In Situ Efficient End Functionalization of Polyisoprene by Epoxide Compounds via Neodymium-Mediated Coordinative Chain Transfer Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures for End Functionalization of Isoprene
2.2. Characterization
2.3. DFT Calculations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mundil, R.; Bravo, C.; Merle, N.; Zinck, P. Coordinative Chain Transfer and Chain Shuttling Polymerization. Chem. Rev. 2024, 124, 210–244. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative chain transfer polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Hu, Y.; Zhang, X. Lanthanide complexes mediated coordinative chain transfer polymerization of conjugated dienes. Sci. China Technol. Sci. 2018, 61, 1286–1294. [Google Scholar] [CrossRef]
- Wang, W.-X.; Zhao, W.-P.; Dong, J.; Zhang, H.-Q.; Wang, F.; Liu, H.; Zhang, X.-Q. Polyisoprene Bearing Dual Functionalized Mini-Blocky Chain-Ends Prepared from Neodymium-mediated Coordinative Chain Transfer Polymerizations. Chin. J. Polym. Sci. 2023, 41, 720–727. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Liu, H.; Hu, Y.; Zhang, X. Randomly Coordinative Chain Transfer Copolymerization of 1,3-Butadiene and Isoprene: A Highly Atom-Economic Way for Accessing Butadiene/Isoprene Rubber. Ind. Eng. Chem. Res. 2020, 59, 10754–10762. [Google Scholar] [CrossRef]
- Fan, C.; Bai, C.; Cai, H.; Dai, Q.; Zhang, X.; Wang, F. Preparation of high cis-1,4 polyisoprene with narrow molecular weight distribution via coordinative chain transfer polymerization. J. Polym. Sci. Polym. Chem. 2010, 48, 4768–4774. [Google Scholar] [CrossRef]
- Tang, Z.; Liang, A.; Liang, H.; Zhao, J.; Xu, L.; Zhang, J. Reversible Coordinative Chain Transfer Polymerization of Butadiene Using a Neodymium Phosphonate Catalyst. Macromol. Res. 2019, 27, 789–794. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Zheng, W.; Guo, J.; Zhang, C.; Zhao, L.; Zhang, H.; Hu, Y.; Bai, C.; Zhang, X. Fully-reversible and semi-reversible coordinative chain transfer polymerizations of 1,3-butadiene with neodymium-based catalytic systems. Polymer 2013, 54, 6716–6724. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, C.; Hu, Y.; Jia, X.; Bai, C.; Zhang, X. Reversible coordinative chain transfer polymerization of isoprene and copolymerization with ε-caprolactone by neodymium-based catalyst. Polymer 2012, 53, 6027–6032. [Google Scholar] [CrossRef]
- Córdova, T.; Enríquez-Medrano, F.J.; Cartagena, E.M.; Villanueva, A.B.; Valencia, L.; Álvarez, E.N.C.; González, R.L.; Díaz-de-León, R. Coordinative Chain Transfer Polymerization of Sustainable Terpene Monomers Using a Neodymium-Based Catalyst System. Polymers 2022, 14, 2907. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Q.; Dong, J.; Wang, F.; Luo, F.; Liu, H.; Zhang, X. Neodymium-based one-precatalyst/dual-cocatalyst system for chain shuttling polymerization to access cis-1,4/trans-1,4 multiblock polybutadienes. Mater. Tod. Commun. 2021, 27, 102453. [Google Scholar] [CrossRef]
- Belaid, I.; Macqueron, B.; Poradowski, M.-N.; Bouaouli, S.; Thuilliez, J.; Cruz-Boisson, F.D.; Monteil, V.; D’Agosto, F.; Perrin, L.; Boisson, C. Identification of a Transient but Key Motif in the Living Coordinative Chain Transfer Cyclocopolymerization of Ethylene with Butadiene. ACS Catal. 2019, 9, 9298–9309. [Google Scholar] [CrossRef]
- Göttker-Schnetmann, I.; Kenyon, P.; Mecking, S. Coordinative Chain Transfer Polymerization of Butadiene with Functionalized Aluminum Reagents. Angew. Chem. Int. Ed. 2019, 58, 17777–17781. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Zinck, P.; Vitorino, M.J.; Mortreux, A.; Visseaux, M. Rare rarths/main group metal alkyls catalytic systems for the 1,4-trans stereoselective coordinative chain transfer polymerization of isoprene. J. Polym. Sci. Polym. Chem. 2010, 48, 4640–4647. [Google Scholar] [CrossRef]
- Hassanian-Moghaddam, D.; Mortazavi, S.M.M.; Ahmadjo, S.; Doveirjavi, M.; Rahmati, A.; Ahmadi, M. Resolving long-chain branch formation in tandem catalytic coordinative chain transfer polymerization of ethylene via thermal analysis. J. Polym. Res. 2021, 29, 3. [Google Scholar] [CrossRef]
