Effects of Titanium Dioxide (TiO2) on Physico-Chemical Properties of Low-Density Polyethylene
Abstract
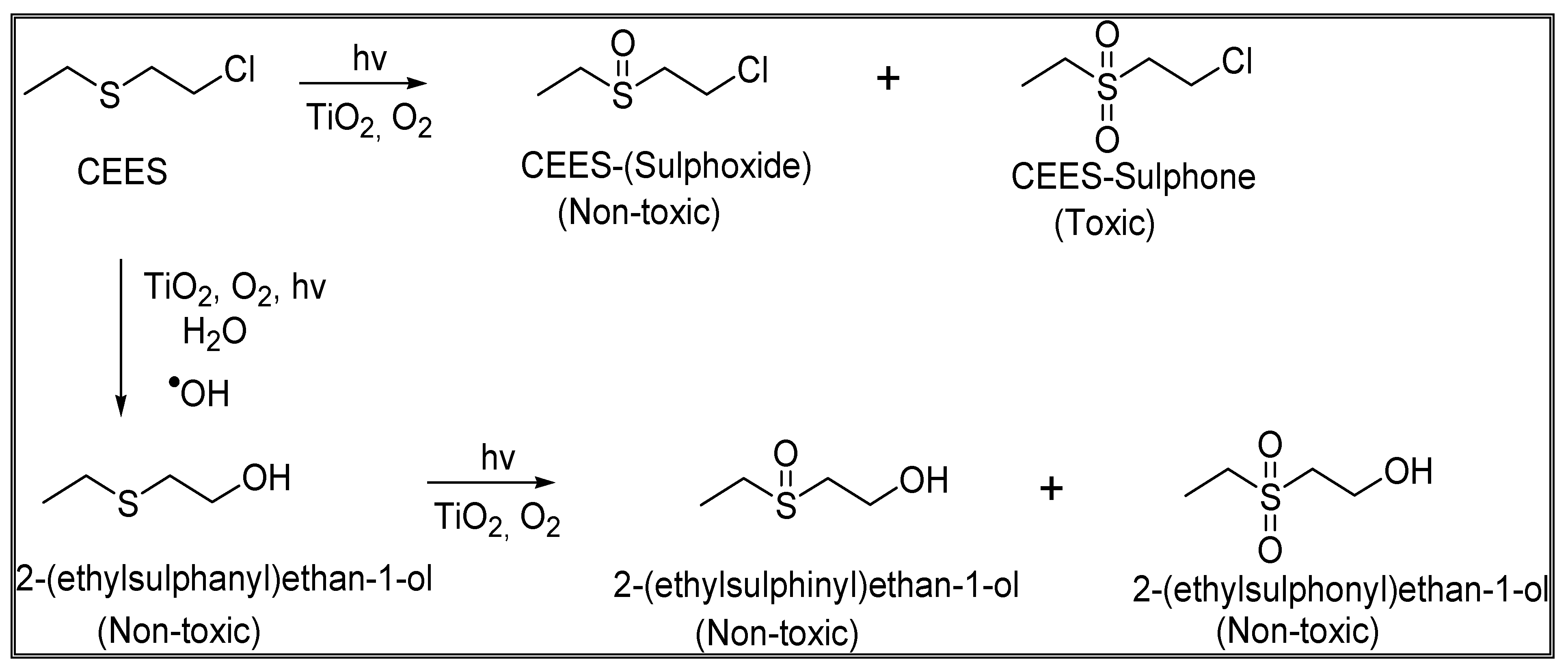
:1. Introduction
- (a)
- Chemical degradation–partial or selective breakdown of the polymer due to the presence of a chemical or various chemical rendering the non-toxic or less toxic;
- (b)
- Chemical penetration–chemical diffuses through the wicking or imperfections and closures in impermeable structures;
- (c)
- Chemical permeation–this is a molecular path of chemicals through the material of the structure; and
- (d)
- Chemical evaporation–the exposed chemical may depend on their repellency, evaporation or the vapour of the chemical droplet may dissipate into the atmosphere or permeate through the CPC.
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
- Titanium dioxide (TiO2)—>99% purity; Anatase; Merck, Johannesburg, South Africa;
- 2-Chloroethyl ethyl sulphide (CEES)—>99% purity, Merck, Johannesburg, South Africa;
- Nitrogen gas—99.999% purity, Afrox supplier, Germiston, South Africa.
2.1.2. Materials
- LDPE pellets—SASOL, Modderfontein, South Africa.
2.1.3. Equipment and Instruments
- Co-rotating twin screw extruder—LabTech Engineering, Phraeksa, Thailand;
- Film blowing twin screw extruder—Nanjing Extrusion Machinery, Nanjing, China;
- Cryo-microtome—Leica UC7, Leica Microsystems, Wezlar, Germany;
- Scanning electron microscope coupled to an energy-dispersive X-ray spectrometer (SEM-EDS)—Zeiss FE-SEM 55VP, Zeiss, Jena, Germany;
- Transmission electron microscope (TEM)—JEM 1010, Joel, Tokyo, Japan;
- Simultaneous Thermal Analyzer (STA) 6000—Perkin Elmer, Waltham, MA, USA;
- Tensile tester—Instron 3345, Norwood, MA, USA;
- UV source (UV-B Lamp; 295–320 nm)—Philips, Amsterdam, The Netherlands.
2.1.4. Software
- Pyris Software for STA 6000—Ver.13;
- Bluehill LE for Instron 3345—Ver.3.77.4940.
