Cross-Linked Metathesis Polynorbornenes Based on Nadimides Bearing Hydrocarbon Substituents: Synthesis and Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods for Characterization of Monomers and Polymers Obtained via Metathesis Polymerization
2.3. Synthetic Part
2.3.1. Synthesis of Monomers
2.3.2. 2,2′-Hexane-1,6-diylbis(3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3-dione) (NB6) [47]
2.3.3. 2,2′-Decane-1,10-diylbis(3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3-dione) (NB10) [48]
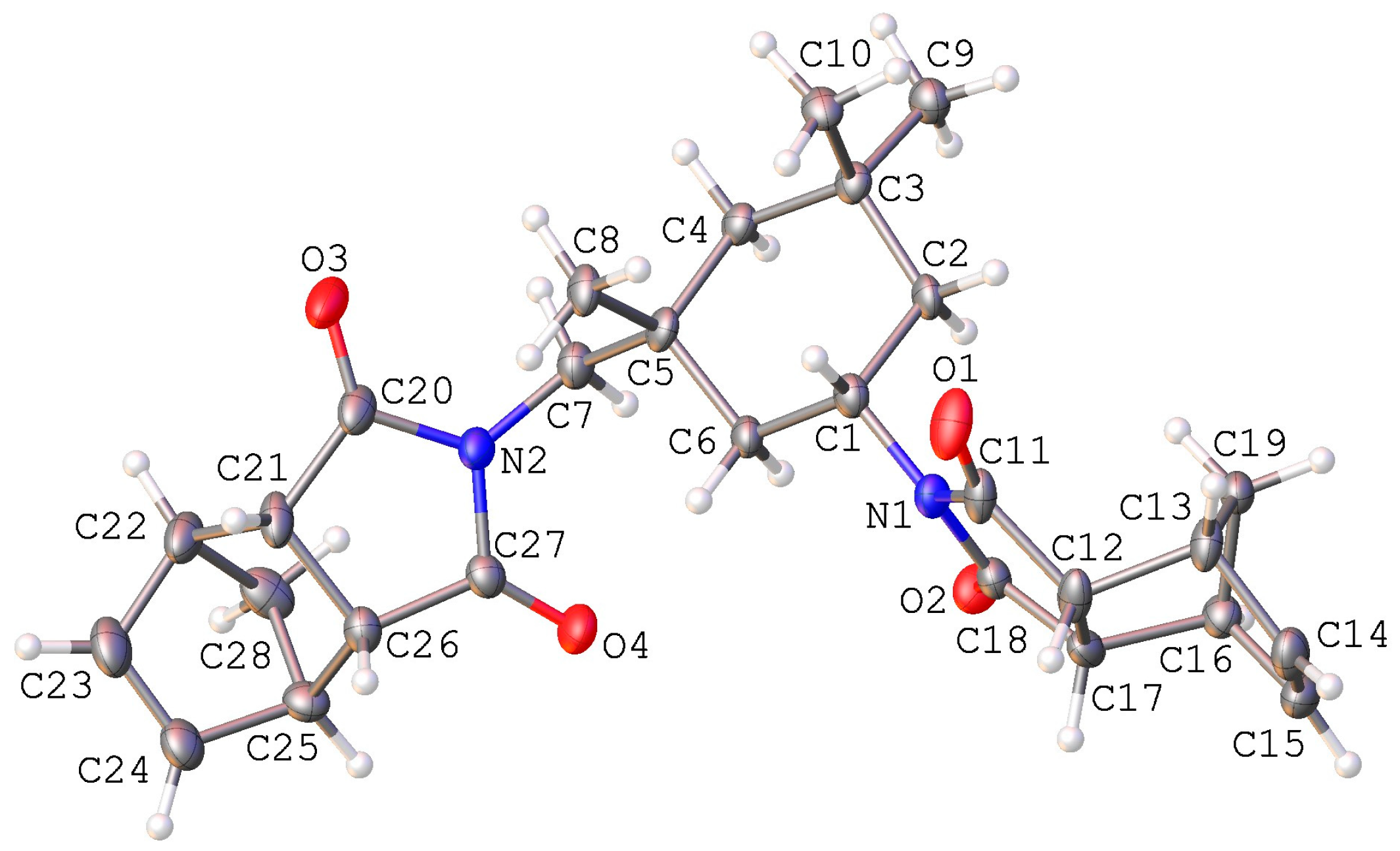
2.3.4. 2-((1R,3S)-3-[(1,3-Dioxohexahydro-1H-4,7-methanoisoindole-2-yl)methyl]-3,5,5-trimethylcyclohexyl)hexahydro-1H-4,7-methanoisoindole-1,3-dione (NBI)
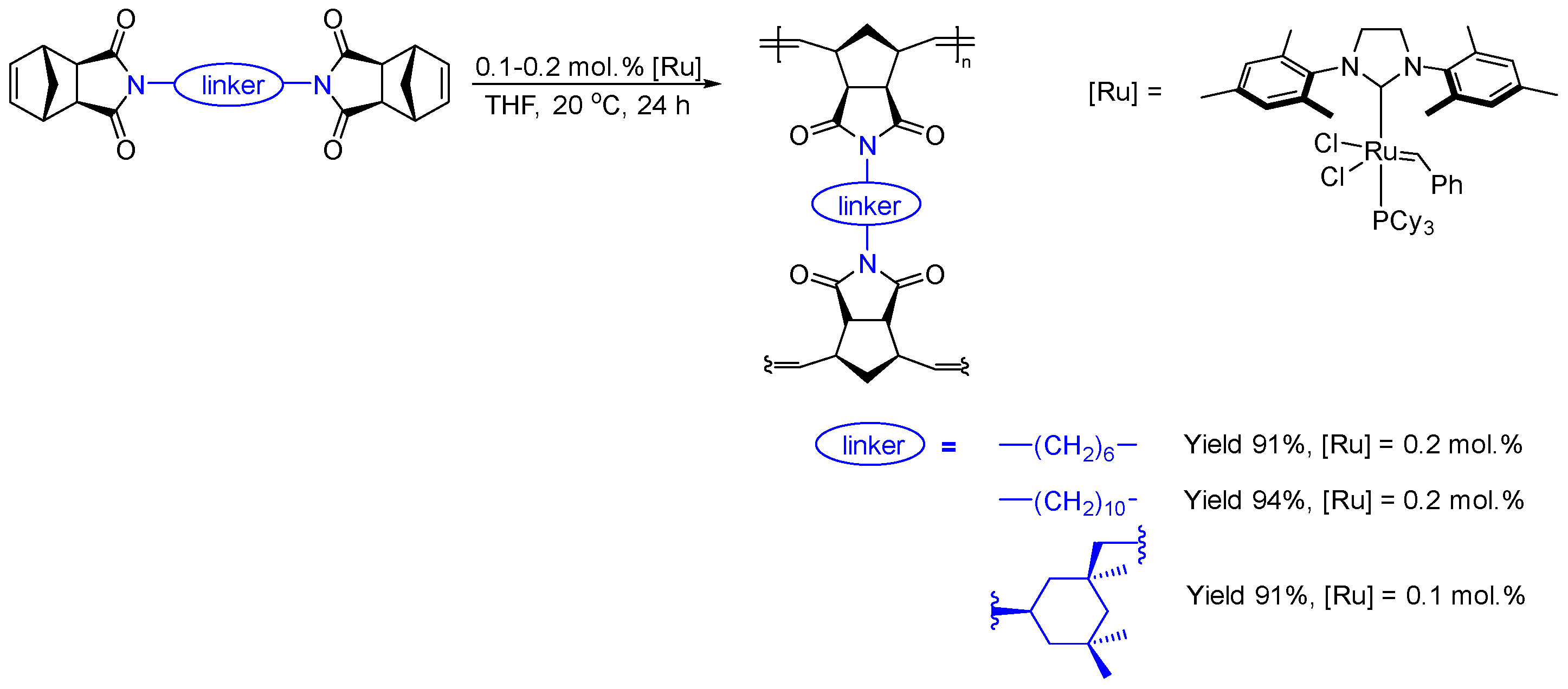
2.3.5. Synthesis of Cross-Linked Homopolymers
2.3.6. Homopolymer Based on NBI
2.3.7. Homopolymer Based on NB6
2.3.8. Homopolymer Based on NB10
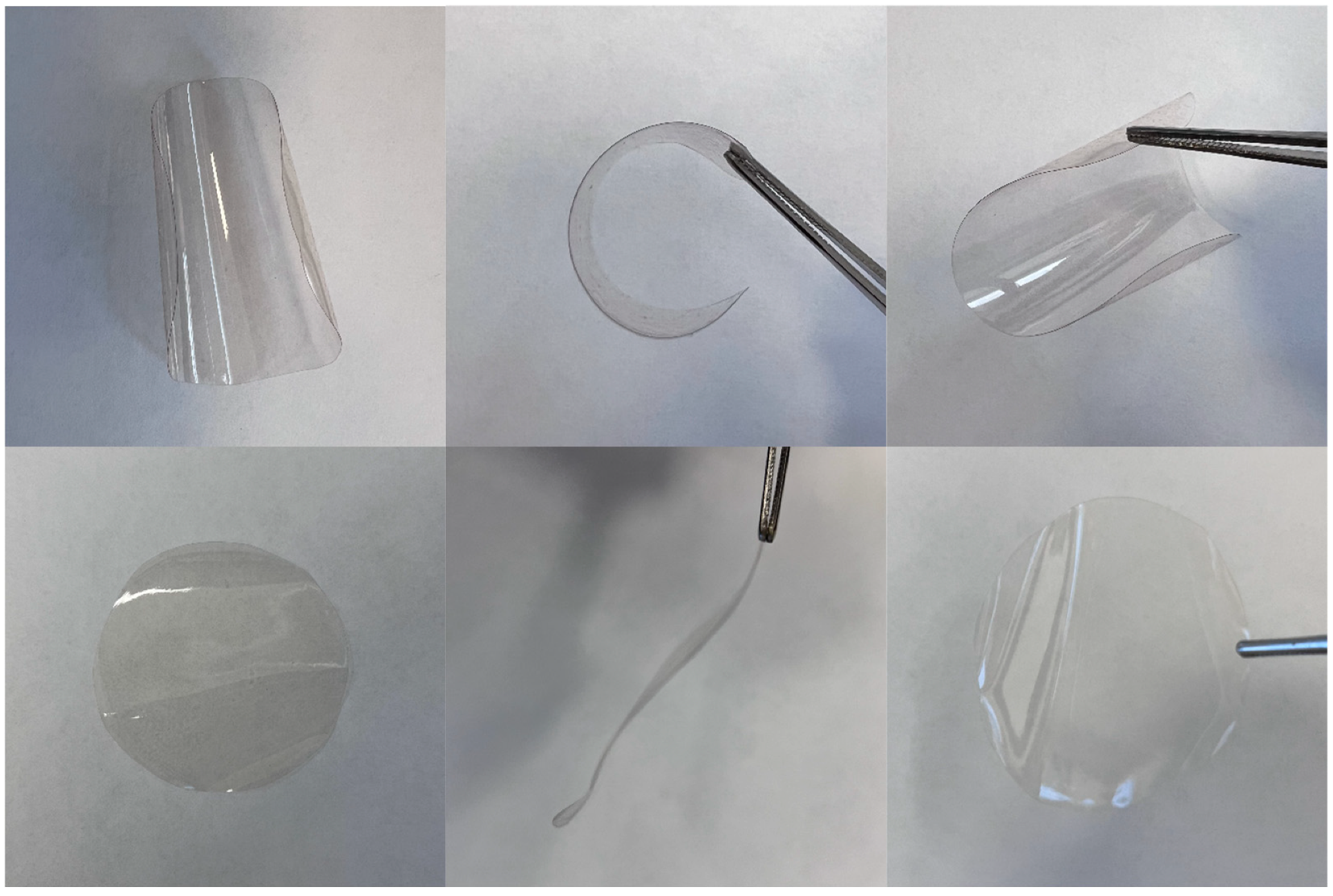
2.4. Synthesis of Membranes Based on Cross-Linked Copolymers
2.5. Permeation Study of 1-Phenylethanol and Mandelic Acid through Membranes Based on the Synthesized Metathesis Copolymers
3. Results and Discussion
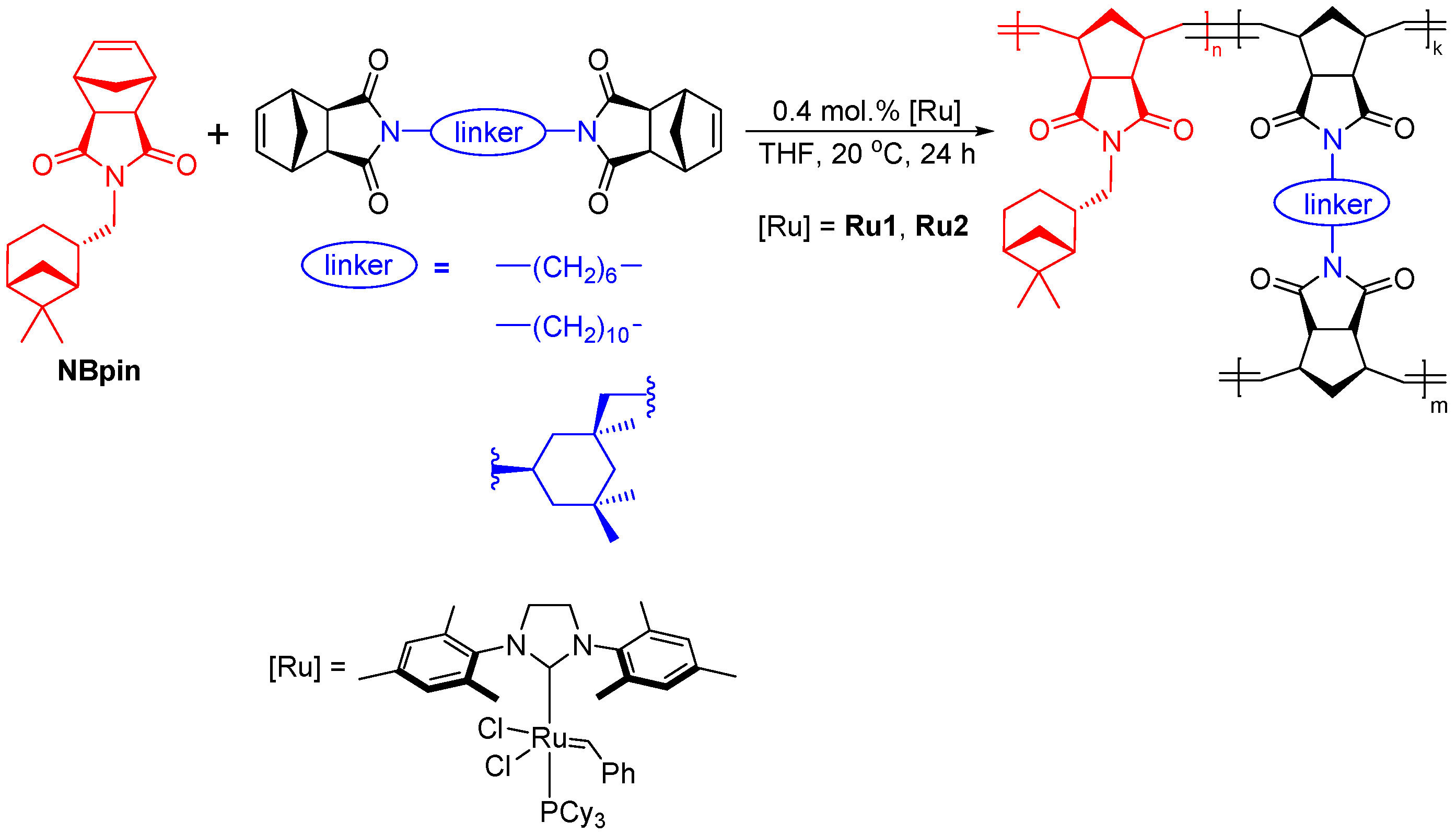
3.1. Synthesis of Metathesis Homopolymers
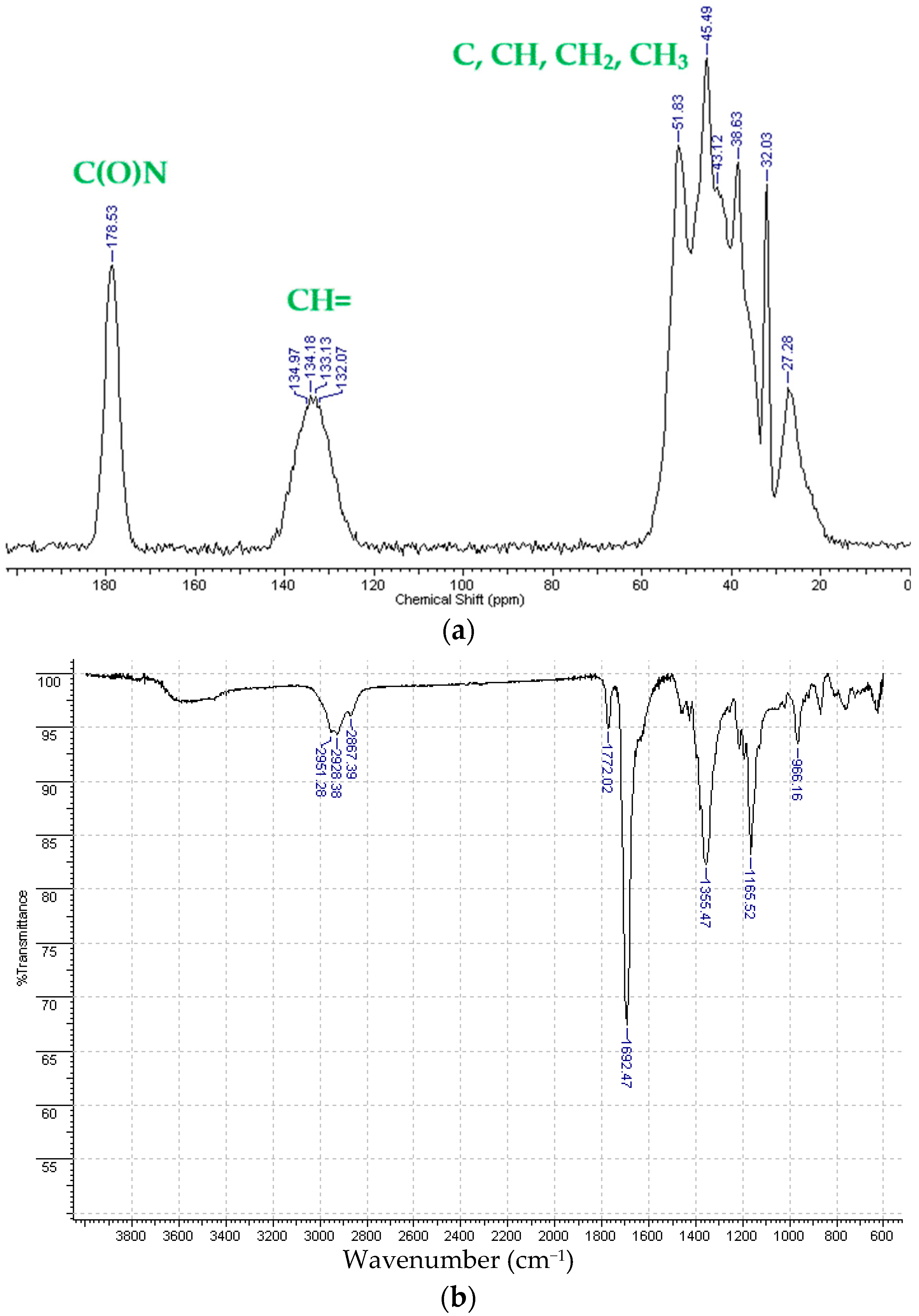
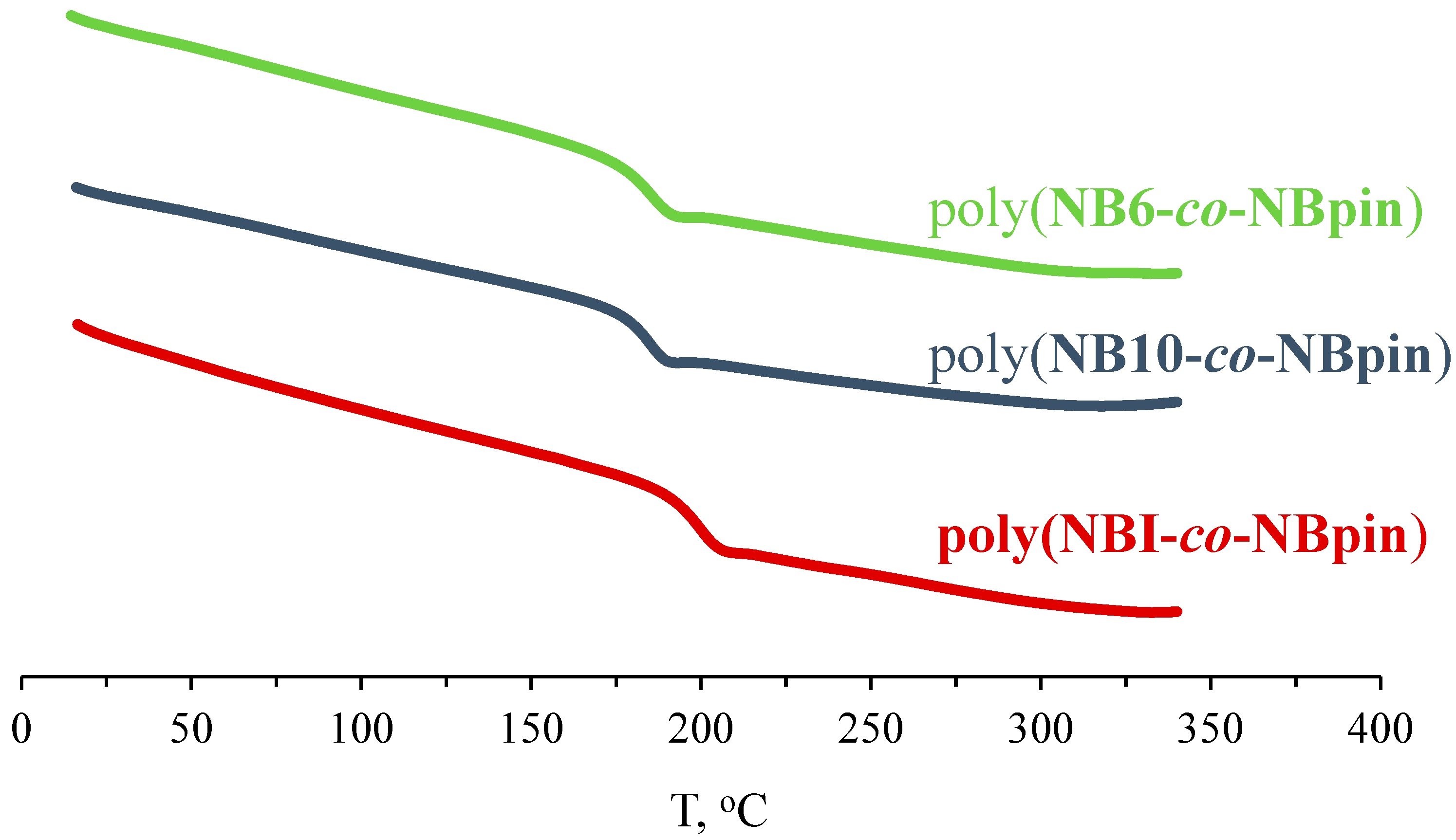
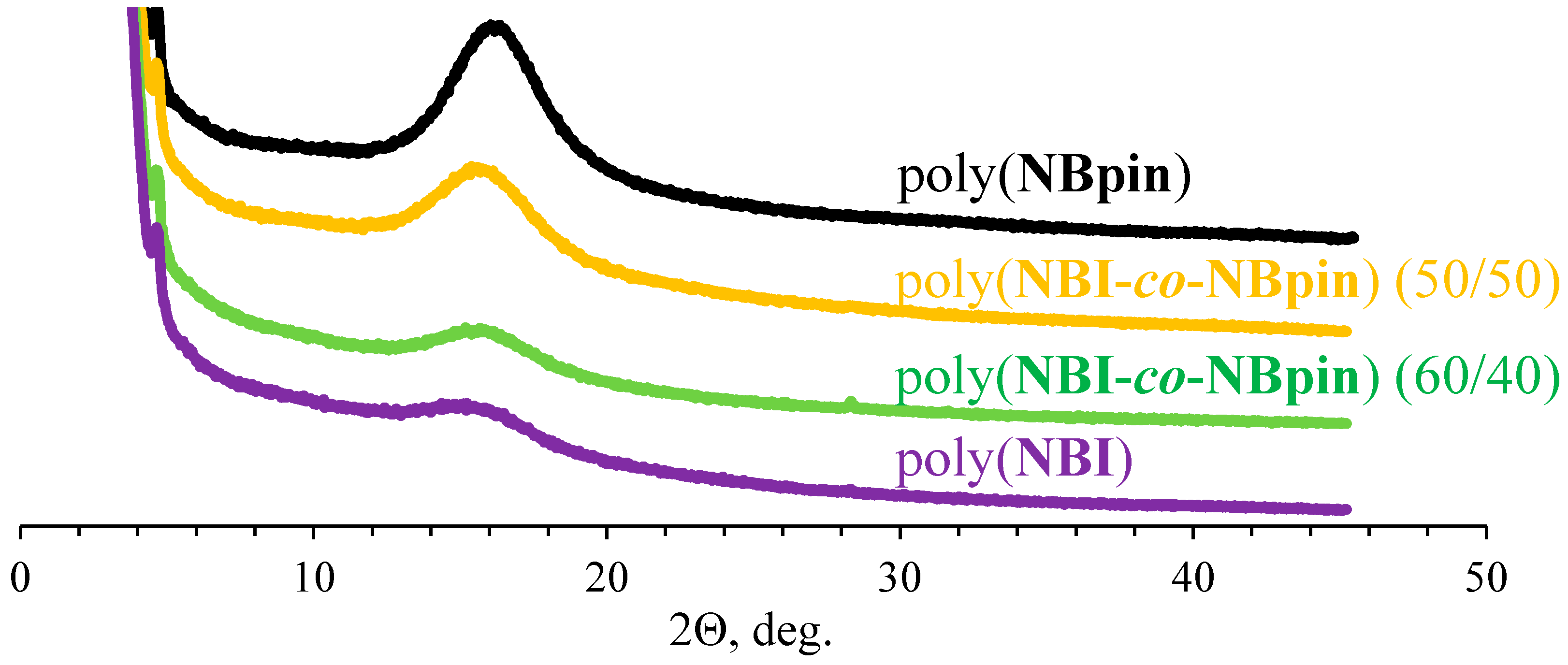
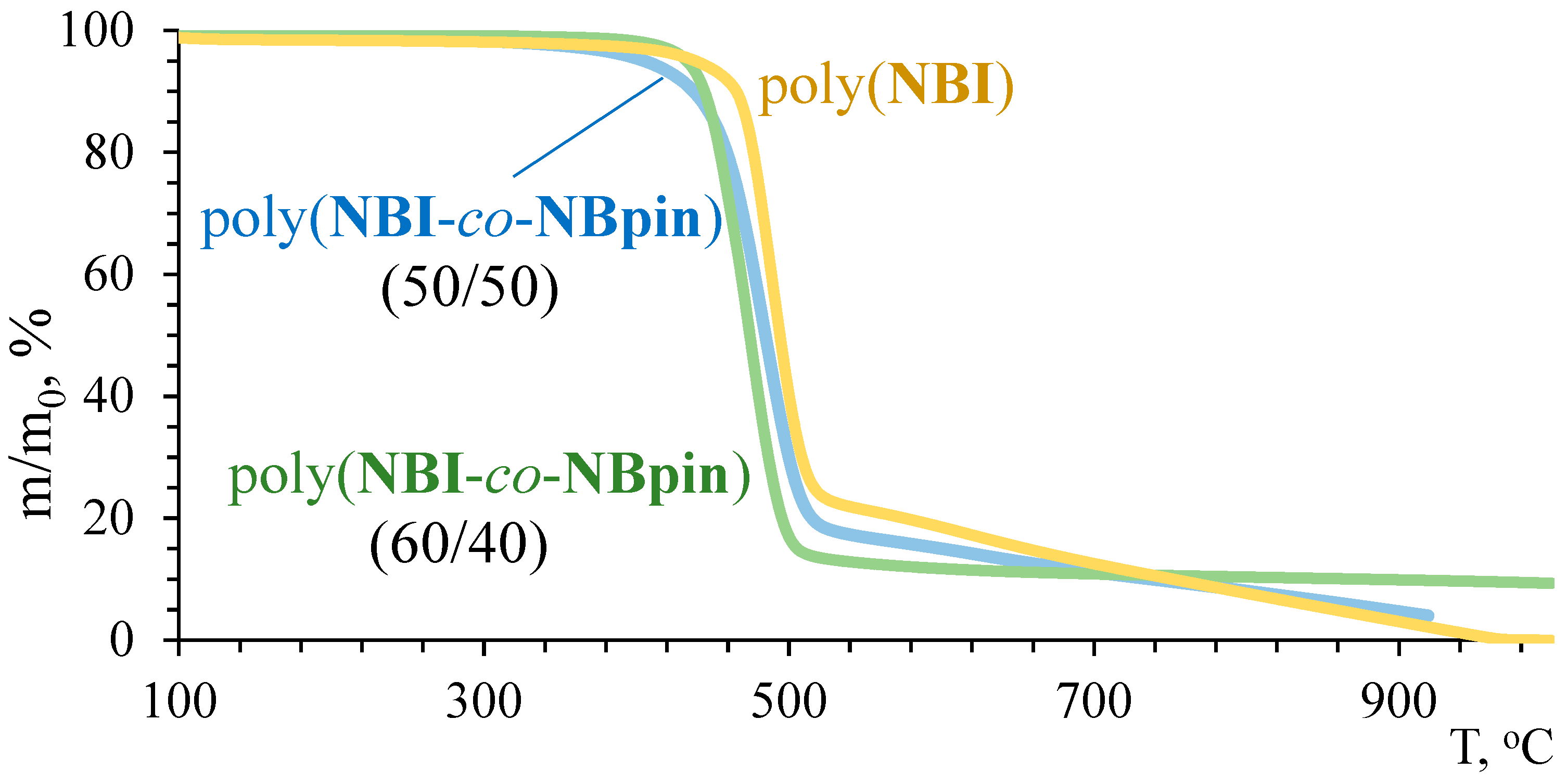
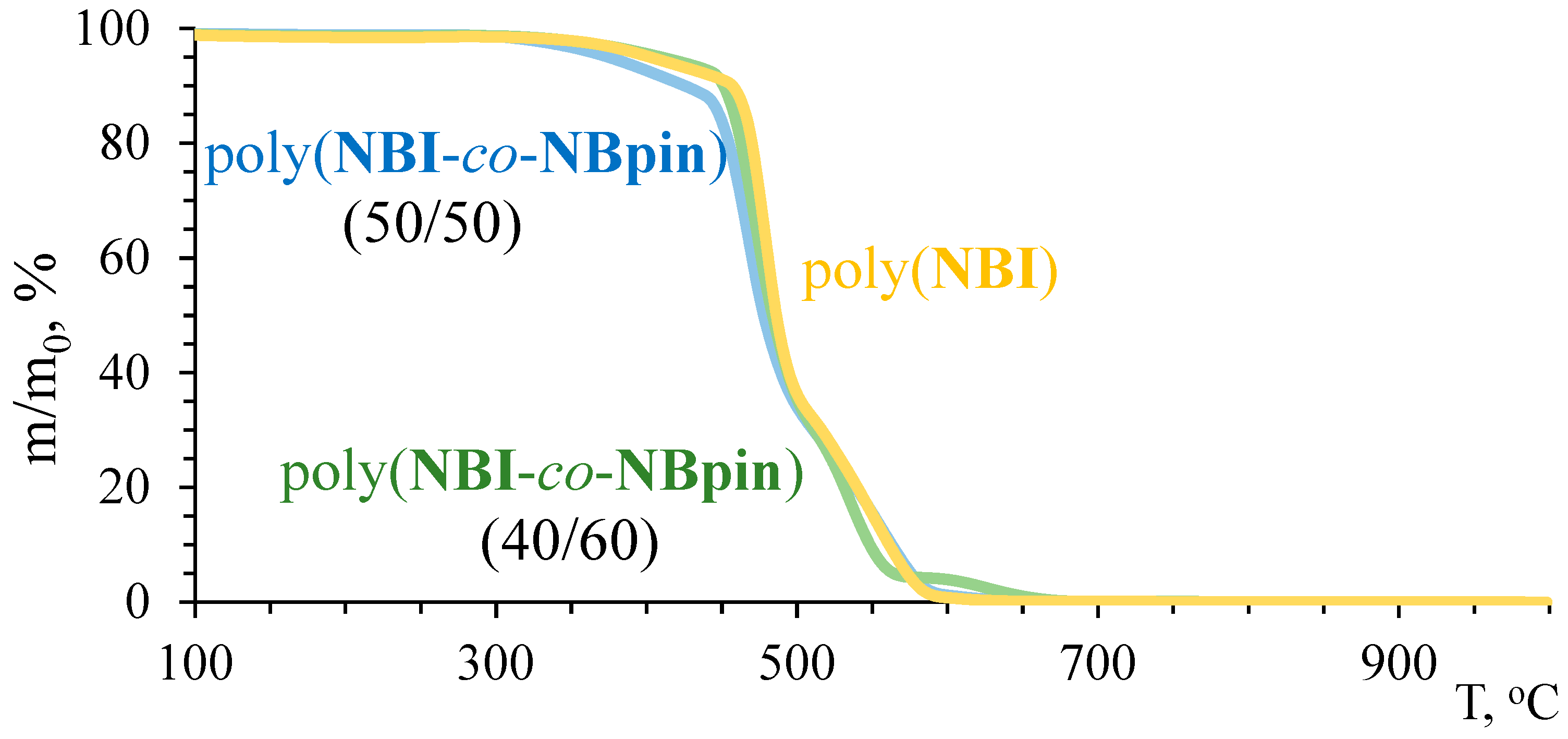
3.2. Physicochemical Properties of the Synthesized Metathesis Homo- and Copolymers
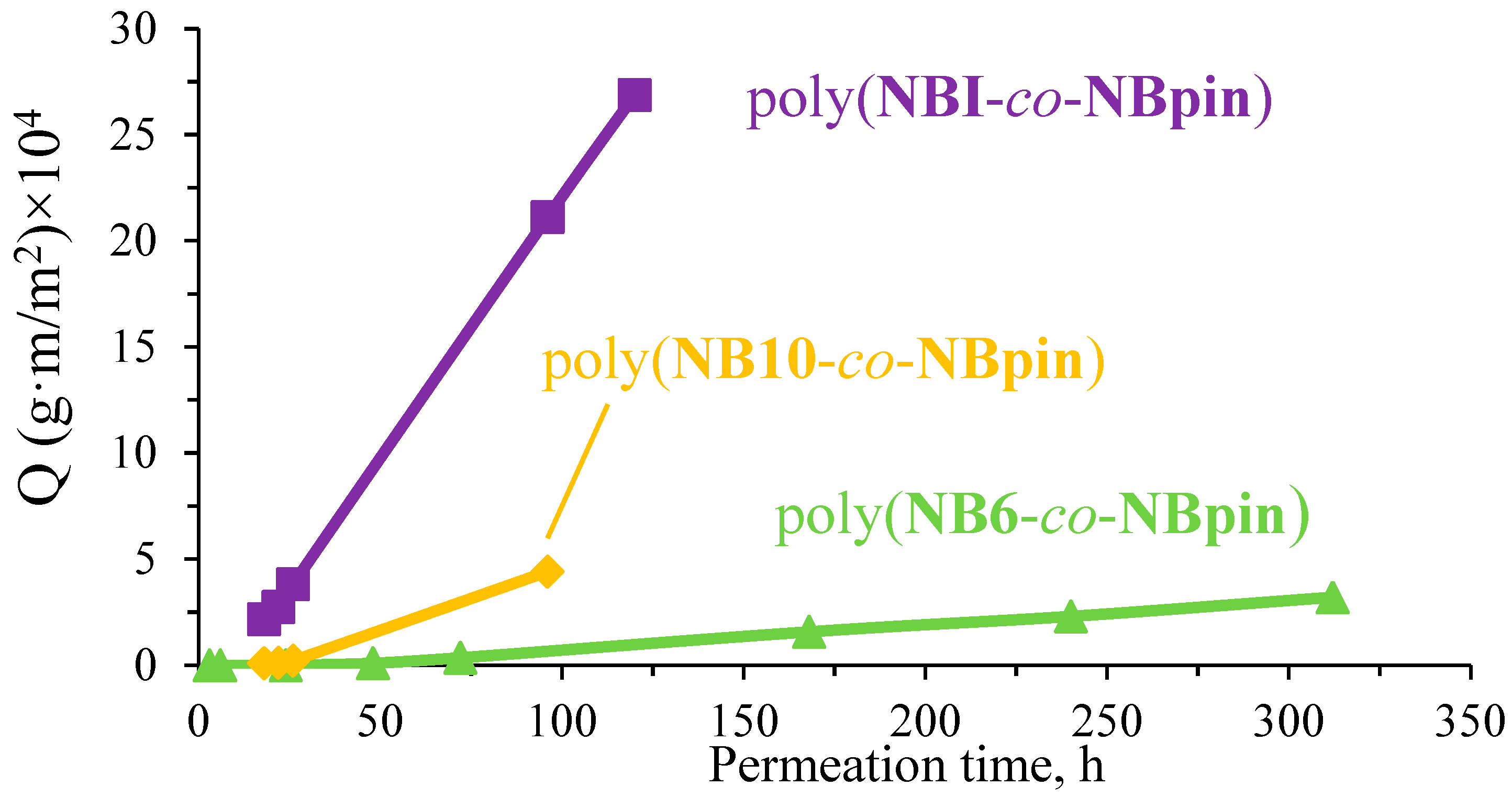
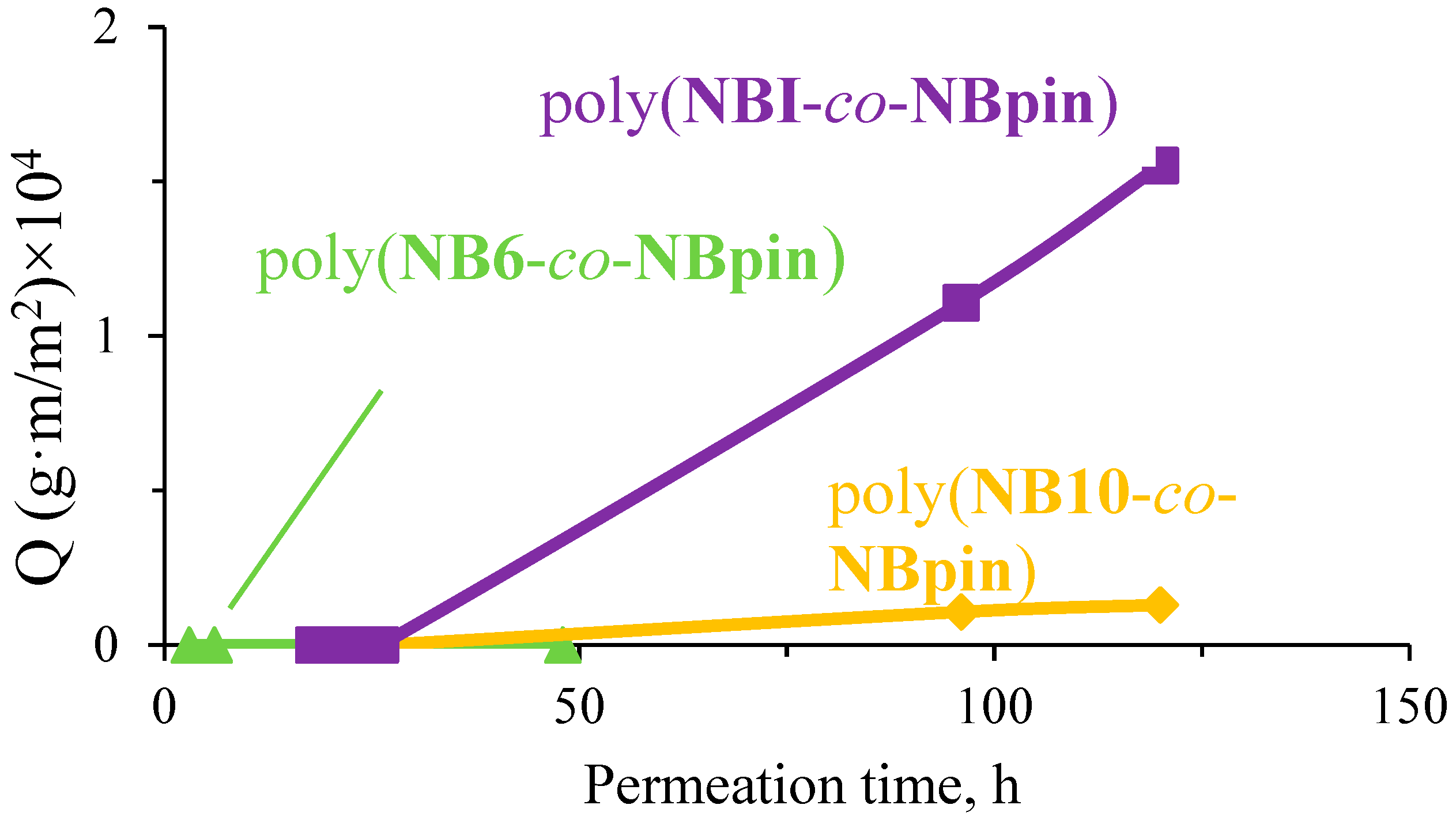
3.3. Permeability Study of the Synthesized Cross-Linked Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flid, V.R.; Gringolts, M.L.; Shamsiev, R.S.; Finkelshtein, E.S. Norbornene, norbornadiene and their derivatives: Promising semi-products for organic synthesis and production of polymeric materials. Russ. Chem. Rev. 2018, 87, 1169–1205. [Google Scholar] [CrossRef]
