Investigation into the Internal Factors for the Catalytic Oxidation of Cyclohexane by Zr(IV)-Based Metal-Organic Frameworks
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
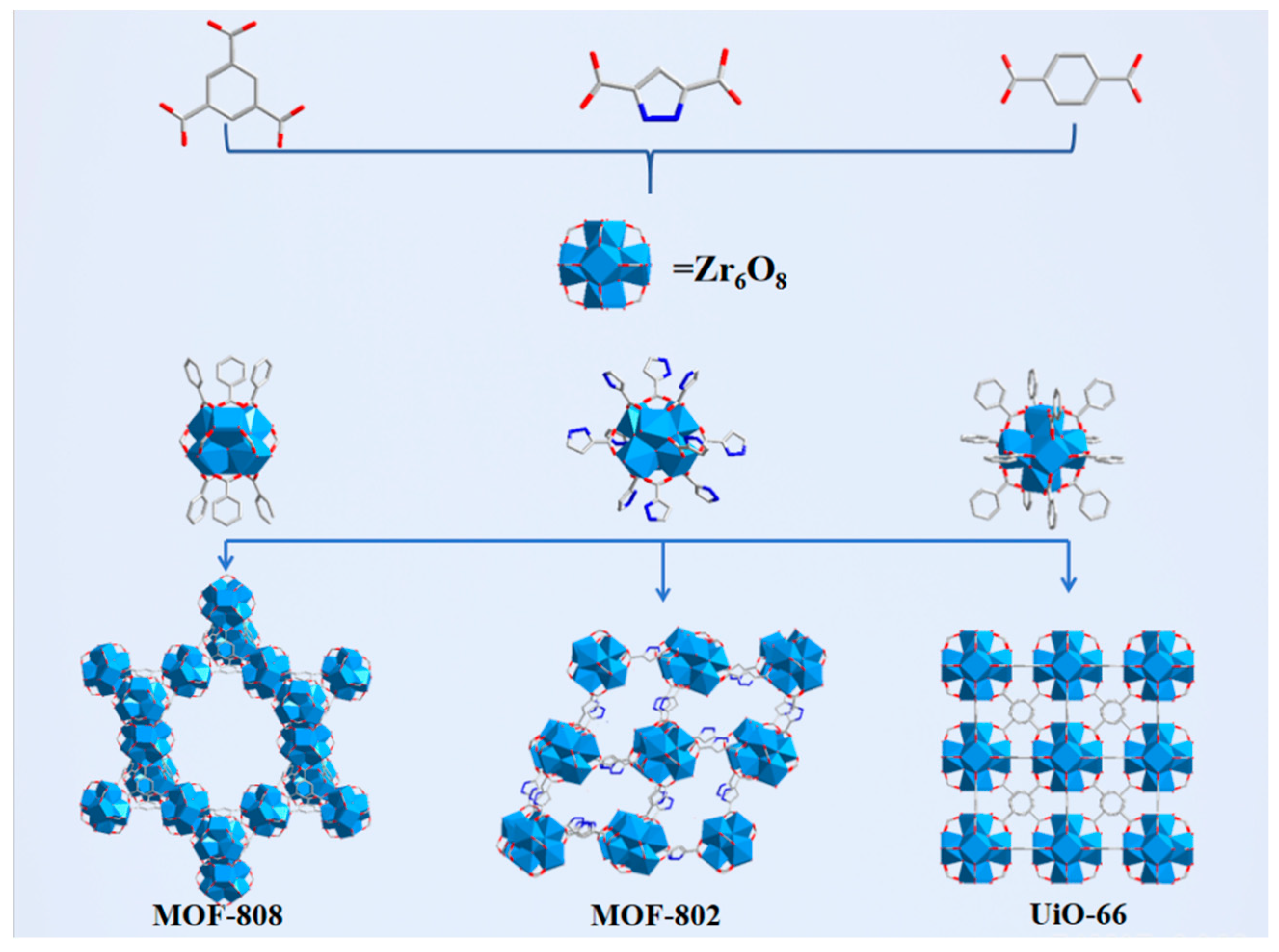
2.2. Catalyst Syntheses
2.3. Characterizations
2.4. Catalytic Test
2.5. Grand Canonical Monte Carlo (GCMC) Simulations
2.6. Free-Radical Capture Methods
3. Results and Discussion
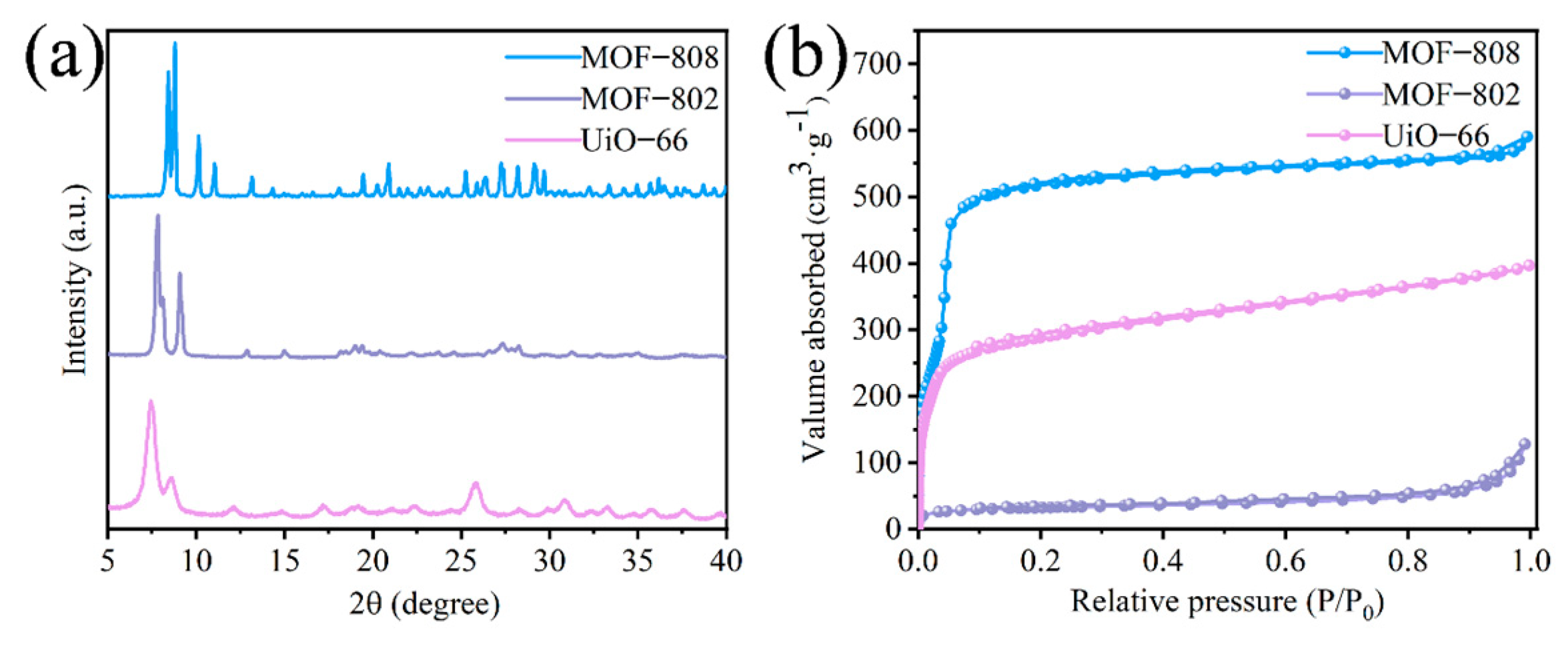
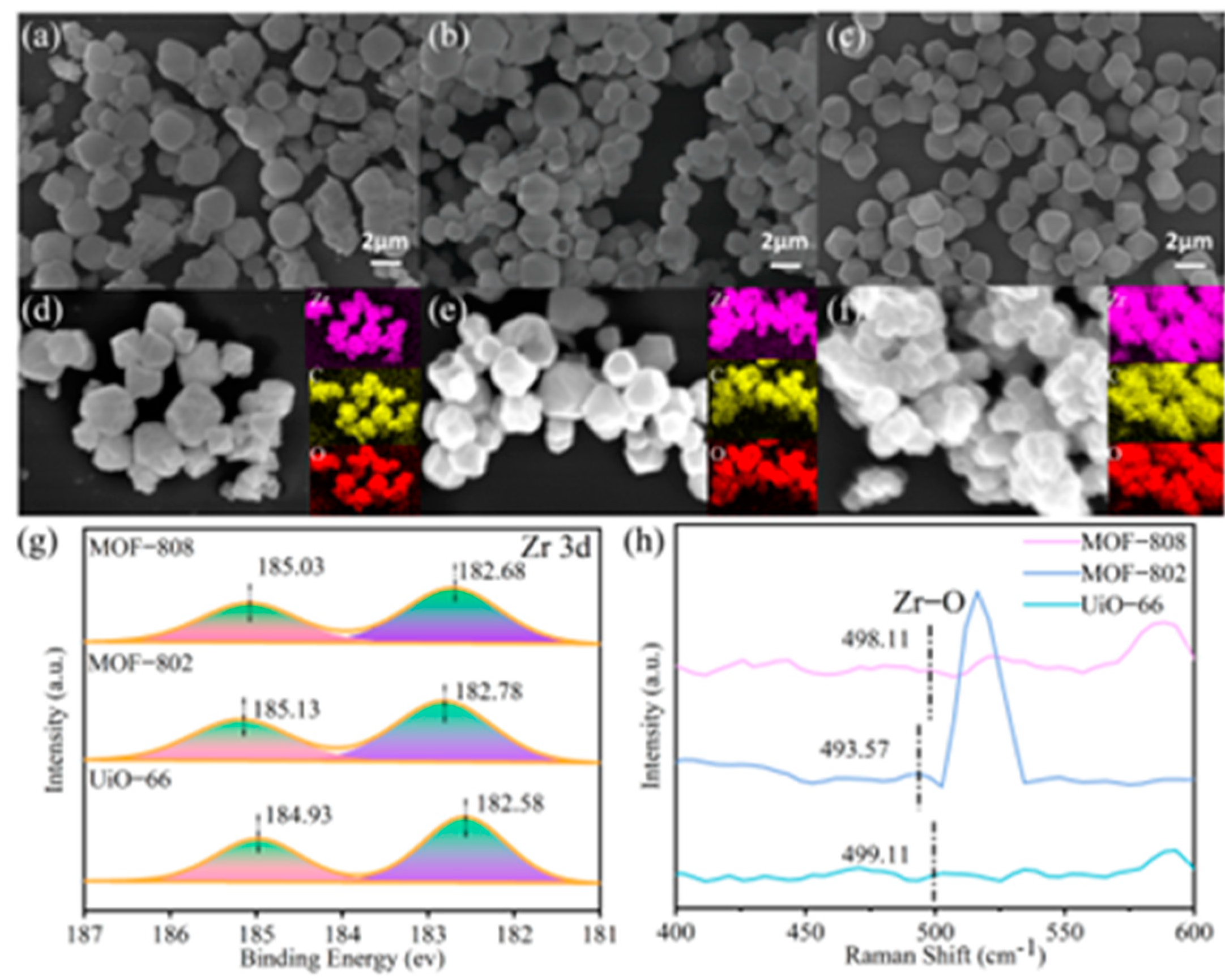
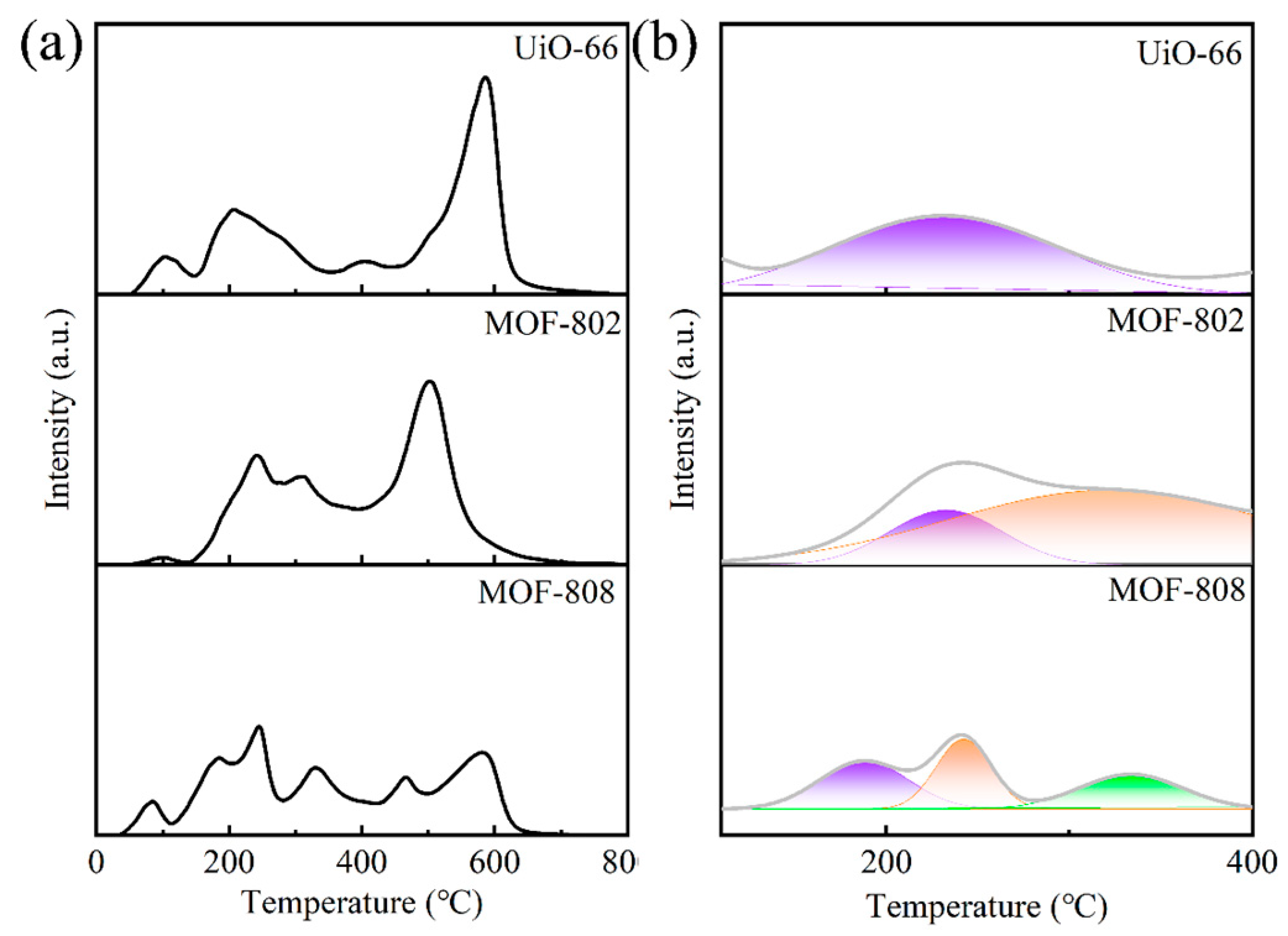
3.1. Characterization of Catalysts
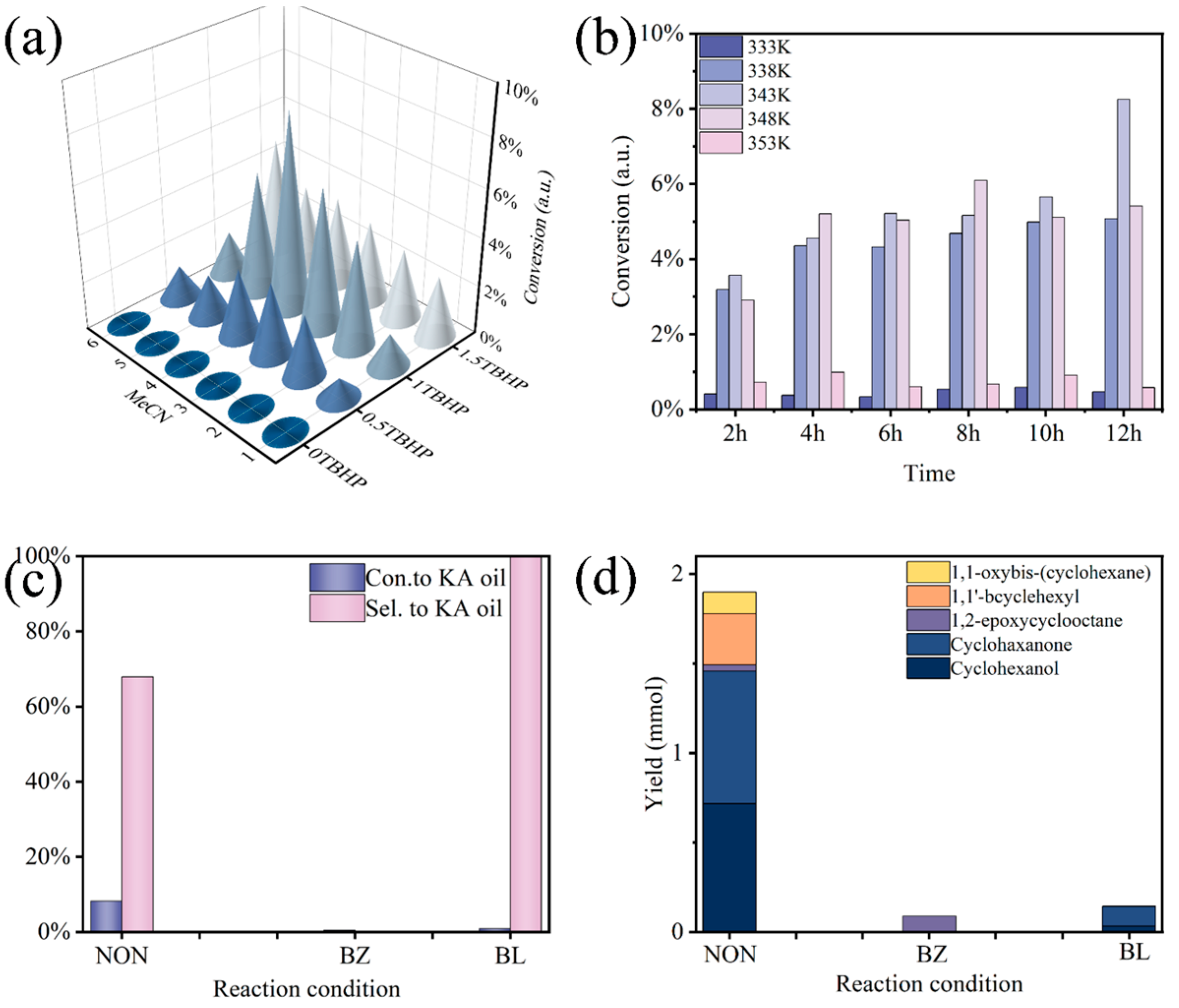
3.2. Investigation of the Effect of Different Linkage Numbers
3.3. Investigation of the Effect of Solvent and Temperature
3.4. Free-Radical Capture Experiments
3.5. Catalytic Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal-organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef]
- Xu, C.; Pan, Y.; Wan, G.; Liu, H.; Wang, L.; Zhou, H.; Yu, S.H.; Jiang, H.L. Turning on Visible-Light Photocatalytic C-H Oxidation over Metal-Organic Frameworks by Introducing Metal-to-Cluster Charge Transfer. J. Am. Chem. Soc. 2019, 141, 19110–19117. [Google Scholar] [CrossRef]
- Sun, Z.B.; Si, Y.N.; Zhao, S.N.; Wang, Q.Y.; Zang, S.Q. Ozone Decomposition by a Manganese-Organic Framework over the Entire Humidity Range. J. Am. Chem. Soc. 2021, 143, 5150–5157. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Z.; Zhang, L.; Liu, J.; Huang, Q.; Lu, M.; Ji, W.X.; Lan, Y.Q. Stable Heterometallic Cluster-Based Organic Framework Catalysts for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2020, 59, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mukherjee, S.; Zahid Hussain, M.; Ye, S.; Wang, X.; Li, W.; Cao, R.; Elsner, M.; Fischer, R.A. Porphyrin-based MOFs for sensing environmental pollutants. Chem. Eng. J. 2024, 492, 152377. [Google Scholar] [CrossRef]
- Lin, Y.; Li, W.H.; Wen, Y.; Wang, G.E.; Ye, X.L.; Xu, G. Layer-by-Layer Growth of Preferred-Oriented MOF Thin Film on Nanowire Array for High-Performance Chemiresistive Sensing. Angew. Chem. Int. Ed. 2021, 60, 25758–25761. [Google Scholar] [CrossRef] [PubMed]
- Kasper, T.; Pavan, M.; Müller-Buschbaum, K. On the validity of rapid optical sensing of dioxygen by means of sensitivity, stability, and reversibility for archetype MOFs post-synthetically modified with Eu3+. J. Mater. Chem. A 2024, 12, 769–780. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Han, Y.; Liu, L.; Han, Z.; Wu, M. Engineering ethane-trapping metal-organic framework for efficient ethylene separation under high humid conditions. Sep. Purif. Technol. 2024, 342, 127011. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, Y.; Yun, Y.S. Highly Effective Removal of Nonsteroidal Anti-Inflammatory Pharmaceuticals from Water by Zr(IV)-Based Metal-Organic Framework: Adsorption Performance and Mechanisms. ACS Appl. Mater. Interfaces 2018, 10, 28076–28085. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Alinia Asli, M.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Hammouda, S.B.; Doshi, B.; Guijarro, N.; Min, X.; Tang, C.J.; Sillanpää, M.; Sivula, K.; Wang, S. MIL-101(Fe)/g-C3N4 for enhanced visible-light-driven photocatalysis toward simultaneous reduction of Cr(VI) and oxidation of bisphenol A in aqueous media. Appl. Catal. B Environ. 2020, 272, 119033. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Wang, H.; Chen, Y.; Zhang, M. MOF-74 as an Efficient Catalyst for the Low-Temperature Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2016, 8, 26817–26826. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.H.M.; Henry, N.; Volkringer, C.; Loiseau, T.; Vezin, H.; Hureau, M.; Moissette, A. Iodine Uptake by Zr-/Hf-Based UiO-66 Materials: The Influence of Metal Substitution on Iodine Evolution. ACS Appl. Mater. Interfaces 2022, 14, 29916–29933. [Google Scholar] [CrossRef] [PubMed]
- Bon, V.; Senkovska, I.; Baburin, I.A.; Kaskel, S. Zr- and Hf-Based Metal-Organic Frameworks: Tracking Down the Polymorphism. Cryst. Growth Des. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal-Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, J.; Zhang, X.; Liao, Y.; Wang, R.; Zhang, K.; Lyu, J.; Farha, O.K.; Hupp, J.T. Node-Accessible Zirconium MOFs. J. Am. Chem. Soc. 2020, 142, 21110–21121. [Google