2.2. Methods
2.2.1. Extrusion of LDPE Films
2.2.2. Morphology
2.2.3. Determination of Thermal Properties
2.2.4. Tensile Testing
2.2.5. Permeation Testing
2.2.6. UV Exposure Testing
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphology Studies
3.2. UV Exposure
3.3. Thermal Analysis
3.4. Tensile Properties
3.5. Permeation Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and Characterization of Polyethylene-Based Composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Fenercioglu, H.; Polat, S.; Guglu, M. Effects of Metal Nanoparticles on the Physical and Migration Properties of Low Density Polyethylene Films. J. Food. Eng. 2018, 229, 32–42. [Google Scholar]
- Dehbi, A.; Mourad, A.I. Durability of Mono-Layer Versus Tri-Layers LDPE Films Used as Greenhouse Cover: Comparative Study. Arab. Chem. 2016, 9, S282–S289. [Google Scholar] [CrossRef]
- Gitchaiwat, A.; Likhitlert, S.; Kositchaiyong, A.; Israngkura, K.; Taptim, K.; Sombatsompop, N. Uses of 2-hydroxypropyl–3-piperazinyl-quinoline carboxylic acid methacrylate and Terbutryn as algaecides in low-density polyethylene mulching films for agricultural applications. J. Plast. Film. Sheet. 2016, 32, 97–118. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.; Hur, H.G. Biodegradation of polyethylene: A Brief Review. Appl. Biol. Chem. 2020, 63, 27–41. [Google Scholar] [CrossRef]
- Sirocic, A.P.; Rescek, A.; Scetar, M.; Krehula, L.K.; Hrnjak-Murgic, Z. Development of Low Density Polyethylene Nanocomposites Films For Packaging. Polym. Bull. 2014, 71, 705–717. [Google Scholar] [CrossRef]
- Redhwi, H.H.; Siddiqui, M.N.; Andrady, A.L.; Hussain, S. Durability of LDPE Nanocomposites with Clay, Silica, and Zinc Oxide–Part I: Mechanical Properties of the Nanocomposite Materials. J. Nanomater. 2013, 2013, 654716. [Google Scholar] [CrossRef]