- Wali Ullah, M.; Haraguchi, N. Asymmetric Diels-Alder Reaction Catalyzed by Facile Recoverable Ionically Core-Corona Polymer Microsphere-Immobilized MacMillan Catalyst. ChemistrySelect 2022, 7, e202202568. [Google Scholar] [CrossRef]
- Petrov, V.A.; Davidson, F.; Krusic, P.J.; Marchione, A.A.; Marshall, W.J. Cycloaddition reaction of quadricyclane and fluoroolefins. J. Fluor. Chem. 2005, 126, 599–608. [Google Scholar] [CrossRef]
- Zotkin, M.A.; Alentiev, D.A.; Shorunov, S.V.; Sokolov, S.E.; Gavrilova, N.N.; Bermeshev, M.V. Microporous polynorbornenes bearing carbocyclic substituents: Structure-property study. Polymer 2023, 269, 125732. [Google Scholar] [CrossRef]
- Kilgallon, L.J.; McFadden, T.P.; Sigman, M.S.; Johnson, J.A. Tricyclononenes and tricyclononadienes as efficient monomers for controlled ROMP: Understanding structure–propagation rate relationships and enabling facile post-polymerization modification. Chem. Sci. 2024, 15, 8334–8345. [Google Scholar] [CrossRef]
- Cater, H.L.; Balynska, I.; Allen, M.J.; Freeman, B.D.; Page, Z.A. User Guide to Ring-Opening Metathesis Polymerization of endo-Norbornene Monomers with Chelated Initiators. Macromolecules 2022, 55, 6671–6679. [Google Scholar] [CrossRef]
- Gringolts, M.L.; Bermeshev, M.V.; Starannikova, L.E.; Rogan, Y.V.; Yampol’skii, Y.P.; Finkel’shtein, E.S. Synthesis and gas separation properties of metathesis polynorbornenes with different positions of one or two SiMe3 groups in a monomer unit. Polym. Sci. Ser. A 2009, 51, 1233–1240. [Google Scholar] [CrossRef]
- Kośnik, W.; Lichosyt, D.; Śnieżek, M.; Janaszkiewicz, A.; Woźniak, K.; Malińska, M.; Trzaskowski, B.; Kajetanowicz, A.; Grela, K. Ruthenium Olefin Metathesis Catalysts Bearing a Macrocyclic N-Heterocyclic Carbene Ligand: Improved Stability and Activity. Angew. Chem. Int. Ed. 2022, 61, e202201472. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.J.; Lin, T.-P.; Grubbs, R.H. Examining the Effects of Monomer and Catalyst Structure on the Mechanism of Ruthenium-Catalyzed Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc. 2019, 141, 17796–17808. [Google Scholar] [CrossRef]
