Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
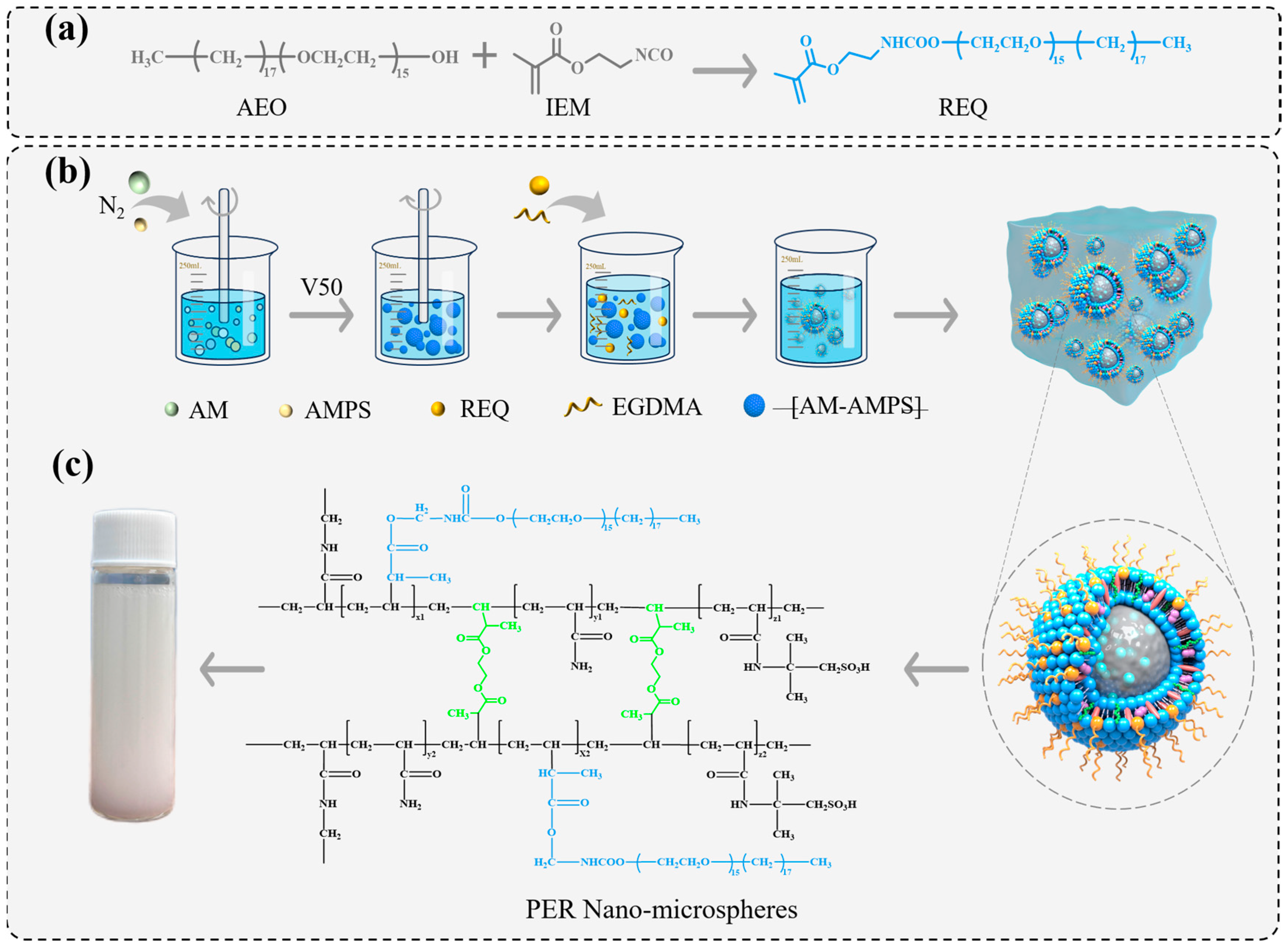
2.3. Synthesis of the REQ Monomer and PER Nanomicrospheres
2.4. Characterization and Performance Testing
2.4.1. Characterization
2.4.2. Performance Testing
2.4.3. Blocking Test
2.4.4. Oil Drive and Recovery Experiment
3. Results and Discussion
3.1. Characterization of Nanomicrospheres
3.1.1. Changes in Temperature Rise during Polymerisation
3.1.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.1.3. NMR of the Monomer REQ
3.1.4. Thermal Stability Test
3.1.5. Laser Particle Size Testing
3.1.6. Environmental SEM Microscopy Testing
3.2. PER Nanomicrospheres Performance Testing
3.2.1. Salt and Temperature Resistance Testing of PER Nanomicrospheres
3.2.2. Experiments on the Blocking Properties of Nanomicrospheres in Cores with Different Permeabilities
3.2.3. Experiments on the Blocking Properties of Nanomicrospheres in Cores for Different Concentrations
3.3. Experiments on Oil Drive and Recovery
3.3.1. Experiment on Oil Recovery and Enhancement by a Microsphere System at 2000 mg/L
3.3.2. Experiment on Oil Recovery and Enhancement by a Microsphere System at 3000 mg/L
3.3.3. Experiment on Oil Recovery and Enhancement by a Microsphere System at 4000 mg/L
3.4. PER Nanomicrospheres Enhanced Oil Recovery Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shagymgereyeva, S.; Sarsenbekuiy, B.; Kang, W.L.; Yang, H.B.; Turtabayev, S. Advances of polymer microspheres and its applications for enhanced oil recovery. Colloids Surf. B Biointerfaces 2024, 233, 113622. [Google Scholar] [CrossRef] [PubMed]
- Baffie, F.; Patias, G.; Shegiwal, A.; Brunel, F.; Monteil, V.; Verrieux, L.; Perrin, L.; Haddleton, D.M.; Dagosto, F. Block Copolymers Based on Ethylene and Methacrylates Using a Combination of Catalytic Chain Transfer Polymerisation (CCTP) and Radical Polymerisation, Angewandte Chemie International Edition. Angew. Chem. Int. Ed. 2021, 133, 25560–25568. [Google Scholar] [CrossRef]
- Preusser, C.; Chovancova, A.; Lacik, L.; Hutchinson, R. Modeling the Radical Batch Homopolymerization ofAcrylamide in Aqueous Solution. Macromol. React. Eng. 2016, 10, 490–501. [Google Scholar] [CrossRef]
- Giz, A.; Giz-Catalgil, H.; Alb, A.; Brousseau, J.L.; Reed, W. Kinetics and Mechanisms of Acrvlamide Polymerization from Absolute, Online Monitoring of Polymerization Reaction. Macromolecules 2001, 34, 1180–1191. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Li, J.J.; Wang, T.T.; Wu, Y.Y.; Luo, H.Z. Precision polymer synthesis by controlled radical polymerization: Fusing the progress from polymer chemistry and reaction engineering. Prog. Polym. Sci. 2022, 130, 101555. [Google Scholar] [CrossRef]
- Lu, R.L.; He, S.Y.; Wang, T.F.; Lai, L.; Zhao, M.W. Low-cost preparation of temperature-resistant and salt-tolerant amphiphilic carbon dots from a nonionic surfactant and its application in enhanced oil recovery. Carbon 2024, 225, 119104. [Google Scholar] [CrossRef]