- Hung, Y.C.; Yemmireddy, V.K. Using Photocatalyst Metal Oxide as Antimicrobial Surface Coatings to Ensure Food Safety-Opportunities and Challenges. Compr. Rev. Food. Sci. Food Safety 2017, 16, 617–631. [Google Scholar]
- Esthappan, S.K.; Kuttappan, S.K.; Joseph, R. Thermal and Mechanical Properties of Polypropylene/Titanium Dioxide Nanocomposite Fibers. Mater. Des. 2012, 37, 537–542. [Google Scholar] [CrossRef]
- Yadav, A.; Vigneshwaran, N.; Prasad, V.; Kathe, A.A.; Raj, S.; Yadav, D.; Sundaramoorthy, C. Functional Finishing in Cotton Fabrics Using Zinc Oxide Nanoparticle. Bull. Mater. Sci. 2006, 29, 641–645. [Google Scholar] [CrossRef]
- Kemp, T.J.; McIntyre, R.A. Mechanism of Action of Titanium Dioxide Pigment in the Photodegradation of Poly(vinyl Chloride) and Other Polymers. Prog. React. Kinet. Mec. 2000, 26, 337–374. [Google Scholar] [CrossRef]
- Khalilova, H.K.; Hasanova, S.A.; Aliyev, F.G. Photocatalytic Removal of Organic Pollutants from Industrial Wastewater Using TiO2 Catalyst. J. Environ. Prot. 2018, 9, 691–698. [Google Scholar] [CrossRef]
- Amin, M.; Scott, G.; Tillekeratne, L.M. Mechanism of the Photo-Initiation Process in the Polyethylene. Europ. Polym. J. 1975, 11, 85–89. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paula, S.; Dytta, J. Visible light photocatalytic Degradation of Microplastic Residues with Zinc Oxide Nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Lipp-Symonowicz, B.; Sztajnowski, S.; Kardas, I. Influence of UV Radiation on the Mechanical Properties of Polyamide and Polypropylene Fibres in Aspect of Their Restructuring. Autex. Res. J. 2006, 6, 191–195. [Google Scholar] [CrossRef]
- Lee, Q.Y.; Li, H. Photocatalytic Degradation of Plastic Waste: A Mini Review. Micromachines 2021, 12, 907. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. SpringerPlus 2013, 2, 398–430. [Google Scholar] [CrossRef]