- Schrock, R.R. High-Oxidation State Molybdenum and Tungsten Complexes Relevant to Olefin Metathesis. In Handbook of Metathesis; Grubbs, R.H., Wenzel, A.G., O’Leary, D.J., Khosravi, E., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–32. [Google Scholar] [CrossRef]
- Masoud, S.M.; Vorobyeva, D.V.; Petropavlovskikh, D.A.; Bruneau, C.; Osipov, S.N. Fluorine-containing ruthenium-based olefin metathesis catalysts. Russ. Chem. Rev. 2021, 90, 419. [Google Scholar] [CrossRef]
- Akmalov, T.R.; Masoud, S.M.; Petropavlovskikh, D.A.; Zotova, M.A.; Nefedov, S.E.; Osipov, S.N. New olefin metathesis catalysts with fluorinated unsymmetrical imidazole-based ligands. Mendeleev Commun. 2018, 28, 609–611. [Google Scholar] [CrossRef]
- Nickel, A.; Edgecombe, B.D. 4.30—Industrial Applications of ROMP. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 749–759. [Google Scholar] [CrossRef]
- Varlas, S.; Lawrenson, S.B.; Arkinstall, L.A.; O’Reilly, R.K.; Foster, J.C. Self-assembled nanostructures from amphiphilic block copolymers prepared via ring-opening metathesis polymerization (ROMP). Prog. Polym. Sci. 2020, 107, 101278. [Google Scholar] [CrossRef]
- Barnhill, S.A.; Bell, N.C.; Patterson, J.P.; Olds, D.P.; Gianneschi, N.C. Phase Diagrams of Polynorbornene Amphiphilic Block Copolymers in Solution. Macromolecules 2015, 48, 1152–1161. [Google Scholar] [CrossRef]
- Lin, X.; Shi, J.; Niwayama, S. Synthesis of polynorbornadienes by ring-opening metathesis polymerization and their saturated derivatives bearing various ester groups and carboxyl groups. RSC Adv. 2023, 13, 3494–3504. [Google Scholar] [CrossRef]
- He, H.; Song, B.; Qiu, G.; Wang, W.; Gu, H. Synthesis, conjugating capacity and biocompatibility evaluation of a novel amphiphilic polynorbornene. Des. Monomers Polym. 2020, 23, 141–154. [Google Scholar] [CrossRef]