Scholar] [CrossRef]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, Structure, and Metalation of Two New Highly Porous Zirconium Metal-Organic Frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, Y.; Tan, Y.; Zhao, X.; Zhang, Y.; Cheng, C.; Yang, M. Porphyrinic Metal-Organic Framework PCN-224 Nanoparticles for Near-Infrared-Induced Attenuation of Aggregation and Neurotoxicity of Alzheimer’s Amyloid-β Peptide. ACS Appl. Mater. Interfaces 2018, 10, 36615–36621. [Google Scholar] [CrossRef]
- Ji, P.; Drake, T.; Murakami, A.; Oliveres, P.; Skone, J.H.; Lin, W. Tuning Lewis Acidity of Metal-Organic Frameworks via Perfluorination of Bridging Ligands: Spectroscopic, Theoretical, and Catalytic Studies. J. Am. Chem. Soc. 2018, 140, 10553–10561. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Hu, J.N.; Tian, L.C.; Liang, J.X.; Lin, J.; Li, L.; Zhu, C.; Wang, X. Zirconium-oxo Nodes of MOFs with Tunable Electronic Properties Provide Effective OH Species for Enhanced Methane Hydroxylation. Angew. Chem. Int. Ed. 2022, 61, e202205077. [Google Scholar] [CrossRef] [PubMed]
- Vomeri, A.; Stucchi, M.; Villa, A.; Evangelisti, C.; Beck, A.; Prati, L. New insights for the catalytic oxidation of cyclohexane to K-A oil. J. Energy Chem. 2022, 70, 45–51. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, B.; Wang, F.; Yan, Y.; Hong, X.; Xu, H.; Xia, M.; Wang, F. Green synthesis of MOF-808 with modulation of particle sizes and defects for efficient phosphate sequestration. Sep. Purif. Technol. 