- Khalil, E. A Technical Overview on Protective Clothing Against Chemical Hazards. AASCIT J. Chem. 2015, 2, 67–76. [Google Scholar]
- Ndibewu, P.P.; Ngobeni, P.; Lefakane, T.E.; Netshiozwi, T.E. Recent Advances in Biopolymers; Parveen, F., Ed.; IntechOpen Limited: London, UK, 2016; pp. 145–187. [Google Scholar]
- Dolez, P.I.; Marsha, S.; McQueen, R.H. Fibers and Textiles for Personal Protective Equipment: Review of Recent Progress and Perspectives on Future Developments. Textiles 2022, 2, 349–381. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2019, 49, 97–138. [Google Scholar] [CrossRef]
- Suhail, M.S. An Overview of Chemical Protective Clothing. Int. J. Adv. Eng. Res. Sci. 2017, 6, 1713–1719. [Google Scholar]
- Dupont Personal Protection, Catalogue. Available online: https://www.dupont.co.in/content/dam/dupont/amer/us/en/personal-protection/public/documents/en/DuPont_Personal_Protection_Catalogue_APAC_EN.pdf (accessed on 15 September 2024).
- Draeger Specification Sheet CPS 7900. Available online: https://en.safetygas.com/chemical-cbrn-suit-cps7900 (accessed on 10 September 2024).
- Kappler, Know What You Are Getting Into. Available online: https://shop.dqeready.com/content/documents/Kappler%20Level%20A%20HazMat%20Suit%20Instructions-User%20Information%20for%20Zytron%20Level%20A%20Garments.pdf (accessed on 10 September 2024).
- Kappert, E.J.; Raaijmakers, M.J.T.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 Polymers Commonly Employed for Solvent-Resistant Nanofiltration Membranes: A Comprehensive Dataset. J. Membrane Sci. 2019, 569, 177–231. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal-Organic Frameworks for the Removal of Toxic Industrial Chemicals and Chemical Warfare Agents. R. Soc. Chem. 