- Wang, X.; Wilson, T.J.; Alentiev, D.; Gringolts, M.; Finkelshtein, E.; Bermeshev, M.; Long, B.K. Substituted polynorbornene membranes: A modular template for targeted gas separations. Polym. Chem. 2021, 12, 2947–2977. [Google Scholar] [CrossRef]
- Zotkin, M.A.; Alentiev, D.A.; Borisov, R.S.; Kozlova, A.A.; Borisov, I.L.; Shalygin, M.G.; Bermeshev, M.V. Polynorbornenes with carbocyclic substituents: A perspective approach to highly permeable gas separation membranes. J. Membr. Sci. 2024, 702, 122786. [Google Scholar] [CrossRef]
- Sundell, B.J.; Lawrence, J.A.; Harrigan, D.J.; Lin, S.; Headrick, T.P.; O’Brien, J.T.; Penniman, W.F.; Sandler, N. Exo-selective, Reductive Heck Derived Polynorbornenes with Enhanced Molecular Weights, Yields, and Hydrocarbon Gas Transport Properties. ACS Macro Lett. 2020, 9, 1363–1368. [Google Scholar] [CrossRef]
- Vargas, J.; Martínez, A.; Santiago, A.A.; Tlenkopatchev, M.A.; Gaviño, R.; Aguilar-Vega, M. The effect of fluorine atoms on gas transport properties of new polynorbornene dicarboximides. J. Fluor. Chem. 2009, 130, 162–168. [Google Scholar] [CrossRef]
- Vargas, J.; Santiago, A.A.; Tlenkopatchev, M.A.; Gaviño, R.; Laguna, M.F.; López-González, M.; Riande, E. Gas Transport and Ionic Transport in Membranes Based on Polynorbornenes with Functionalized Imide Side Groups. Macromolecules 2007, 40, 563–570. [Google Scholar] [CrossRef]
- Medentseva, E.I.; Khrychikova, A.P.; Bermesheva, E.V.; Borisov, I.L.; Petukhov, D.I.; Karpov, G.O.; Morontsev, A.A.; Nesterova, O.V.; Bermeshev, M.V. CO2-separation performance of vinyl-addition polynorbornenes with ester functionalities. J. Membr. Sci. 2024, 705, 122916. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Bermesheva, E.V.; Petukhov, D.I.; Lunin, A.O.; Borisov, I.L.; Shantarovich, V.P.; Bekeshev, V.G.; Alentiev, D.A.; Bermeshev, M.V. The Magic of Spiro-Epoxy Moiety: An Easy Way to Improve CO2-Separation Performance of Polymer Membrane. Adv. Funct. Mater. 2024, 34, 2405461. [Google Scholar] [CrossRef]