- Li, X.R.; Gao, D.G.; Liu, M.C.; Zheng, L.J.; Li, P.Y. Synthesis of amphiphilic Janus SiO2/styrene butyl acrylate polymer microspheres and their application in oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132076. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.B.; Gao, K.J.; Zhou, J.S.; Zhou, Y.H.; Yang, D.Z. Preparation and performance of graphene oxide-polyacrylamide based nanoscale microsphere emulsion for profile control. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131508. [Google Scholar] [CrossRef]
- You, Q.; Wen, Q.Y.; Fang, J.C.; Guo, M.; Zhang, Q.S.; Dai, C.L. Experimental study on lateral flooding for enhanced oil recovery in bottom-water reservoir with high water cut. J. Pet. Sci. Eng. 2019, 174, 747–756. [Google Scholar] [CrossRef]
- Jia, H.; He, J.; Wang, Q.X.; Xu, Y.B.; Zhang, L.Y.; Jia, H.D.; Song, L.; Wang, Y.B.; Xie, Q.Y.; Wu, H.Y. Investigation on novel redox-responsive ferrocenyl surfactants with reversible interfacial behavior and their recycling application for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129971. [Google Scholar] [CrossRef]
- Chen, Q.B.; Zheng, J.W. Adsorption of zwitterionic oligomer-grafted silica nanoparticles on rock surface in enhanced oil recovery studied by molecular dynamics simulations. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133013. [Google Scholar] [CrossRef]
- Cao, H.; Li, Y.Q.; Gao, W.B.; Cao, J.X.; Sun, B.Y.; Zhang, J. Experimental investigation on the effect of interfacial properties of chemical flooding for enhanced heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132335. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, Y.Q.; Luan, H.X.; Gao, W.B.; Guo, Y.; Chen, Y.H. Pore scale and macroscopic visual displacement of oil-in-water emulsions for enhanced oil recovery. Chem. Eng. Sci. 2019, 197, 404–414. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Q.; Tan, S.; Wang, C.H.; Wu, Y. Mesomorphic Polymer Hydrogel Stabilizing Ionic Surfactant Self-Assembly for Fuel Cells. Ind. Eng. Chem. Res. 2022, 61, 8792–8799. [Google Scholar] [CrossRef]
- Lu, X.K.; Wang, M.R. High-Performance Nanogel-in-Oils as Emulsion Evolution Controller for Displacement Enhancement in Porous Media. ACS Appl. Mater. Interfaces 2023, 15, 49554–49566. [Google Scholar] [CrossRef]
- Scholz, C.; Ldov, A.; Pöschel, T.; Engel, M.; Löwen, H. Surfactants and rotelles in active chiralfuids. Sci. Adv. 2021, 7, eabf8998. [Google Scholar] [CrossRef]
- Durkin, T.; Barua, B.; Savagatrup, S. Modification of amphiphilic block copolymers for responsive and biologically active surfactants in complex droplets. Giant 2023, 13, 100134. [Google Scholar] [CrossRef]
- Tangparitkul, S.; Jiang, J.T.; Jeraal, M.T.; Charpentier, T.J.; Harbottle, D. Competitive Adsorption of Interfacially Active Nanoparticles and Anionic Surfactant at the Crude Oil-Water Interface. Langmuir 2023, 39, 2483–2490. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, D.; Long, Y.F.; Wang, L.Z. Highly Robust Nanogels from Thermal-Responsive Nanoparticles with Controlled Swelling for Engineering Deployments. ACS Appl. Mater. Interfaces 2023, 15, 11175–11184. [Google Scholar] [CrossRef]