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, A. Chapter 12: Re-Use, Recycling and Degradation of Composites. In Green Composites: Polymer Composites and the Environment; Elsevier: Amsterdam, The Netherlands, 2004; pp. 252–271. [Google Scholar]
- Alvarado, J.; Acosta, G.; Perez, F. Study of the Effect of the Dispersion of Functionalized Nanoparticles TiO2 with Photocatalytic Activity In LDPE. Polym. Degrad. Stab. 2016, 134, 376–382. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The Effect of UV Light on the Chemical and Mechanical Properties of a Transparent Epoxy-Diamine System in the Presence of an Organic UV Absorber. Material 2017, 10, 180. [Google Scholar] [CrossRef]
- Poh, L.; Wu, Q.; Chen, Y.; Narimissa, E. Characterization of Industrial Low-Density Polyethylene: A Thermal, Dynamic Mechanical, and Rheological Investigation. Rheol. Acta 2022, 61, 701–720. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, X.R. Surface Characteristics and Biotoxicity of Airborne Microplastics. Compr. Anal. Chem. 2022, 100, 117–164. [Google Scholar]
- Nguyen, T.K.N.; Kim, H.G.; Kwac, L.K.; Bui, Q.B.; Nguyen, D.M.; Jeong, H. Titanium Dioxide-Benzophenone Hybrid As An Effective Catalyst For Enhanced Photochemical Degradation of Low Density Polyethylene. e-Polymers 2018, 18, 501–510. [Google Scholar] [CrossRef]
- Li, D.; Zhou, L.; Wang, X.; He, L.; Yang, X. Effect of Crystallinity of Polyethylene with Different Densities on Breakdown Strength and Conductance Property. Materials 2019, 12, 1746. [Google Scholar] [CrossRef]
- Tuasikal, M.A.; Alothman, O.Y.; Luqman, M.; Al-Zahrani, S.M.; Jawaid, M. Influence of Natural and Accelerated Weathering on the Mechanical Properties of Low-Density Polyethylene Films. Int. J. Polym. Anal. Charact. 2014, 19, 189–203. [Google Scholar] [CrossRef]