- Valente, A.; Zinck, P.; Mortreux, A.; Bria, M.; Visseaux, M. Half-lanthanocene/dialkylmagnesium-mediated coordinative chain transfer copolymerization of styrene and hexene. J. Polym. Sci. Polym. Chem. 2011, 49, 3778–3782. [Google Scholar] [CrossRef]
- Valente, A.; Zinck, P.; Mortreux, A.; Visseaux, M. Borohydrido rare earth based coordinative chain transfer copolymerization: A convenient tool for tuning the microstructure of isoprene/styrene copolymers. J. Polym. Sci. Polym. Chem. 2011, 49, 1615–1620. [Google Scholar] [CrossRef]
- Zinck, P.; Valente, A.; Bonnet, F.; Violante, A.; Mortreux, A.; Visseaux, M.; Ilinca, S.; Duchateau, R.; Roussel, P. Reversible Coordinative Chain Transfer Polymerization of Styrene by Rare Earth Borohydrides, Chlorides/Dialkylmagnesium Systems. J. Polym. Sci. Polym. Chem. 2010, 48, 802–814. [Google Scholar] [CrossRef]
- Cavalcante de Sá, M.C.; Córdova, A.M.T.; Díaz de León Gómez, R.E.; Pinto, J.C. Modeling of Isoprene Solution Coordinative Chain Transfer Polymerization. Macromol. React. Eng. 2021, 15, 2100005. [Google Scholar] [CrossRef]
- Gao, H.; Lu, X.; Chen, S.; Du, B.; Yin, X.; Kang, Y.; Zhang, K.; Liu, C.; Pan, L.; Wang, B.; et al. Preparation of Well-Controlled Isotactic Polypropylene-Based Block Copolymers with Superior Physical Performance via Efficient Coordinative Chain Transfer Polymerization. Macromolecules 2022, 55, 5038–5048. [Google Scholar] [CrossRef]
- Georges, S.; Touré, A.O.; Visseaux, M.; Zinck, P. Coordinative chain transfer copolymerization and terpolymerization of conjugated dienes. Macromolecules 2014, 47, 4538–4547. [Google Scholar] [CrossRef]
- Valente, A.; Stoclet, G.; Bonnet, F.; Mortreux, A.; Visseaux, M.; Zinck, P. Isoprene–styrene chain shuttling copolymerization mediated by a lanthanide half-sandwich complex and a lanthanidocene: Straightforward access to a new type of thermoplastic elastomers. Angew. Chem. Int. Ed. 2014, 53, 4638–4641. [Google Scholar] [CrossRef]