- Yan, H.; Yu, X.R.; Ji, R.J. Preparation of core-shell polymer microspheres by the dry water method and their performance evaluation. Fine Chem. 2023, 40, 192–199. [Google Scholar] [CrossRef]
- Liu, J.T.; Guan, X.X.; He, T.Y. Evaluation of the performance of nanopolymer microspheres as anatomical agents. Pet. Nat. Gas Chem. Eng. 2023, 52, 77–82. [Google Scholar]
- Duan, R.; Chen, J.; Hou, G.; Ke, J. Preparation and characterization of cross-linked chlorinated polystyrene microspheres. In Applied Sciences in Graphic Communication and Packaging; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Yamauchi, A.; Shirai, A.; Kawabe, K.; Iwamoto, T.; Wakiya, T.; Nishiyama, H.; Inagi, S.; Tomita, I. Well-defined polymer microspheres formed by living dispersion polymerization: Precisely functionalized crosslinked polymer microspheres from monomers possessing cumulated double bonds. NPG Asia Mater. 2016, 8, e307. [Google Scholar] [CrossRef]
- Niu, G.; Wang, L.C.; Hua, M.Q. Adsorption performance of copper-based nitrogen-carbon microspheres on tetracycline hydrochloride in wastewater. Fine Chem. 2024, 10, 1–17. [Google Scholar] [CrossRef]
- Ju, M.; Li, Y.; Yu, L.; Wang, C.; Zhang, L. Preparation of size-controllable monodispersed carbon@silica core-shell microspheres and hollow silica microspheres. Microporous Mesoporous Mater. 2017, 247, 75–85. [Google Scholar] [CrossRef]
- Ye, J.; Ru, Y.; Weng, H.; Fu, L.; Chen, J.; Chen, F.; Xiao, Q.; Xiao, A. Rational design of agarose/dextran composite microspheres with tunable core–shell microstructures for chromatographic application. Int. J. Biol. Macromol. 2024, 263, 130051. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.S.; Liu, F.; Cheng, R.; Bai, Y.; Wang, R.; Wang, J.; Geng, Y.; Jin, Y.; Ma, Z. Micro-nano polymer microspheres as a blocking agent in oil-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131808. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, H.; Chen, C.; Wang, R.; Su, L.; Guo, B. Synthesis and characterization of phase change materials microcapsules with paraffin core/cross-linked hybrid polymer shell for thermal energy storage. J. Energy Storage 2020, 32, 101897. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, W.; Zhou, H.; Wang, C.; Fu, S. Morphological investigation of styrene and acrylamide polymer microspheres prepared by dispersion copolymerization. Colloid. Polym. Sci. 2000, 278, 509–516. [Google Scholar] [CrossRef]
- Shi, X.D.; Yue, X.A. Migration and blocking mechanisms of self-aggregated microspheres as a novel profile control. J. Pet. Sci. Eng. 2020, 184, 106458. [Google Scholar] [CrossRef]
- Wang, H.Y. Study on the Relationship between Structure and Properties of Acrylamide Copolymerized Microspheres. Ph.D. Thesis, China University of Petroleum, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, R.; Zhou, J.; Chen, H.; Tan, Y. A novel hyper-cross-linked polymer for high-efficient fluid-loss control in oil-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127004. [Google Scholar] [CrossRef]