2022, 300, 181825. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Kong, Y.R.; Luo, H.B.; Liu, M.; Liu, Y.; Ren, X.M. Efficiently Boosting Moisture Retention Capacity of Porous Superprotonic Conducting MOF-802 at Ambient Humidity via Forming a Hydrogel Composite Strategy. ACS Appl. Mater. Interfaces 2021, 13, 37231–37238. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Y.; Bediako, J.K.; Cho, C.W.; Sarkar, A.K.; Lim, C.R.; Yun, Y.S. Structure-controlled recovery of palladium(II) from acidic aqueous solution using metal-organic frameworks of MOF-802, UiO-66 and MOF-808. Chem. Eng. J. 2019, 362, 280–286. [Google Scholar] [CrossRef]
- Lv, H.J.; Zhang, J.W.; Jiang, Y.C.; Li, S.N.; Hu, M.C.; Zhai, Q.G. Micropore Regulation in Ultrastable [Sc3O]-Organic Frameworks for Acetylene Storage and Purification. Inorg. Chem. 2022, 61, 3553–3562. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, Z.; Yu, L.; Huang, J.; Shen, S.; Zhao, S.; Wu, Y.; Tian, X.; Wang, J.; Xia, Q. Boosting trace SO2 adsorption and separation performance by the modulation of the SBU metal component of iron-based bimetal MOFs. J. Mater. Chem. A 2023, 11, 14728–14737. [Google Scholar] [CrossRef]