- Georges, S.; Bonnet, F.; Roussel, P.; Visseaux, M.; Zinck, P. β-Diketiminate-supported magnesium alkyl: Synthesis, structure and application as co-catalyst for polymerizations mediated by a lanthanum half-sandwich complex. Appl. Organomet. Chem. 2016, 30, 26–31. [Google Scholar] [CrossRef]
- Valente, A.; Zinck, P.; Mortreux, A.; Visseaux, M. Catalytic Chain Transfer (co-)Polymerization: Unprecedented Polyisoprene CCG and a New Concept to Tune the Composition of a Statistical Copolymer. Macromol. Rapid. Comm. 2009, 30, 528–531. [Google Scholar] [CrossRef]
- Phuphuak, Y.; Bonnet, F.; Stoclet, G.; Bria, M.; Zinck, P. Isoprene chain shuttling polymerisation between cis and trans regulating catalysts: Straightforward access to a new material. Chem. Commun. 2017, 53, 5330–5333. [Google Scholar] [CrossRef]
- Jian, Z.; Cui, D.; Hou, Z.; Li, X. Living catalyzed-chain-growth polymerization and block copolymerization of isoprene by rare-earth metal allyl precursors bearing a constrained-geometry-conformation ligand. Chem. Commun. 2010, 46, 3022–3024. [Google Scholar] [CrossRef] [PubMed]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Coordinative chain transfer polymerization of 1-decene in the presence of a Ti-based diamine bis(phenolate) catalyst: A sustainable approach to produce low viscosity PAOs. Green Chem. 2020, 22, 4617–4626. [Google Scholar] [CrossRef]
- Hanifpour, A.; Ahmadi, M.; Nekoomanesh-Haghighi, M.; Bahri-Laleh, N. Effect of different chain transfer agents in the coordinative chain transfer oligomerization of dec-1-ene. J. Mol. Struct. 2022, 1263, 133157. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Dong, J.; Li, W.; Li, X.; Liu, H.; Zhang, X.; Wang, F. Facile and efficient in-situ end-functionalization of neodymium-based polybutadiene by isocyanate via a coordinative chain transfer polymerization strategy. Polymer 2024, 305, 127166. [Google Scholar] [CrossRef]
