 16, 2670. https://doi.org/10.3390/polym16182670
Tsirigotis-Maniecka M, Górska E, Mazurek-Hołys A, Pawlaczyk-Graja I. Unlocking the Potential of Food Waste: A Review of Multifunctional Pectins. Polymers. 2024; 16(18):2670. https://doi.org/10.3390/polym16182670
Chicago/Turabian StyleTsirigotis-Maniecka, Marta, Ewa Górska, Aleksandra Mazurek-Hołys, and Izabela Pawlaczyk-Graja. 2024. "Unlocking the Potential of Food Waste: A Review of Multifunctional Pectins" Polymers 16, no. 18: 2670. https://doi.org/10.3390/polym16182670








