Magnetic Molecularly Imprinted Polymer Combined with Solid-Phase Extraction for Purification of Schisandra chinensis Lignans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Evaluation of Mt-MIPs
2.2.1. Chromatographic Circumstances
2.2.2. Preparation of DES Functional Monomers
2.2.3. Optimization of Polymer Preparation Conditions
2.2.4. Synthesis of Mt-MIPs
2.3. Preparation and Evaluation of St-MIPs
2.3.1. Vinyl-Modified Fe3O4 Was Prepared in One Step
2.3.2. Synthesis of St-MIPs
2.4. Characterization Methods of Mt-MIP and St-MIP
2.5. Adsorption Performance Evaluation of Mt-MIP and St-MIP
2.6. S. chinensis Lignans Separation by St-MIP Combined with SPE
3. Results
3.1. Standard Curves of S. chinensis Lignans
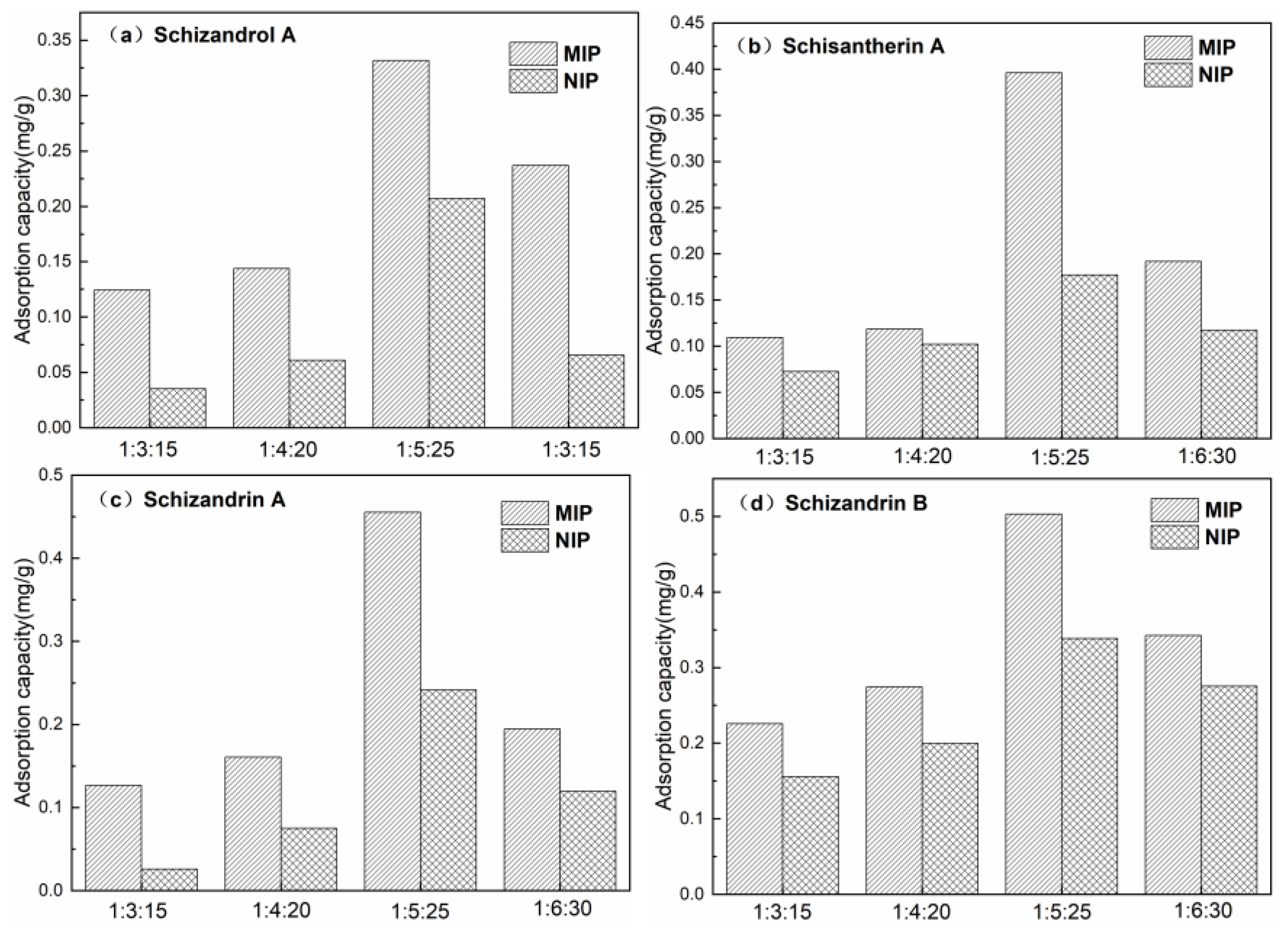