- Liu, P.; Yang, X.; Li, H.; Zhang, S.; Hu, Y.; Zhou, G.; Hadjichristidis, N. Synthesis of α,ω-End Functionalized Polydienes: Allylic-Bearing Heteroleptic Aluminums for Selective Alkylation and Transalkylation in Coordinative Chain Transfer Polymerization. Angew. Chem. Int. Ed. 2024, 63, e202317494. [Google Scholar] [CrossRef]
- Chen, M.-K.; Zhao, Y.-H.; Zhang, R.; Yang, Y.; Cao, J.; Tang, M.-Z.; Huang, G.; Xu, Y.-X. Carbonate nanophase guided by terminally functionalized polyisoprene leading to a super tough, recyclable and transparent rubber. Chem. Eng. J. 2023, 452, 139130. [Google Scholar] [CrossRef]
- Ling, F.-W.; Luo, M.-C.; Chen, M.-K.; Zeng, J.; Li, S.-Q.; Yin, H.-B.; Wu, J.-R.; Xu, Y.-X.; Huang, G. Terminally and randomly functionalized polyisoprene lead to distinct aggregation behaviors of polar groups. Polymer 2019, 178, 121629. [Google Scholar] [CrossRef]
- Li, S.; Tang, M.; Huang, C.; Zhang, R.; Wu, J.; Ling, F.; Xu, Y.-X.; Huang, G. Branching function of terminal phosphate groups of polyisoprene chain. Polymer 2019, 174, 18–24. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, R.; Li, S.; Zeng, J.; Luo, M.; Xu, Y.-X.; Huang, G. Towards a Supertough Thermoplastic Polyisoprene Elastomer Based on a Biomimic Strategy. Angew. Chem. Int. Ed. 2018, 57, 15836–15840. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Yin, H.-B.; Bai, S.-J.; Zhang, R.; Li, C.-H.; Tang, M.-Z.; Xu, Y.-X. Probe the terminal interactions and their synergistic effects on polyisoprene properties by mimicking the structure of natural rubber. Polymer 2021, 237, 124362. [Google Scholar] [CrossRef]
- Tang, M.; Bai, S.-j.; Xu, R.; Zhang, R.; Li, S.-Q.; Xu, Y.-X. Oligopeptide binding guided by spacer length lead to remarkably strong and stable network of polyisoprene elastomers. Polymer 2021, 233, 124185. [Google Scholar] [CrossRef]