- Sadrnia, H.; Zamanian, M.; Khojastehpour, M.; Hossein, F.; Thibault, J. Effect of TiO2 Nanoparticles on Barrier and Mechanical Properties of PVA Films. J. Membr. Sci. Res. 2021, 7, 67–73. [Google Scholar]
- Kumosa, M.; Lu, T.; Solis-Ramos, E.; Yi, Y. UV Degradation Model for Polymers and Polymer Matrix Composites. Polym. Degrad. Stabil. 2018, 154, 203–210. [Google Scholar]
- Zhao, J.; Cai, G.; Cui, L.; Larbi, A.S.; Tsavdaridis, K.D. Deterioration of Basic Properties of the Materials in FRP-Strengthening RC Structures Under Ultraviolet Exposure. Polymers 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Munir, N. Determination of Low-Density Polyethylene Water Permeability, Transport Activation Energy and Mechanical Properties after Thermal Oxidation and Immersion in Water. Master’s Thesis, University of Minnesota Duluth, Duluth, MN, USA, 2019. [Google Scholar]
- Mao, F. Permeation of Hydrocarbons through Polyvinyl Chloride (PVC) and Polyethylene (PE) Pipes and Pipe Gaskets. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2008. [Google Scholar]
- Siracusa, V.; Genovese, L.; Ingrao, C.; Munari, A.; Lotti, N. Barrier Properties of Poly(Propylene Cyclohexanedicarboxylatee) Random Eco-Friendly Copolyesters. Polymers 2018, 10, 502. [Google Scholar] [CrossRef]
- Fallis, I.A.; Griffiths, P.C.; Cosgrove, T.; Dreiss, C.A.; Govan, N.; Heenan, R.K. Locus Specific Microemulsion Catalysts for Sulfur Mustard (HD) Chemical Warfare Agent Decontamination. J. Am. Chem. Soc. 2009, 131, 9746–9771. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A.J.; Hupp, T.; Farha, O.K. Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal-Organic Framework. Angew. Chem. Int. Ed. Engl. 2015, 54, 9001–9005. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, J.R.; Bustone, G.P.; Clark, E.R. Decontamination and Remediation of the Sulfur Mustard Simulant CEES with “ Off-the-Shelf” Reagents in Solution and Gel States: A Proof-of-Concept Study. Chem. Open 2017, 6, 497–500. [Google Scholar] [CrossRef]
- Ergerton, T.A.; Yang, R.; Christensen, P.A.; White, J.R.; Malt, A. Spectroscopic Studies of Photodegradation of Polyethylene Films Containing TiO2 Nanoparticles. J. App. Polym. Sci. 2011, 119, 1330–1338. [Google Scholar]
- Yang, R.; Christensen, P.A.; Egerton, T.A.; White, J.R. Degradation Products Formed During UV Exposure of Polyethylene-ZnO Nano-Composites. Polym. Degrad. Stabil. 2010, 95, 1533–1541. [Google Scholar] [CrossRef]
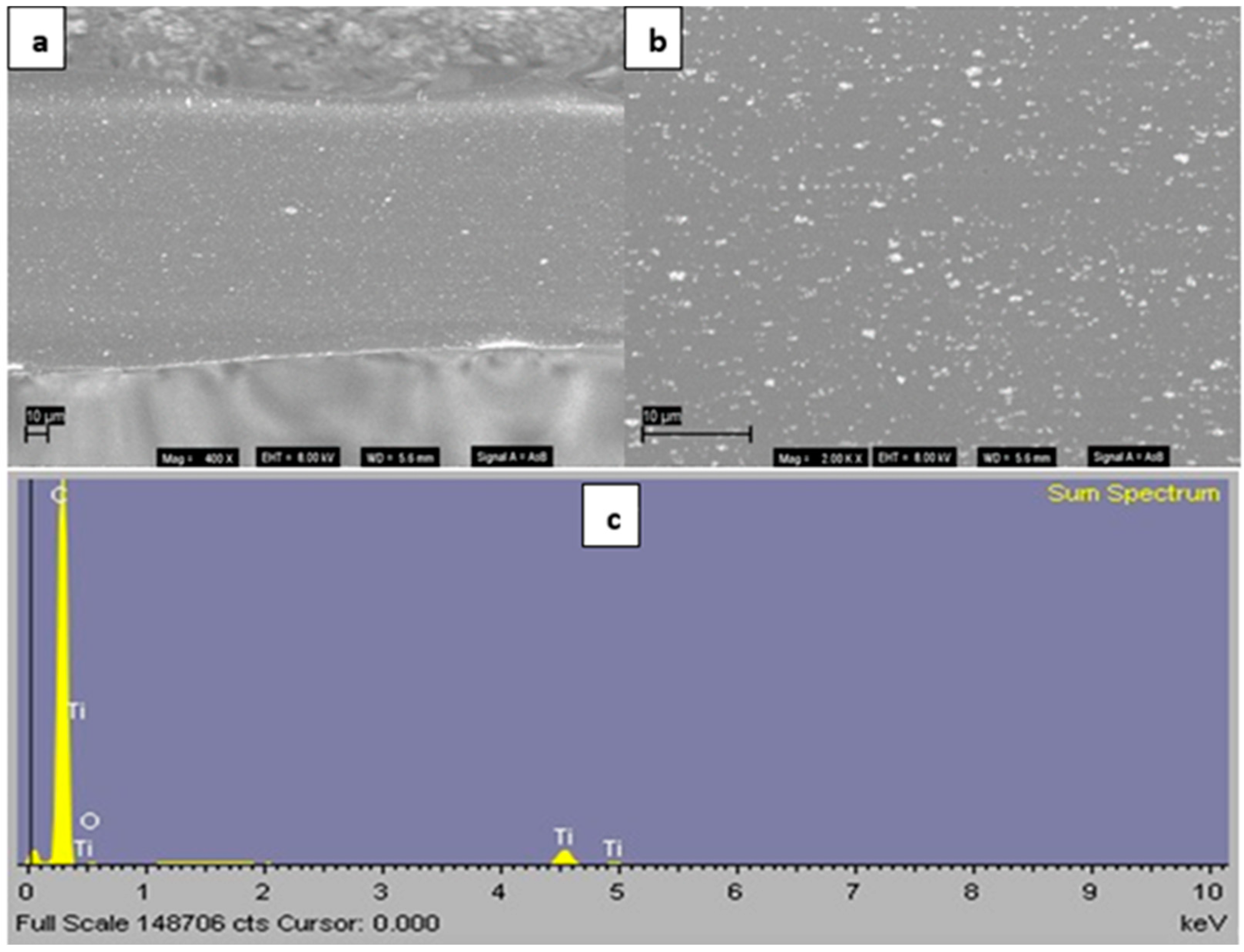
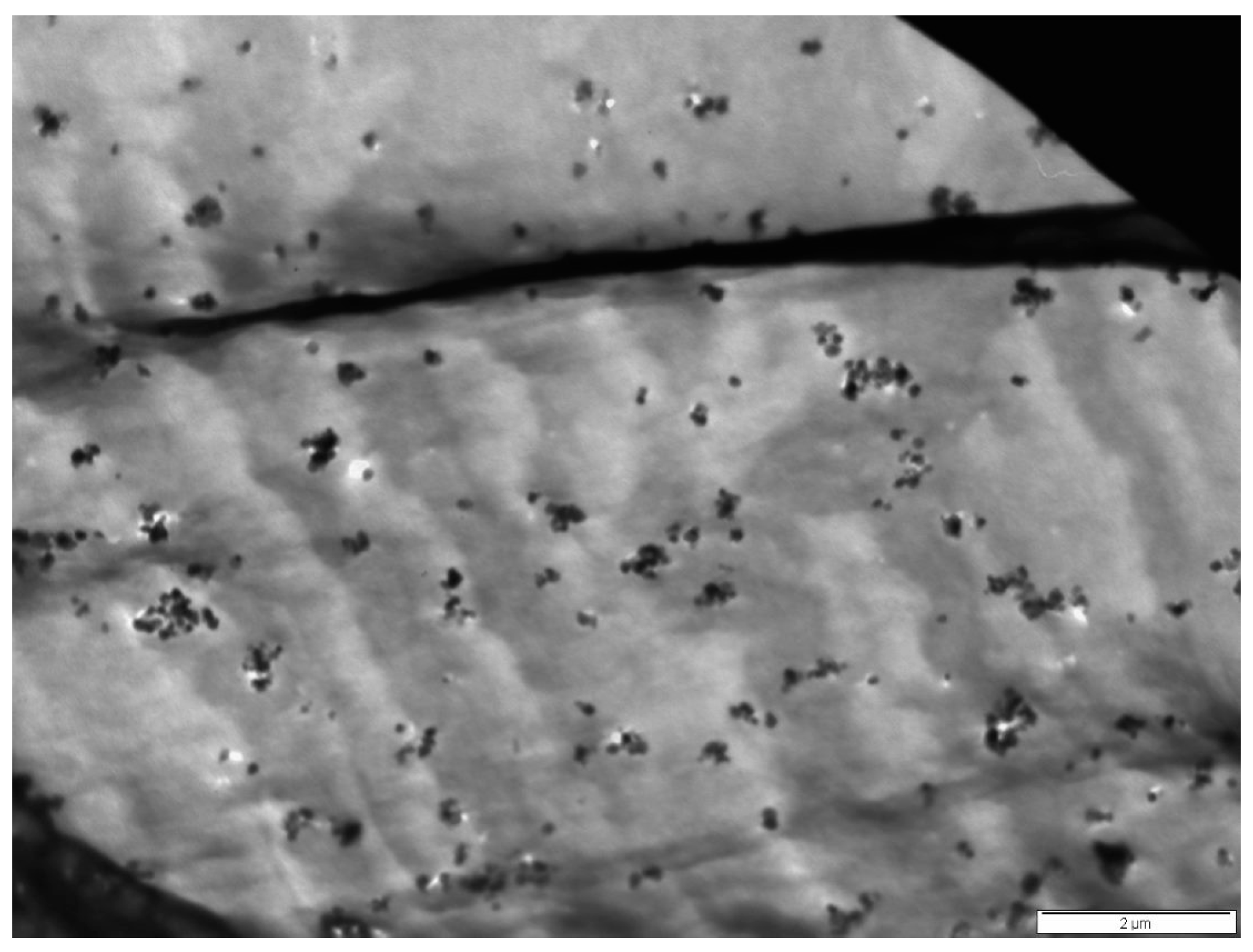
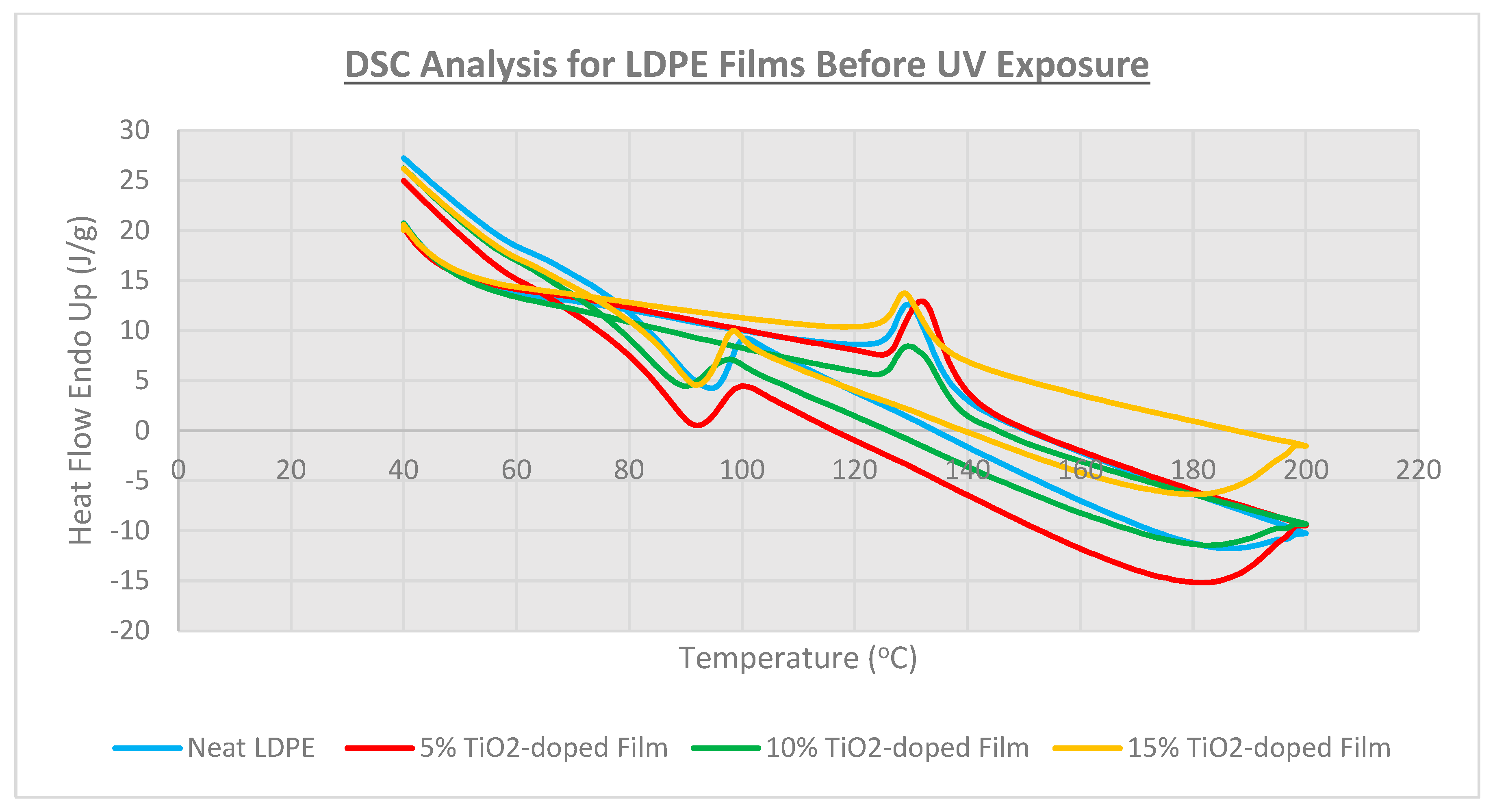
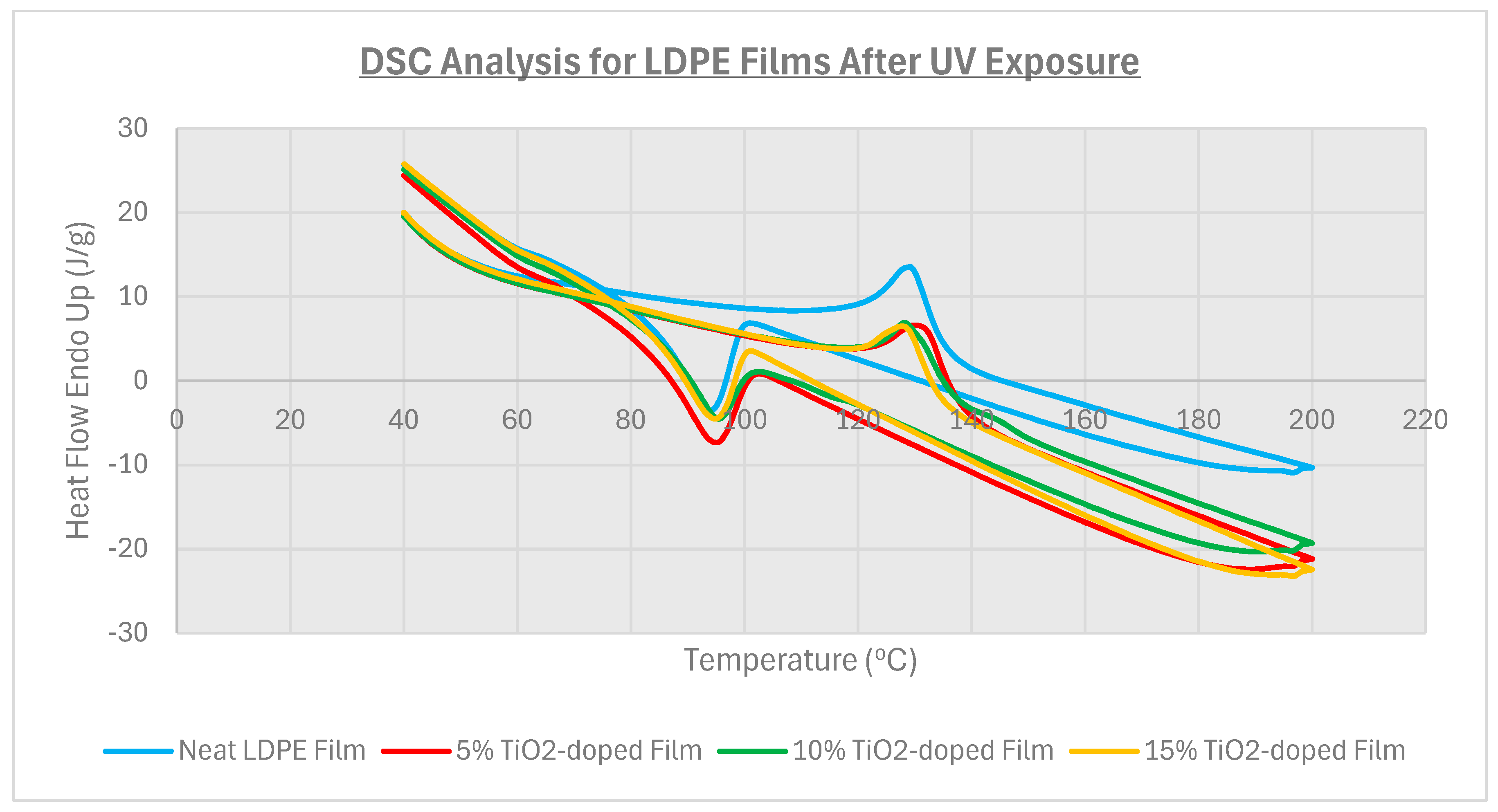
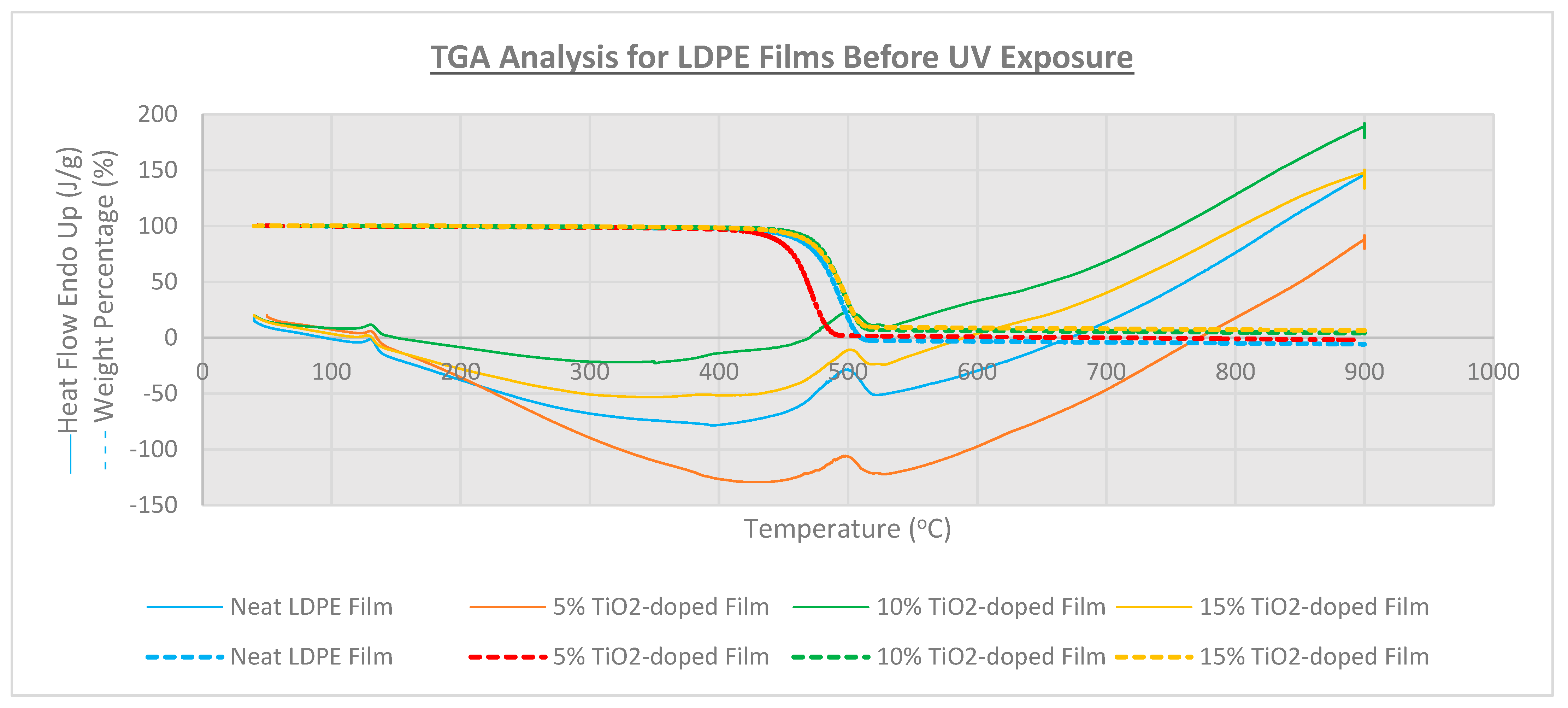
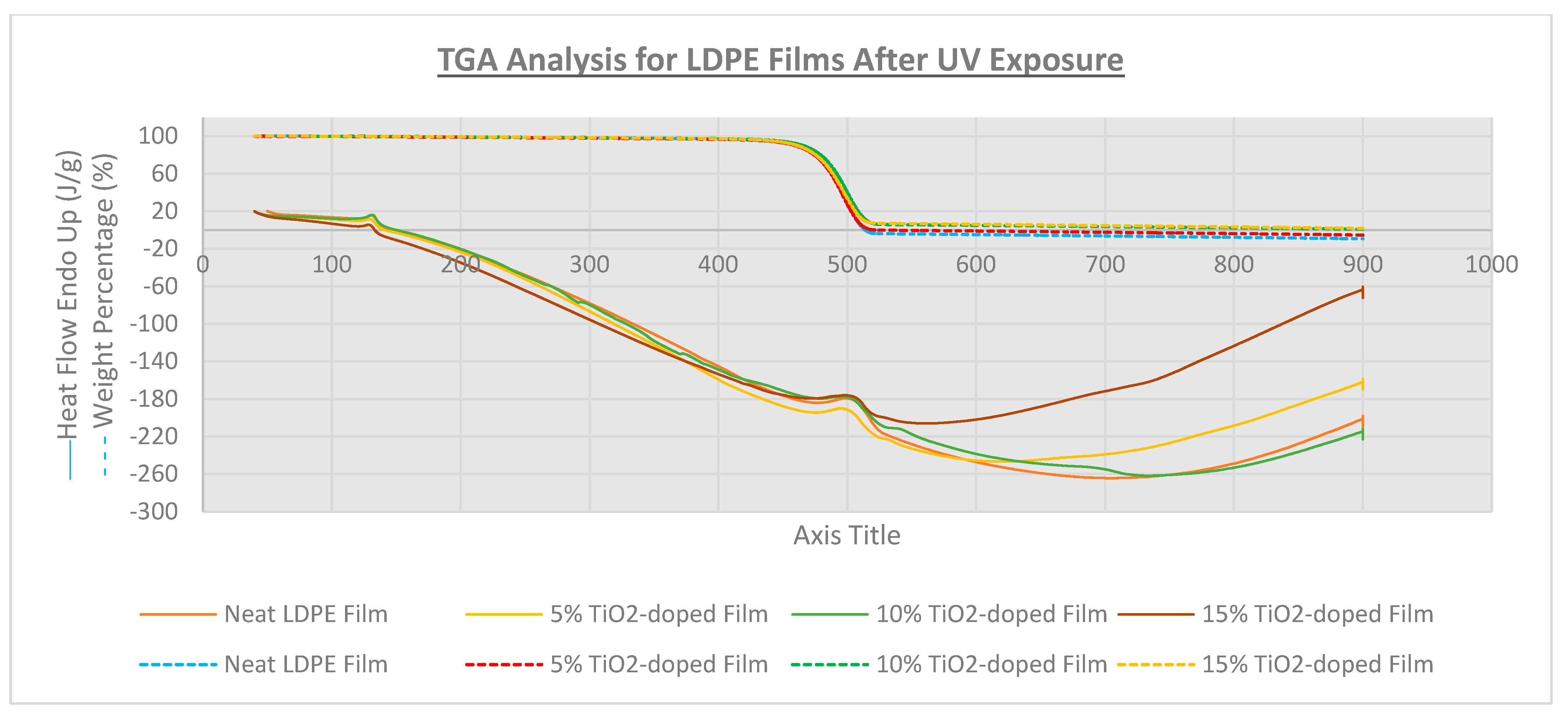

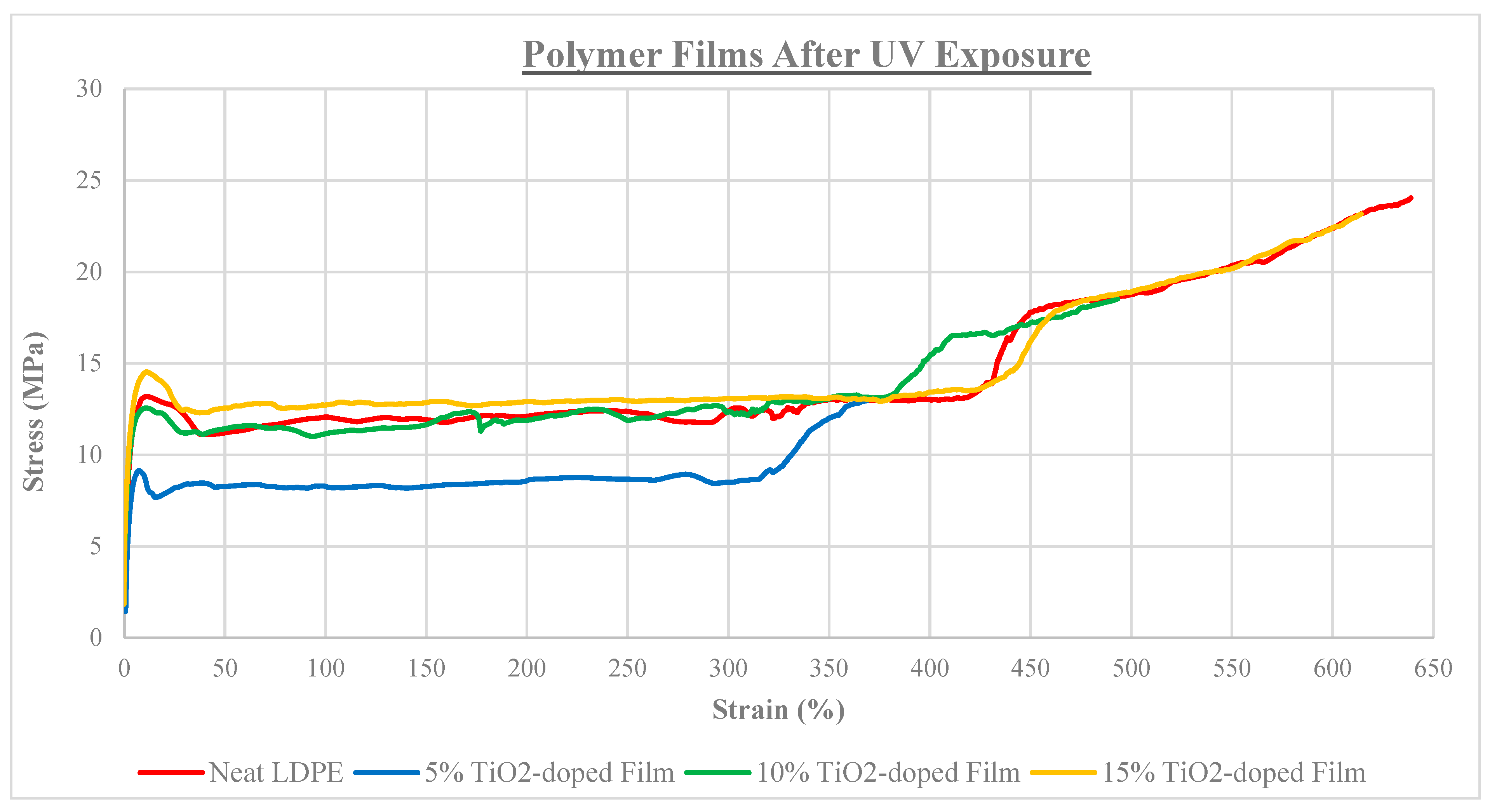
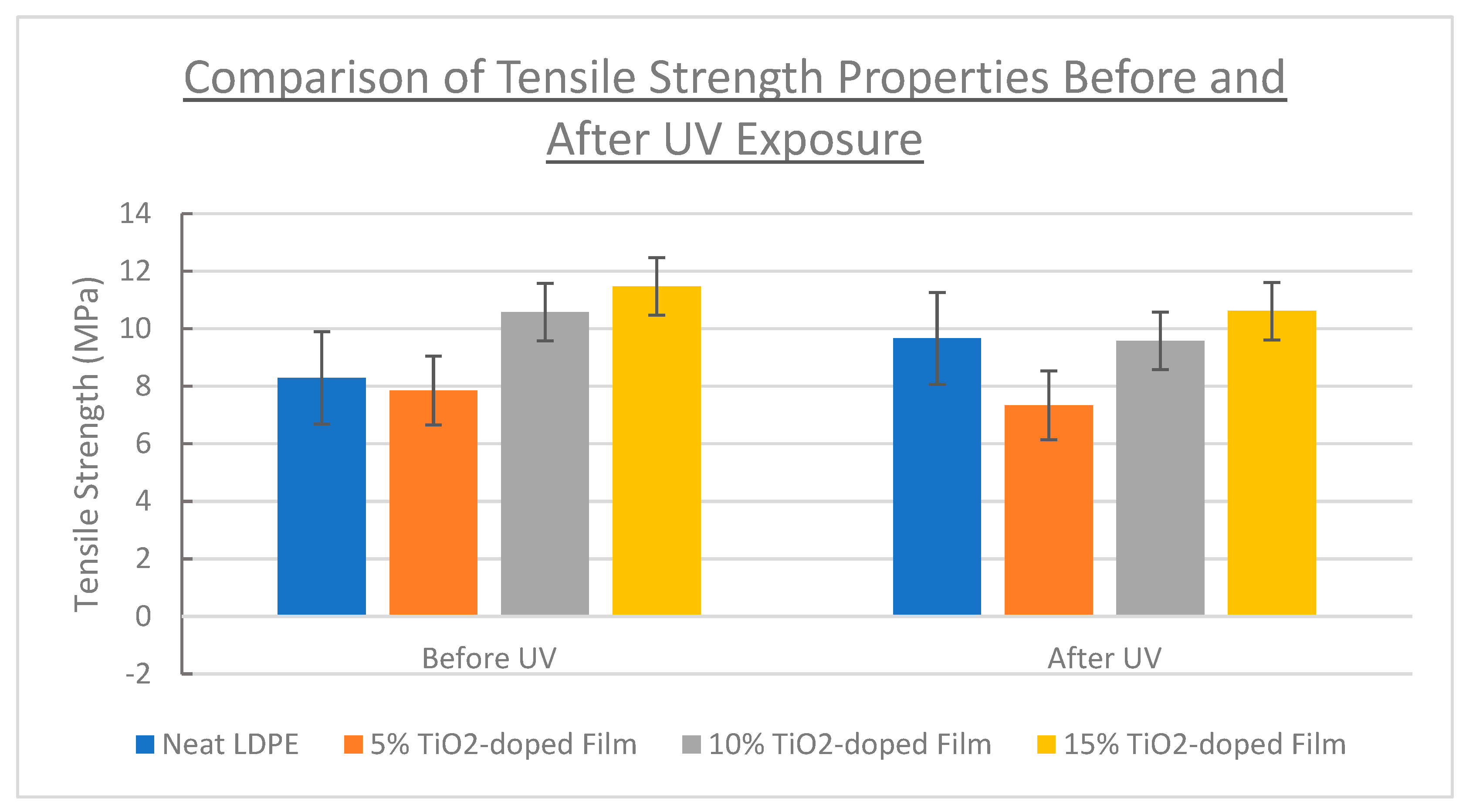
| Film Identity | Melt Onset Tc (°C) | Melt Peak Temp. Tm (°C) | Enthalpy (J/g) | Crystallinity (%) | Mass Loss (%) | Degradation Temp. (°C) |
|---|---|---|---|---|---|---|
| Before UV Exposure | ||||||
| Neat LDPE | 95.9 | 109.7 | 40 | 13.7 | - | 478 |
| 5% TiO2-doped | 93.7 | 112.1 | 39 | 13.3 | - | 478 |
| 10% TiO2-doped | 91.8 | 111.0 | 30 | 10.2 | 95.9 | 482 |
| 15% TiO2-doped | 93.7 | 109.1 | 28 | 9.6 | 93.6 | 482 |
| After UV Exposure | ||||||
| Neat LDPE | 95.9 | 109.9 | 52 | 17.7 | - | 475 |
| 5% TiO2-doped | 96.8 | 112.1 | 55 | 18.8 | - | 475 |
| 10% TiO2-doped | 97.2 | 108.8 | 46 | 15.8 | 99.0 | 474 |
| 15% TiO2-doped | 95.9 | 108.5 | 40 | 13.7 | 98.2 | 479 |