3.2. Results of Optimization of Polymer Preparation Conditions
3.3. Template Molecular Screening of St-MIPs
3.4. Characterization of Mt-MIP and St-MIP
3.4.1. FT-IR Spectroscopy Analysis
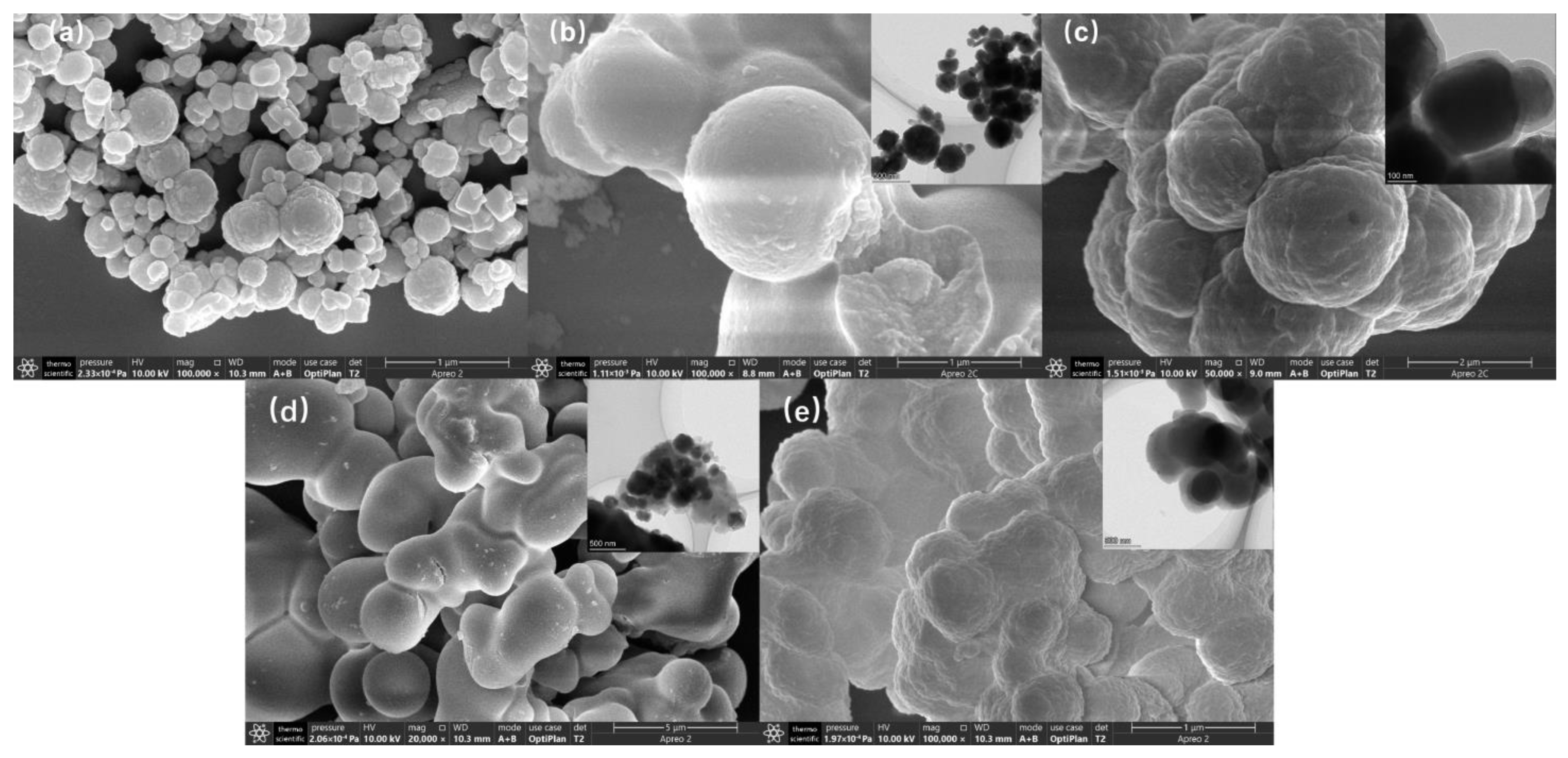
3.4.2. Surface Morphology Analysis of Mt-MIP and St-MIP