- Li, S.-Q.; Tang, M.-Z.; Huang, C.; Zhang, R.; Huang, G.-S.; Xu, Y.-X. The Relationship between Pendant Phosphate Groups and Mechanical Properties of Polyisoprene Rubber. Chin. J. Polym. Sci. 2021, 39, 465–473. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, R.; Tang, M.; Huang, G.-S.; Xu, Y.-X. The Effect of Branching Structure on the Properties of Entangled or Non-covalently Crosslinked Polyisoprene. Chin. J. Polym. Sci. 2020, 39, 113–121. [Google Scholar] [CrossRef]
- Burkey, A.A.; Fischbach, D.M.; Wentz, C.M.; Beers, K.L.; Sita, L.R. Highly Versatile Strategy for the Production of Telechelic Polyolefins. ACS Macro Lett. 2022, 11, 402–409. [Google Scholar] [CrossRef]
- Thomas, T.S.; Hwang, W.; Sita, L.R. End-Group-Functionalized Poly(α-olefinates) as Non-Polar Building Blocks: Self-Assembly of Sugar–Polyolefin Hybrid Conjugates. Angew. Chem. Int. Ed. 2016, 55, 4683–4687. [Google Scholar] [CrossRef]
- Cueny, E.S.; Sita, L.R.; Landis, C.R. Quantitative Validation of the Living Coordinative Chain-Transfer Polymerization of 1-Hexene Using Chromophore Quench Labeling. Macromolecules 2020, 53, 5816–5825. [Google Scholar] [CrossRef]
- Briquel, R.; Mazzolini, J.; Le Bris, T.; Boyron, O.; Boisson, F.; Delolme, F.; D’Agosto, F.; Boisson, C.; Spitz, R. Polyethylene building blocks by catalyzed chain growth and efficient end functionalization strategies, including click chemistry. Angew. Chem. Int. Edit. 2008, 47, 9311–9313. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, J.; Espinosa, E.; D’Agosto, F.; Boisson, C. Catalyzed chain growth (CCG) on a main group metal: An efficient tool to functionalize polyethylene. Polym. Chem. 2010, 1, 793. [Google Scholar] [CrossRef]
- Han, C.J.; Lee, M.S.; Byun, D.J.; Kim, S.Y. Synthesis of hydroxy-terminated polyethylene via controlled chain transfer reaction and poly(ethylene-b-caprolactone) block copolymer. Macromolecules 2002, 35, 8923–8925. [Google Scholar] [CrossRef]