- Davoodi, S.; Sa, A.R.; Jamshidi, S.; Jahromi, A.F. A novel field applicable mud formula with enhanced fluid loss properties in high pressure-high temperature well condition containing pistachio shell powder. J. Pet. Sci. Eng. 2018, 162, 378–385. [Google Scholar] [CrossRef]
- Du, W.H.; Wu, P.W.; Zhao, Z.X.; Zhang, X. Facile preparation and characterization of temperature-responsive hydrophilic crosslinked polymer microspheres by aqueous dispersion polymerization. Eur. Polym. J. 2020, 128, 109610. [Google Scholar] [CrossRef]
- Ahmadi, M.; Aliabadian, E.; Liu, B.J.; Lei, X.T.; Khalipoorkordi, P.; Hou, Q.F.; Wang, Y.Y.; Chen, Z.X. Comprehensivereviw of the interfacial behavior of water/oil/surfactant systems using dissipative particle dynamics simulation. Adv. Colloid Interface Sci. 2022, 309, 102774. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Guo, J.; Yang, X.J.; Nicolai, T. Water-in-water-in-water emulsions formed by cooling mixtures of guar, amylopectin and gelatin. Food Hydrocoll. 2021, 118, 106763. [Google Scholar] [CrossRef]
- Tangparitkul, S.; Sukee, A.; Jiang, J.T.; Harbottle, D. Crude oil displacement enhanced by interfacially active nanoparticles and their coupling effect with low-salinity brines. J. Mol. Liq. 2024, 408, 125362. [Google Scholar] [CrossRef]







| Permeability (×10−3 µm2). | Pressure Measurement Interval | Resistance Coefficient, Rf | Residual Resistance Coefficient, Rff | Blocking Rate (%) |
|---|---|---|---|---|
| 800 | P0–P1 | 4.51 | 7.82 | 86.07 |
| P1–P2 | ||||
| P2–P3 | ||||
| P3–P4 | ||||
| 2000 | P0–P1 | 3.47 | 6.01 | 79.13 |
| P1–P2 | ||||
| P2–P3 | ||||
| P3–P4 | ||||
| 4000 | P0–P1 | 2.55 | 4.53 | 68.21 |
| P1–P2 | ||||
| P2–P3 | ||||
| P3–P4 |
| Concentration (mg/L) | Pressure Measurement Interval | Resistance Coefficient, Rf | Residual Resistance Coefficient, Rff | Blocking Rate (%) |
|---|---|---|---|---|
| 2000 | P0–P1 | 4.33 | 8.70 | 83.03 |
| P1–P2 | ||||
| P2–P3 | ||||
| P3–P4 | ||||
| 3000 | P0–P1 | 5.60 | 8.23 | 86.77 |
| P1–P2 | ||||
| P2–P3 | ||||
| P3–P4 | ||||
| 4000 | P0–P1 | 5.71 | 9.75 | 90.32 |
| P1–P2 | ||||
| P2–P3 | ||||
| P3–P4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Lai, X.; Wang, L.; Shi, H.; Li, H.; Chen, J.; Wen, X.; Li, Y.; Song, X.; Wang, W. Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking. Polymers 2024, 16, 2884. https://doi.org/10.3390/polym16202884
Yang W, Lai X, Wang L, Shi H, Li H, Chen J, Wen X, Li Y, Song X, Wang W. Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking. Polymers. 2024; 16(20):2884. https://doi.org/10.3390/polym16202884
Chicago/Turabian StyleYang, Wenwen, Xiaojuan Lai, Lei Wang, Huaqiang Shi, Haibin Li, Jiali Chen, Xin Wen, Yulong Li, Xiaojiang Song, and Wenfei Wang. 2024. "Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking" Polymers 16, no. 20: 2884. https://doi.org/10.3390/polym16202884
APA StyleYang, W., Lai, X., Wang, L., Shi, H., Li, H., Chen, J., Wen, X., Li, Y., Song, X., & Wang, W. (2024). Synthesis of Polyacrylamide Nanomicrospheres Modified with a Reactive Carbamate Surfactant for Efficient Profile Control and Blocking. Polymers, 16(20), 2884. https://doi.org/10.3390/polym16202884