- Fujiwara, T.; Shinba, Y.; Sugimoto, K.; Mori, Y.; Tomikawa, M. Novel High Tg Low Dielectric Constant Coil-Shaped Polymer. J. Photopolym. Sci. Technol. 2005, 18, 289–295. [Google Scholar] [CrossRef]
- Karpov, G.O.; Alentiev, D.A.; Wozniak, A.I.; Bermesheva, E.V.; Lounev, I.V.; Gusev, Y.A.; Shantarovich, V.P.; Bermeshev, M.V. Dielectric properties of addition and metathesis polynorbornenes with bulky side-substituents. Polymer 2020, 203, 122759. [Google Scholar] [CrossRef]
- Sakata, K.; Fujimoto, H. Origin of the endo Selectivity in the Diels–Alder Reaction between Cyclopentadiene and Maleic Anhydride. Eur. J. Org. Chem. 2016, 2016, 4275–4278. [Google Scholar] [CrossRef]
- Barther, D.; Moatsou, D. Ring-Opening Metathesis Polymerization of Norbornene-Based Monomers Obtained via the Passerini Three Component Reaction. Macromol. Rapid Commun. 2021, 42, 2100027. [Google Scholar] [CrossRef]
- Ma, W.; Wright, N.; Wang, Y. Norbornene Dicarboximide: A Green Alternative for Thiol-Norbornene Photopolymers. ACS Macro Lett. 2024, 13, 915–920. [Google Scholar] [CrossRef]
- Nazarov, I.V.; Zarezin, D.P.; Solomatov, I.A.; Danshina, A.A.; Nelyubina, Y.V.; Ilyasov, I.R.; Bermeshev, M.V. Chiral Polymers from Norbornenes Based on Renewable Chemical Feedstocks. Polymers 2022, 14, 5453. [Google Scholar] [CrossRef]
- Nazarov, I.V.; Khrychikova, A.P.; Medentseva, E.I.; Bermesheva, E.V.; Borisov, I.L.; Yushkin, A.A.; Volkov, A.V.; Wozniak, A.I.; Petukhov, D.I.; Topchiy, M.A.; et al. CO2-selective vinyl-addition polymers from nadimides: Synthesis and performance for membrane gas separation. J. Membr. Sci. 2023, 677, 121624. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Medentseva, E.I.; Khrychikova, A.P.; Wozniak, A.I.; Guseva, M.A.; Nazarov, I.V.; Morontsev, A.A.; Karpov, G.O.; Topchiy, M.A.; Asachenko, A.F.; et al. Air-Stable Single-Component Pd-Catalysts for Vinyl-Addition Polymerization of Functionalized Norbornenes. ACS Catal. 2022, 12, 15076–15090. [Google Scholar] [CrossRef]