- Kaneyoshi, H.; Inoue, Y.; Matyjaszewski, K. Synthesis of block and graft copolymers with linear polyethylene segments by combination of degenerative transfer coordination polymerization and atom transfer radical polymerization. Macromolecules 2005, 38, 5425–5435. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.; Cong, R.; Dong, J.; Wu, G.; Wang, F.; Liu, H.; Zhang, X. In-situ Block Copolymerization of 1,3-Butadiene with Cyclohexene Oxide and Trimethylene Carbonate via Combination of Coordinative Chain Transfer Polymerization and Ring Opening Polymerization by Neodymium-based Catalyst System. Eur. Polym. J. 2021, 148, 110355. [Google Scholar] [CrossRef]
- Wang, F.; Dong, B.; Liu, H.; Guo, J.; Zheng, W.; Zhang, C.; Zhao, L.; Bai, C.; Hu, Y.; Zhang, X. Synthesis of block copolymers containing polybutadiene segments by combination of coordinative chain transfer polymerization, ring-opening polymerization, and atom transfer radical polymerization. Macromol. Chem. Phys. 2015, 216, 321–328. [Google Scholar] [CrossRef]
- Zheng, W.; Yan, N.; Zhu, Y.; Zhao, W.; Zhang, C.; Zhang, H.; Bai, C.; Hu, Y.; Zhang, X. Highly trans-1,4-stereoselective coordination chain transfer polymerization of 1,3-butadiene and copolymerization with cyclic esters by a neodymium-based catalyst system. Polym. Chem. 2015, 6, 6088–6095. [Google Scholar] [CrossRef]
- Georges, S.; Hashmi, O.H.; Bria, M.; Zinck, P.; Champouret, Y.; Visseaux, M. Efficient One-Pot Synthesis of End-Functionalized trans-Stereoregular Polydiene Macromonomers. Macromolecules 2019, 52, 1210–1219. [Google Scholar] [CrossRef]
- Frisch, M.J.; H. B. Schlegel, G.W.T.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Program; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Liu, N.; Long, S.; Wu, C.; Cui, D. Highly cis-1,4-selective coordination polymerization of polar 2-(4-methoxyphenyl)-1,3-butadiene and copolymerization with isoprene using a β-diketiminato yttrium bis(alkyl) complex. Polym. Chem. 2016, 7, 1264–1270. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Tang, T.; Zhang, X. cis-1,4 Selective Coordination Polymerization of 1,3-Butadiene and Copolymerization with Polar 2-(4-Methoxyphenyl)-1,3-butadiene by Acenaphthene-Based α-Diimine Cobalt Complexes Featuring Intra-Ligand π-π Stacking Interactions. Polymers 2021, 13, 3329. [Google Scholar] [CrossRef]