| Film Identity | Thickness (µm) | * Tensile Strength (MPa) | * Strain (%) | * Elastic Modulus (MPa) |
|---|---|---|---|---|
| Neat LDPE | 200 | 8.29 ± 1.8 | 370 ± 88 | 5.5 ± 0.4 |
| 5% TiO2-doped | 200 | 7.86 ± 1.2 | 367 ± 51 | 5.4 ± 1.0 |
| 10% TiO2-doped | 150 | 10.6 ± 1.0 | 449 ± 62 | 7.6 ± 0.7 |
| 15% TiO2-doped | 140 | 11.5 ± 1.0 | 405 ± 48 | 8.7 ± 0.6 |
| Film Identity |
Thickness µm |
* Tensile Strength
(MPa) |
* Strain
% |
* Elastic Modulus
(MPa) |
|---|---|---|---|---|
| Neat LDPE | 170 | 9.67 ± 1.7 | 438 ± 57 | 6.3±0.9 |
| 5% TiO2-doped | 200 | 7.34 ± 0.7 | 340 ± 36 | 5.5 ± 1.0 |
| 10% TiO2-doped | 140 | 9.58 ± 1.2 | 424 ± 41 | 7.3 ± 0.7 |
| 15% TiO2-doped | 130 | 10.6 ± 1.1 | 395 ± 30 | 8.5 ± 0.8 |
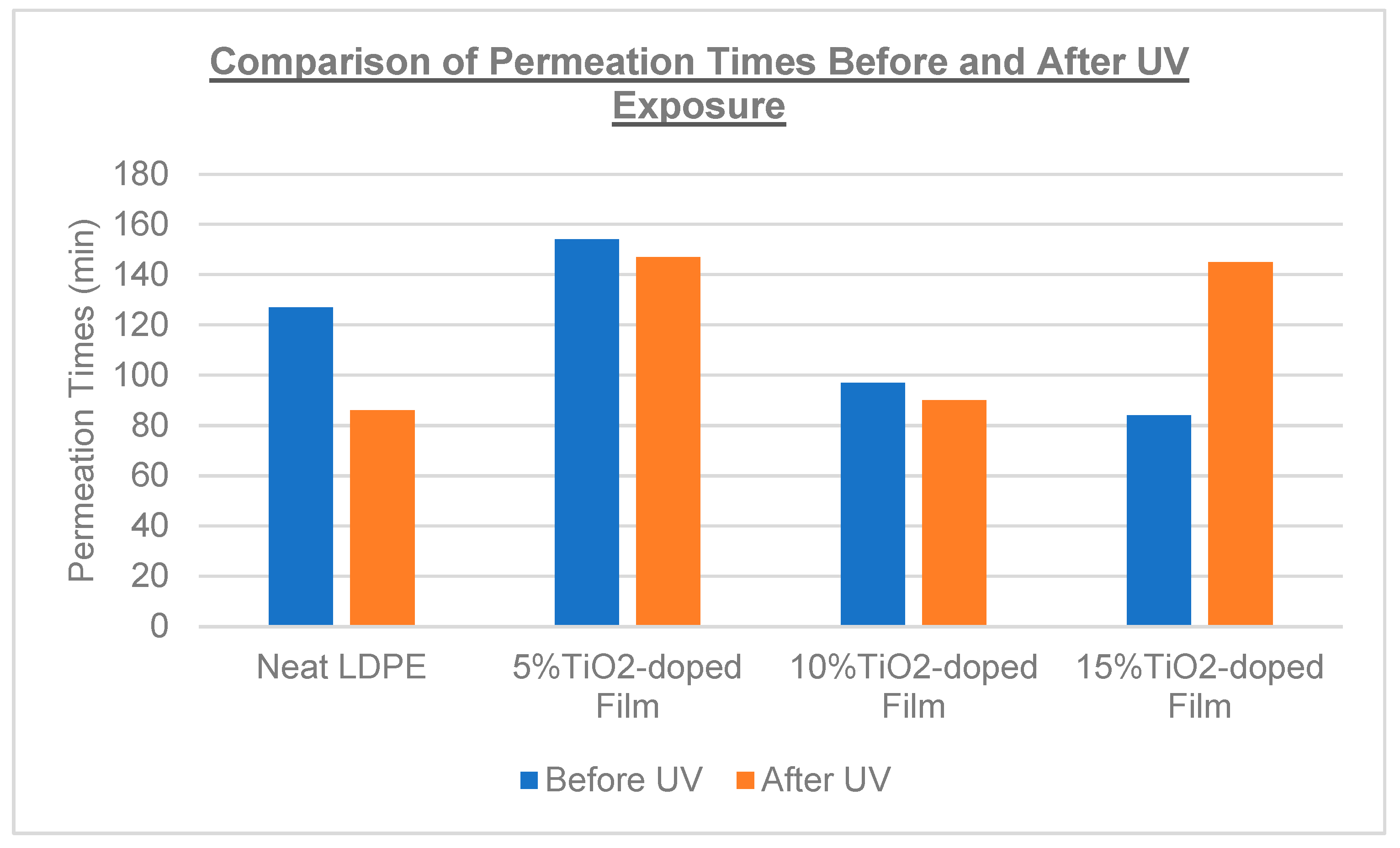
| Film Identity | Thickness (µm) | * Normalized Permeation Time (min) | Thickness (µm) | * Normalized Permeation Time (min) |
|---|---|---|---|---|
| Before UV Exposure | After UV Exposure | |||
| Neat LDPE | 160 | 127 | 150 | 86 |
| 5% TiO2-doped | 200 | >154 | 210 | >147 |
| 10% TiO2-doped | 130 | 97 | 140 | 90 |
| 15% TiO2-doped | 150 | 84 | 140 | 145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndibewu, P.P.; Lefakane, T.E.; Netshiozwi, T.E. Effects of Titanium Dioxide (TiO2) on Physico-Chemical Properties of Low-Density Polyethylene. Polymers 2024, 16, 2788. https://doi.org/10.3390/polym16192788
Ndibewu PP, Lefakane TE, Netshiozwi TE. Effects of Titanium Dioxide (TiO2) on Physico-Chemical Properties of Low-Density Polyethylene. Polymers. 2024; 16(19):2788. https://doi.org/10.3390/polym16192788
Chicago/Turabian StyleNdibewu, Peter P., Tina E. Lefakane, and Taki E. Netshiozwi. 2024. "Effects of Titanium Dioxide (TiO2) on Physico-Chemical Properties of Low-Density Polyethylene" Polymers 16, no. 19: 2788. https://doi.org/10.3390/polym16192788