- Cruz-Morales, J.A.; Vargas, J.; Santiago, A.A.; Vásquez-García, S.R.; Tlenkopatchev, M.A.; Lys, T.d.; López-González, M. Synthesis and gas transport properties of new polynorbornene dicarboximides bearing trifluoromethyl isomer moieties. High Perform. Polym. 2016, 28, 1246–1262. [Google Scholar] [CrossRef]
- Vargas, J.; Santiago, A.A.; Cruz-Morales, J.A.; Tlenkopatchev, M.A.; De Lys, T.; Lõpez-González, M.; Riande, E. Gas transport properties of hydrogenated and fluorinated polynorbornene dicarboximides. Macromol. Chem. Phys. 2013, 214, 2607–2615. [Google Scholar] [CrossRef]
- Kim, D.; Hossain, I.; Kim, Y.; Choi, O.; Kim, T.-H. PEG/PPG-PDMS-Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane. Polymers 2020, 12, 1674. [Google Scholar] [CrossRef]
- Hossain, I.; Husna, A.; Chaemchuen, S.; Verpoort, F.; Kim, T.H. Cross-Linked Mixed-Matrix Membranes Using Functionalized UiO-66-NH2 into PEG/PPG-PDMS-Based Rubbery Polymer for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2020, 12, 57916–57931. [Google Scholar] [CrossRef]
- Kabir, M.H.; Kannan, S.; Veetil, K.A.; Sun, E.K.; Kim, T.-H. Enhancing CO2 Transport Across the PEG/PPG-Based Crosslinked Rubbery Polymer Membranes with a Sterically Bulky Carbazole-Based ROMP Comonomer. Macromol. Rapid Commun. 2024, 2400296. [Google Scholar] [CrossRef]
- Vedovello, P.; Marcio Paranhos, C.; Fernandes, C.; Elizabeth Tiritan, M. Chiral polymeric membranes: Recent applications and trends. Sep. Purif. Technol. 2022, 280, 119800. [Google Scholar] [CrossRef]
- Betzenbichler, G.; Huber, L.; Kräh, S.; Morkos, M.-L.K.; Siegle, A.F.; Trapp, O. Chiral stationary phases and applications in gas chromatography. Chirality 2022, 34, 732–759. [Google Scholar] [CrossRef]