- Gong, D.; Tang, F.; Xu, Y.; Hu, Z.; Luo, W. Cobalt catalysed controlled copolymerization: An efficient approach to bifunctional polyisoprene with enhanced properties. Polym. Chem. 2021, 12, 1653–1660. [Google Scholar] [CrossRef]
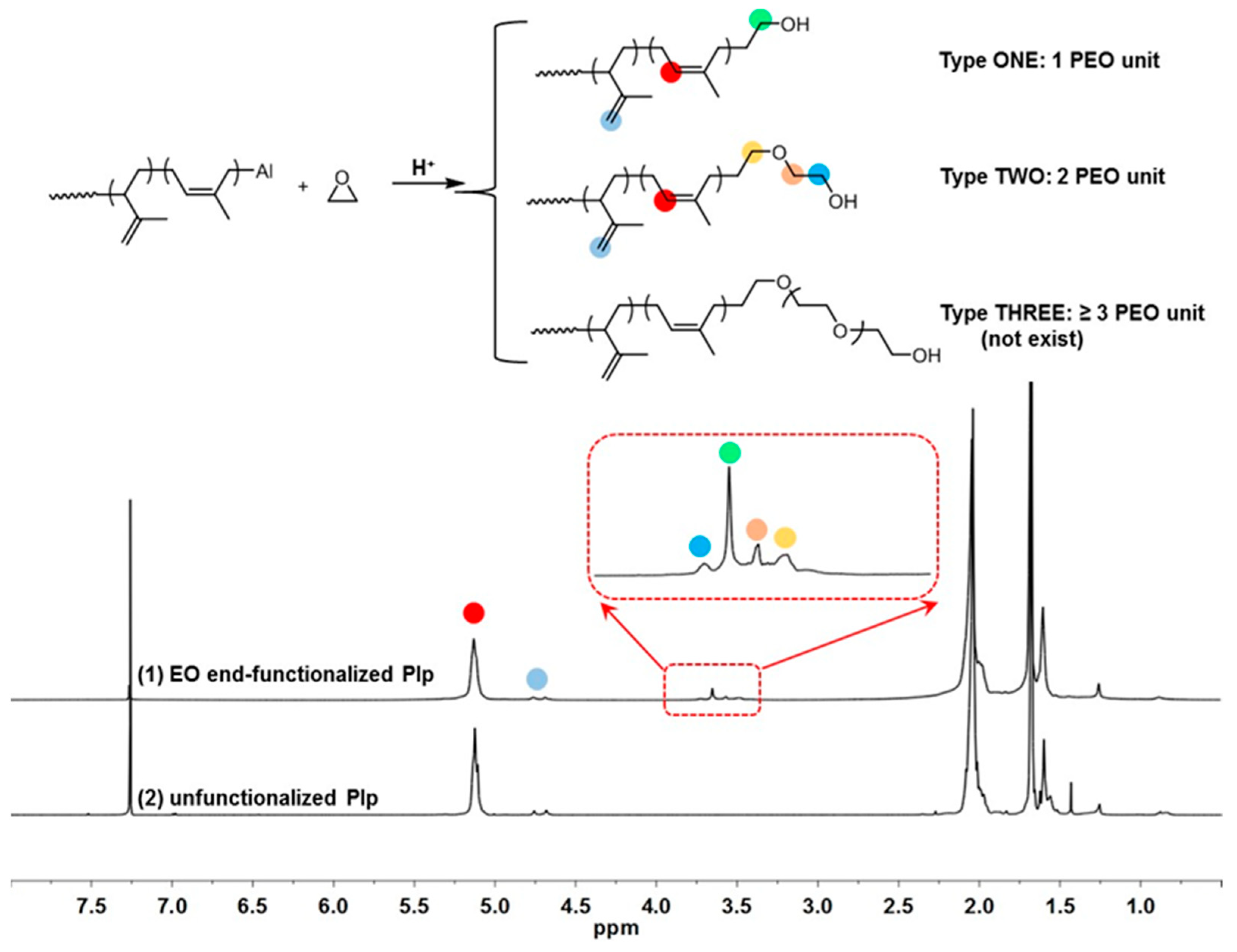

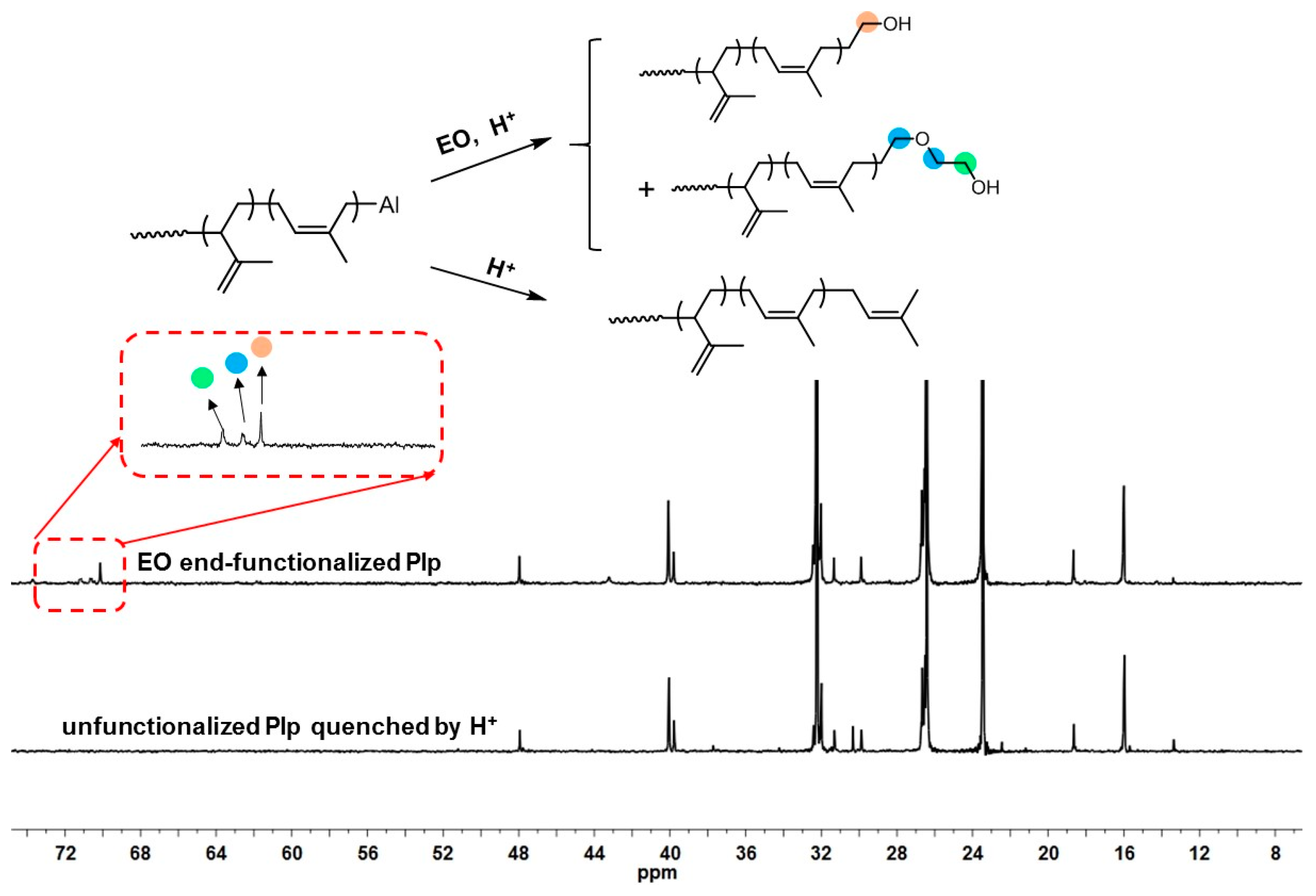


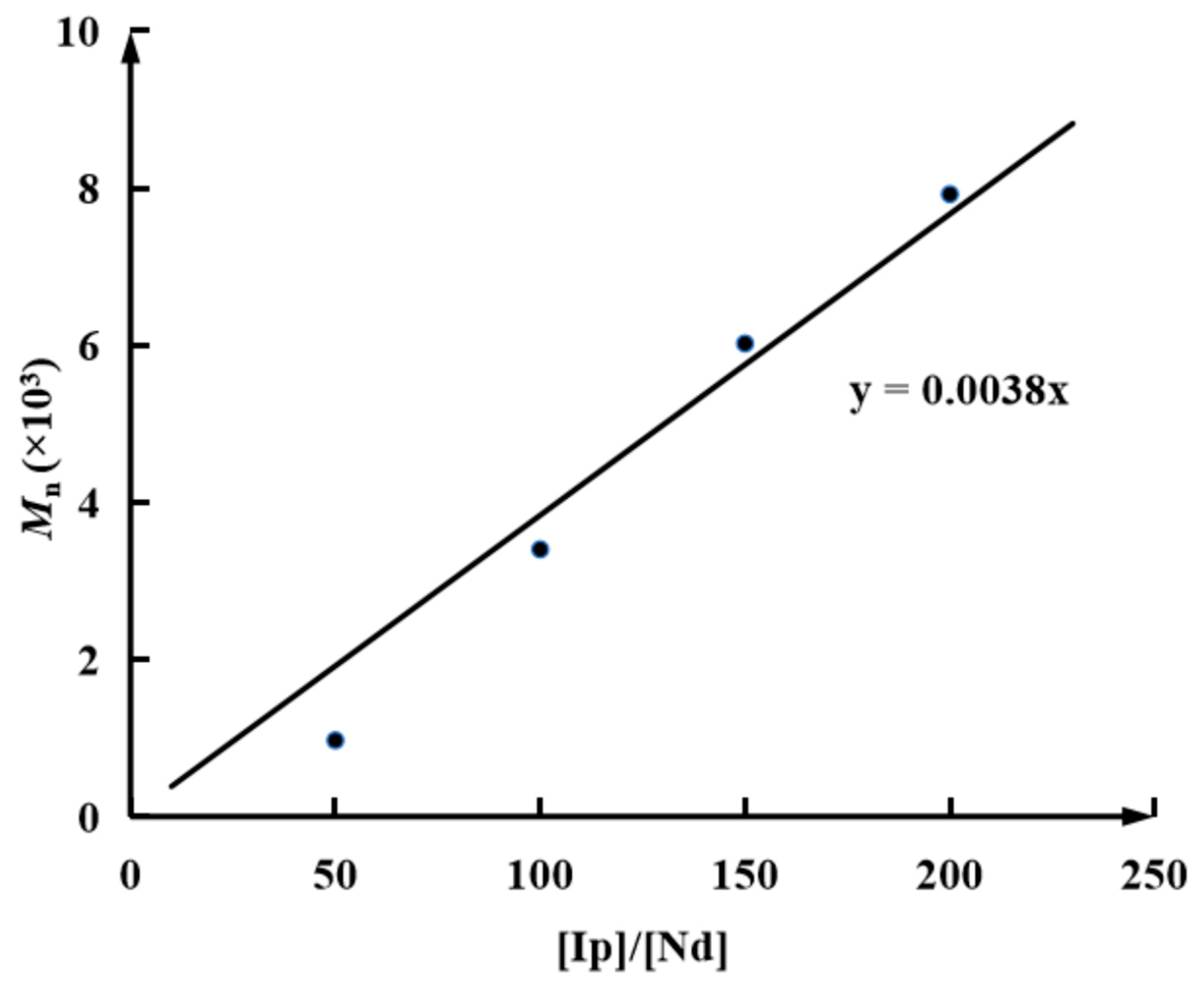
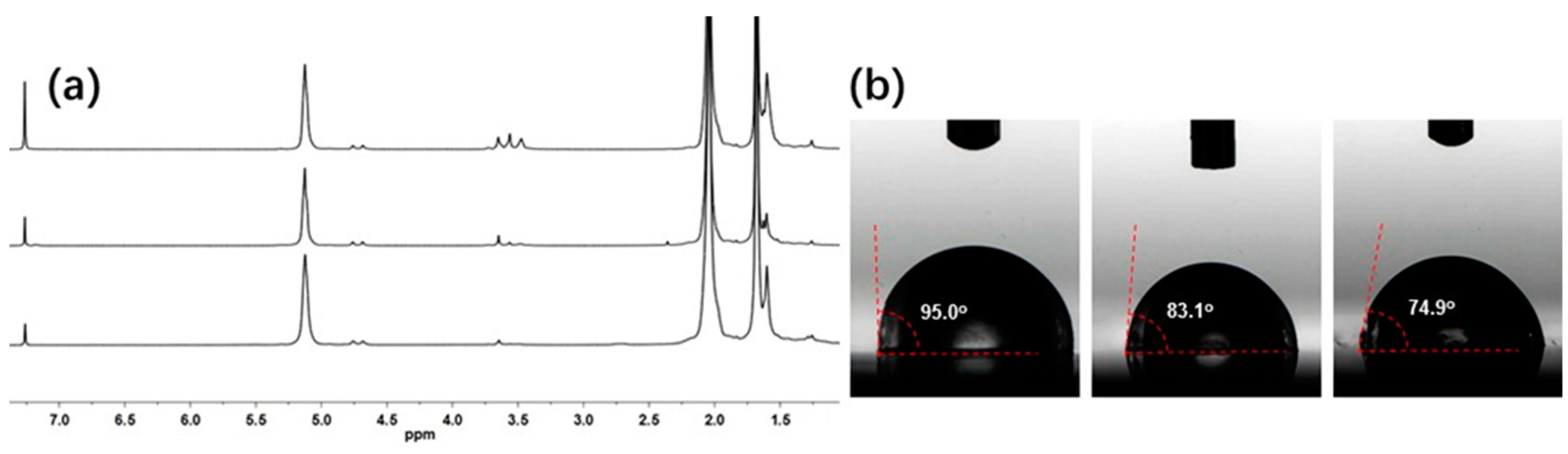
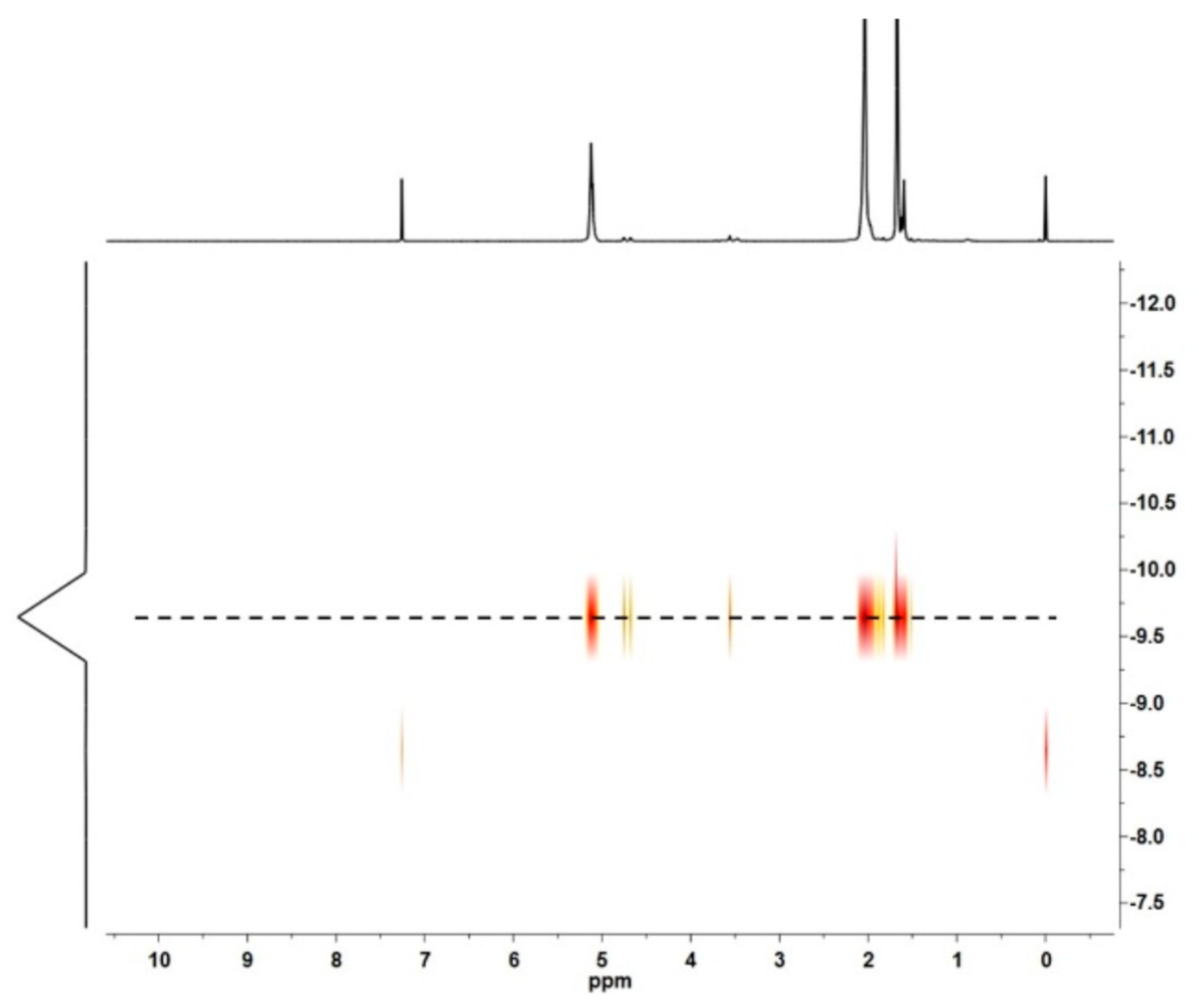
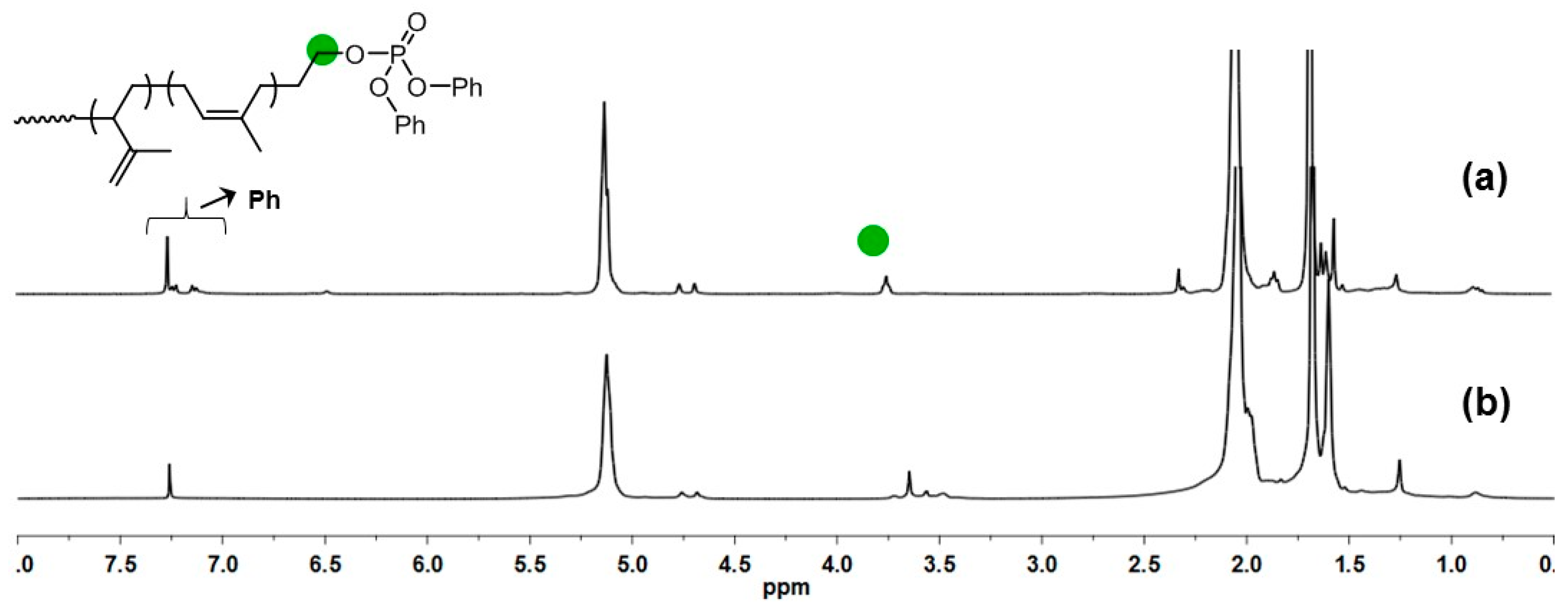
| Run | [Ip]/[Nd] | Yield (%) | Mn ×103 b | Mw ×103 b | Đ b | 1,4-% c | 3,4-% c | f d |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 100 | 0.97 | 1.62 | 1.66 | 96.5 | 3.5 | 92.7 |
| 2 | 100 | 100 | 3.40 | 6.76 | 1.98 | 96.9 | 3.1 | 80.1 |
| 3 | 150 | 100 | 6.02 | 16.3 | 2.73 | 97.3 | 2.7 | 77.2 |
| 4 | 200 | 100 | 7.92 | 30.0 | 3.79 | 96.7 | 3.3 | 74.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Dong, J.; Wang, F.; Zhang, X.; Liu, H. In Situ Efficient End Functionalization of Polyisoprene by Epoxide Compounds via Neodymium-Mediated Coordinative Chain Transfer Polymerization. Polymers 2024, 16, 2672. https://doi.org/10.3390/polym16182672
Zhang X, Dong J, Wang F, Zhang X, Liu H. In Situ Efficient End Functionalization of Polyisoprene by Epoxide Compounds via Neodymium-Mediated Coordinative Chain Transfer Polymerization. Polymers. 2024; 16(18):2672. https://doi.org/10.3390/polym16182672
Chicago/Turabian StyleZhang, Xiuhui, Jing Dong, Feng Wang, Xuequan Zhang, and Heng Liu. 2024. "In Situ Efficient End Functionalization of Polyisoprene by Epoxide Compounds via Neodymium-Mediated Coordinative Chain Transfer Polymerization" Polymers 16, no. 18: 2672. https://doi.org/10.3390/polym16182672
APA StyleZhang, X., Dong, J., Wang, F., Zhang, X., & Liu, H. (2024). In Situ Efficient End Functionalization of Polyisoprene by Epoxide Compounds via Neodymium-Mediated Coordinative Chain Transfer Polymerization. Polymers, 16(18), 2672. https://doi.org/10.3390/polym16182672