- He, X.; Looker, B.G.; Dinh, K.T.; Stubbs, A.W.; Chen, T.; Meyer, R.J.; Serna, P.; Román-Leshkov, Y.; Lancaster, K.M.; Dincă, M. Cerium(IV) Enhances the Catalytic Oxidation Activity of Single-Site Cu Active Sites in MOFs. ACS Catal. 2020, 10, 7820–7825. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Chen, S.; Hou, X.; Liu, B.; Lu, J.; Jiang, H.L. Boosting selective oxidation of cyclohexane over a metal-organic framework by hydrophobicity engineering of pore walls. Chem. Commun. 2017, 53, 10026–10029. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Long, J.; Huang, J.; Yu, G.; Wang, Y. Can the commonly used quenching method really evaluate the role of reactive oxygen species in pollutant abatement during catalytic ozonation? Water Res. 2022, 215, 118275. [Google Scholar] [CrossRef]
- Shen, H.M.; Wang, X.; Ning, L.; Guo, A.B.; Deng, J.H.; She, Y.B. Efficient oxidation of cycloalkanes with simultaneously increased conversion and selectivity using O2 catalyzed by metalloporphyrins and boosted by Zn(AcO)2: A practical strategy to inhibit the formation of aliphatic diacids. Appl. Catal. A Gen. 2021, 609, 117904. [Google Scholar] [CrossRef]
- He, H.H.; Yuan, J.P.; Cai, P.Y.; Wang, K.Y.; Feng, L.; Kirchon, A.; Li, J.; Zhang, L.L.; Zhou, H.-C.; Fang, Y. Yolk-Shell and Hollow Zr/Ce-UiO-66 for Manipulating Selectivity in Tandem Reactions and Photoreactions. J. Am. Chem. Soc. 2023, 145, 17164–17175. [Google Scholar] [CrossRef] [PubMed]