- Brown, H.C.; Kim, K.-W.; Srebnik, M.; Bakthan, S. Organoboranes for synthesis. 7. An improved general synthesis of primary amines from alkenes via hydroboration-organoborane chemistry. Tetrahedron 1987, 43, 4071–4078. [Google Scholar] [CrossRef]
- Lezhnin, P.P.; Nazarov, I.V.; Bermeshev, M.V. Synthesis and Metathesis Polymerization of Nadimide Derived from β-(–)-Pinene. Russ. J. Appl. Chem. 2023, 96, 794–800. [Google Scholar] [CrossRef]
- Berkessel, A.; Roland, K.; Schröder, M.; Neudörfl, J.M.; Lex, J. Enantiomerically Pure Isophorone Diamine [3-(Aminomethyl)-3,5,5-trimethylcyclohexylamine]: A Chiral 1,4-Diamine Building Block Made Available on Large Scale. J. Org. Chem. 2006, 71, 9312–9318. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard D1708-96; Standard Test Method for Tensile Properties of Plastics By Use of Microtensile Specimens. ASTM International: Washington, DC, USA, 2017. Available online: https://www.astm.org/d1708-96.html (accessed on 30 August 2024).
- Zhao, Y.; Zhu, W.; Wu, Y.; Qu, L.; Liu, Z.; Zhang, K. An aggregation-induced emission star polymer with pH and metal ion responsive fluorescence. Polym. Chem. 2016, 7, 6513–6520. [Google Scholar] [CrossRef]
- Learsch, R.; Miyake, G.M. Arm-first synthesis of star polymers with polywedge arms using ring-opening metathesis polymerization and bifunctional crosslinkers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 732–740. [Google Scholar] [CrossRef]
- Belov, N.A.; Gringolts, M.L.; Morontsev, A.A.; Starannikova, L.E.; Yampolskii, Y.P.; Finkelstein, E.S. Gas-transport properties of epoxidated metathesis polynorbornenes. Polym. Sci. Ser. B 2017, 59, 560–569. [Google Scholar] [CrossRef]




| Bifunctional Comonomer | CH3CN | MeOH | Acetone | CH2Cl2 | CHCl3 | Toluene | Et2O | n-Hexane |
|---|---|---|---|---|---|---|---|---|
| NBI | ± | + | ± | − | − | − | + | + |
| NB6 | ± | + | ± | − | − | − | + | + |
| NB10 | ± | + | ± | − | − | − | + | + |
| Bifunctional Comonomer | Tg, °C a | Td5%, °C b | d, g cm−3 | Tensile Strength, σ, MPa | Elongation at Break, ε, % | Young’s Modulus, E, MPa |