- Montjoy, D.G.; Wilson, E.A.K.; Hou, H.; Graves, J.D.; Kotov, N.A. Photocatalytic cyclohexane oxidation and epoxidation using hedgehog particles. Nat. Commun. 2023, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Z.; Zou, X.; Zhang, Z.; Fu, G.; Li, L.; Zhang, X.; Luo, F. Enhancing catalytic aerobic oxidation performance of cyclohexaneviasize regulation of mixed-valence {V16} cluster-based metal–organic frameworks. New J. Chem. 2019, 43, 14527–14535. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Ji, G.; He, C.; Liu, S.; Duan, C. Vanadium(VIV)–Porphyrin-Based Metal–Organic Frameworks for Synergistic Bimetallic Activation of Inert C(sp3)–H Bonds. ACS Appl. Mater. Interfaces 2022, 14, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic Oxidation of Cyclohexane Catalyzed by Size-Controlled Au Clusters on Hydroxyapatite: Size Effect in the Sub-2 nm Regime. ACS Catal. 2010, 1, 2–6. [Google Scholar] [CrossRef]
- Li, X.-H.; Chen, J.-S.; Wang, X.; Sun, J.; Antonietti, M. Metal-Free Activation of Dioxygen by Graphene/g-C3N4Nanocomposites: Functional Dyads for Selective Oxidation of Saturated Hydrocarbons. J. Am. Chem. Soc. 2011, 133, 8074–8077. [Google Scholar] [CrossRef]
- Ji, G.; Zhao, L.; Wang, Y.; Tang, Y.; He, C.; Liu, S.; Duan, C. A Binuclear Cerium-Based Metal–Organic Framework as an Artificial Monooxygenase for the Saturated Hydrocarbon Aerobic Oxidation with High Efficiency and High Selectivity. ACS Catal. 2022, 12, 7821–7832. [Google Scholar] [CrossRef]



| Catalyst | Connected | Con. | Sel. | TON |
|---|---|---|---|---|
| MOF-808 | 6 | 8.30% | 98% | 39.9 |
| MOF-802 | 10 | 0.53% | 83% | 3.27 |
| UiO-66 | 12 | 0.36% | 76% | 2.24 |
| Temperature | Con. | Sel. | TON | |
|---|---|---|---|---|
| A | K | |||
| 60 °C | 0.47% | 38.93% | 36.87% | 2.93 |
| 65 °C | 5.08% | 41.65% | 39.57% | 31.59 |
| 70 °C | 8.24% | 31.06% | 32.08% | 39.28 |
| 75 °C | 3.68% | 29.13% | 44.46% | 22.74 |
| 80 °C | 0.58% | 20.54% | 61.56% | 3.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Tao, Z.-P.; Chu, J.-Q.; Wang, S.-M.; Han, Z.-B. Investigation into the Internal Factors for the Catalytic Oxidation of Cyclohexane by Zr(IV)-Based Metal-Organic Frameworks. Polymers 2024, 16, 3114. https://doi.org/10.3390/polym16223114
Wang K, Tao Z-P, Chu J-Q, Wang S-M, Han Z-B. Investigation into the Internal Factors for the Catalytic Oxidation of Cyclohexane by Zr(IV)-Based Metal-Organic Frameworks. Polymers. 2024; 16(22):3114. https://doi.org/10.3390/polym16223114
Chicago/Turabian StyleWang, Kechao, Zhi-Peng Tao, Jia-Qi Chu, Shi-Ming Wang, and Zheng-Bo Han. 2024. "Investigation into the Internal Factors for the Catalytic Oxidation of Cyclohexane by Zr(IV)-Based Metal-Organic Frameworks" Polymers 16, no. 22: 3114. https://doi.org/10.3390/polym16223114
APA StyleWang, K., Tao, Z.-P., Chu, J.-Q., Wang, S.-M., & Han, Z.-B. (2024). Investigation into the Internal Factors for the Catalytic Oxidation of Cyclohexane by Zr(IV)-Based Metal-Organic Frameworks. Polymers, 16(22), 3114. https://doi.org/10.3390/polym16223114