|---|---|---|---|---|---|---|
| NBI | >350 | 406 | 1.147 | 20 | 14 | 960 |
| NB6 | >350 | 403 | 1.140 | 33 | 16 | - |
| NB10 | 272 | 414 | 1.135 | 29 | 12 | 1170 |
| Parameter | Homopolymer | ||||
|---|---|---|---|---|---|
| poly(NBI) | poly(NB6) | poly(NB10) | poly(NBpin) [41] | poly(NB) [49] | |
| 2θ1, ° | 15.3 | 16.9 | 17.5 | 16.2 | 18.1 |
| d1, Å | 5.8 | 5.3 | 5.1 | 5.4 | 4.9 |
| Parameter | Polymer | ||||
|---|---|---|---|---|---|
| poly(NBpin) [41] | poly(NBI-co-NBpin) (10/90) a | poly(NBI-co-NBpin) (50/50) a | poly(NBI-co-NBpin) (60/40) a | poly(NBI) | |
| 2θ1, ° | 15.3 | 16.9 | 17.5 | 16.2 | 18.1 |
| d1, Å | 5.8 | 5.3 | 5.1 | 5.4 | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadovnikov, K.S.; Nazarov, I.V.; Zhigarev, V.A.; Danshina, A.A.; Makarov, I.S.; Bermeshev, M.V. Cross-Linked Metathesis Polynorbornenes Based on Nadimides Bearing Hydrocarbon Substituents: Synthesis and Physicochemical Properties. Polymers 2024, 16, 2671. https://doi.org/10.3390/polym16182671
Sadovnikov KS, Nazarov IV, Zhigarev VA, Danshina AA, Makarov IS, Bermeshev MV. Cross-Linked Metathesis Polynorbornenes Based on Nadimides Bearing Hydrocarbon Substituents: Synthesis and Physicochemical Properties. Polymers. 2024; 16(18):2671. https://doi.org/10.3390/polym16182671
Chicago/Turabian StyleSadovnikov, Kirill S., Ivan V. Nazarov, Vsevolod A. Zhigarev, Anastasia A. Danshina, Igor S. Makarov, and Maxim V. Bermeshev. 2024. "Cross-Linked Metathesis Polynorbornenes Based on Nadimides Bearing Hydrocarbon Substituents: Synthesis and Physicochemical Properties" Polymers 16, no. 18: 2671. https://doi.org/10.3390/polym16182671
APA StyleSadovnikov, K. S., Nazarov, I. V., Zhigarev, V. A., Danshina, A. A., Makarov, I. S., & Bermeshev, M. V. (2024). Cross-Linked Metathesis Polynorbornenes Based on Nadimides Bearing Hydrocarbon Substituents: Synthesis and Physicochemical Properties. Polymers, 16(18), 2671. https://doi.org/10.3390/polym16182671