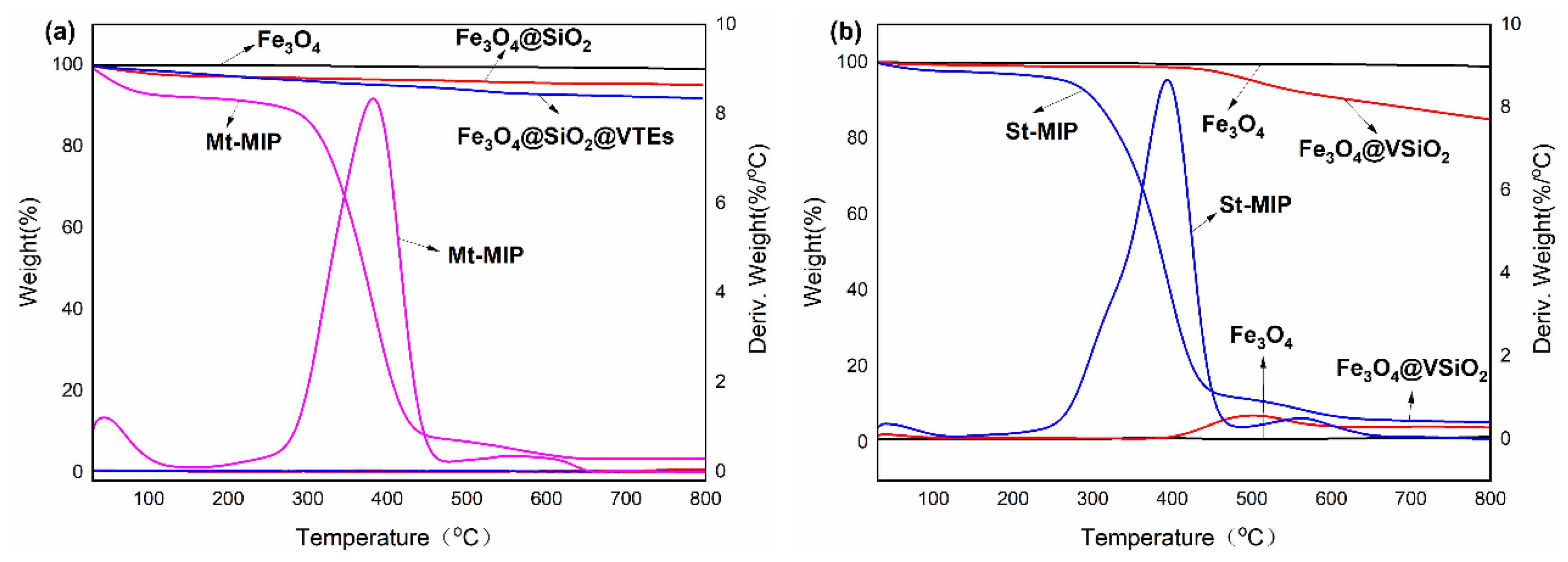
3.4.3. TG and DTG Analysis of Mt-MIP and St-MIP
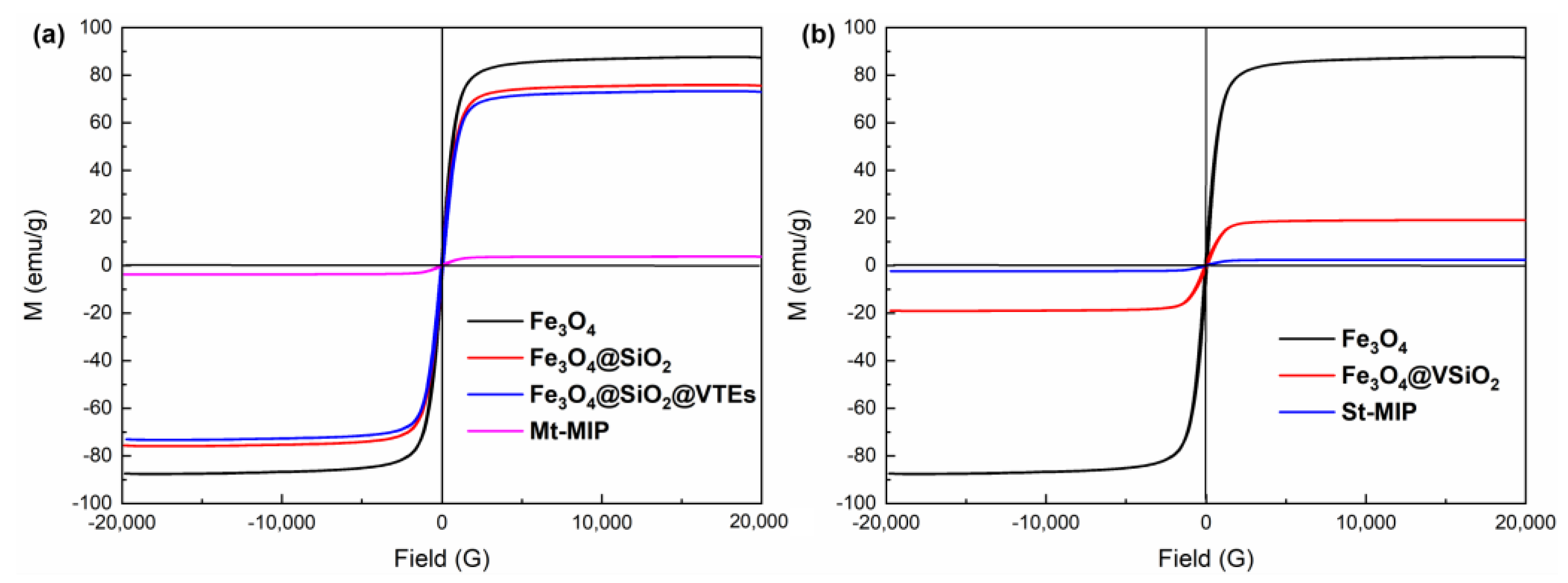
3.4.4. VSM Analysis of Mt-MIP and St-MIP
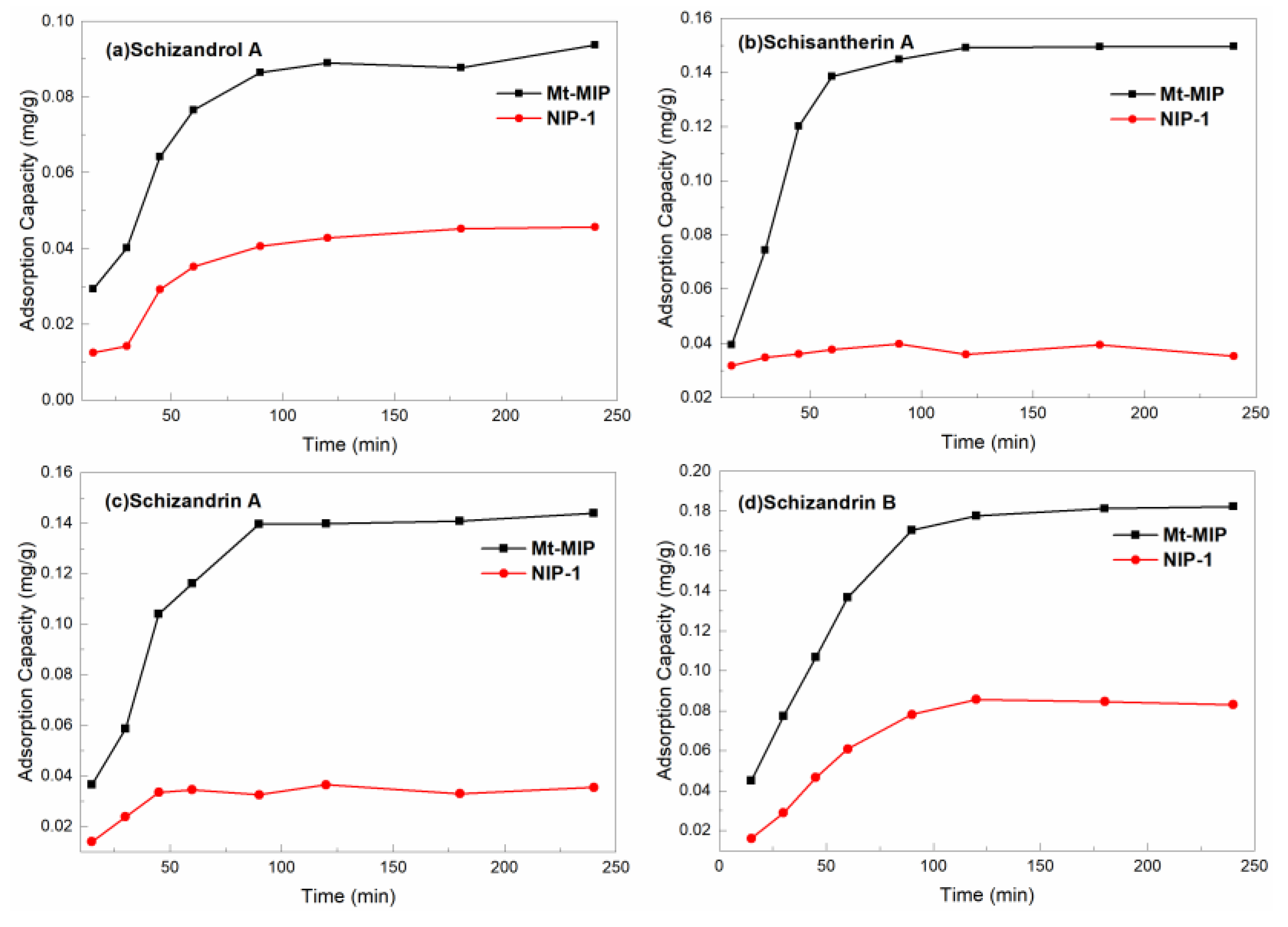
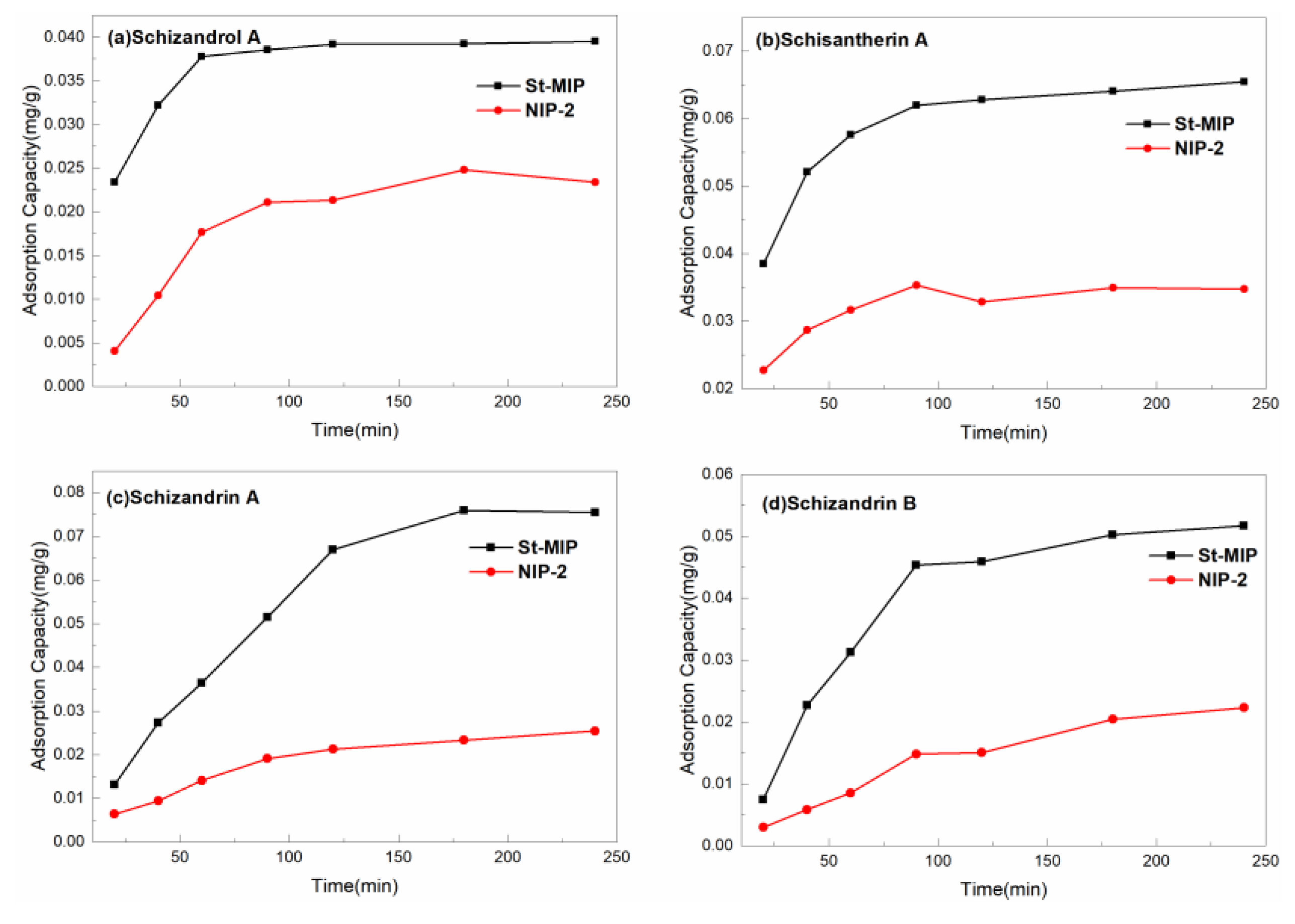
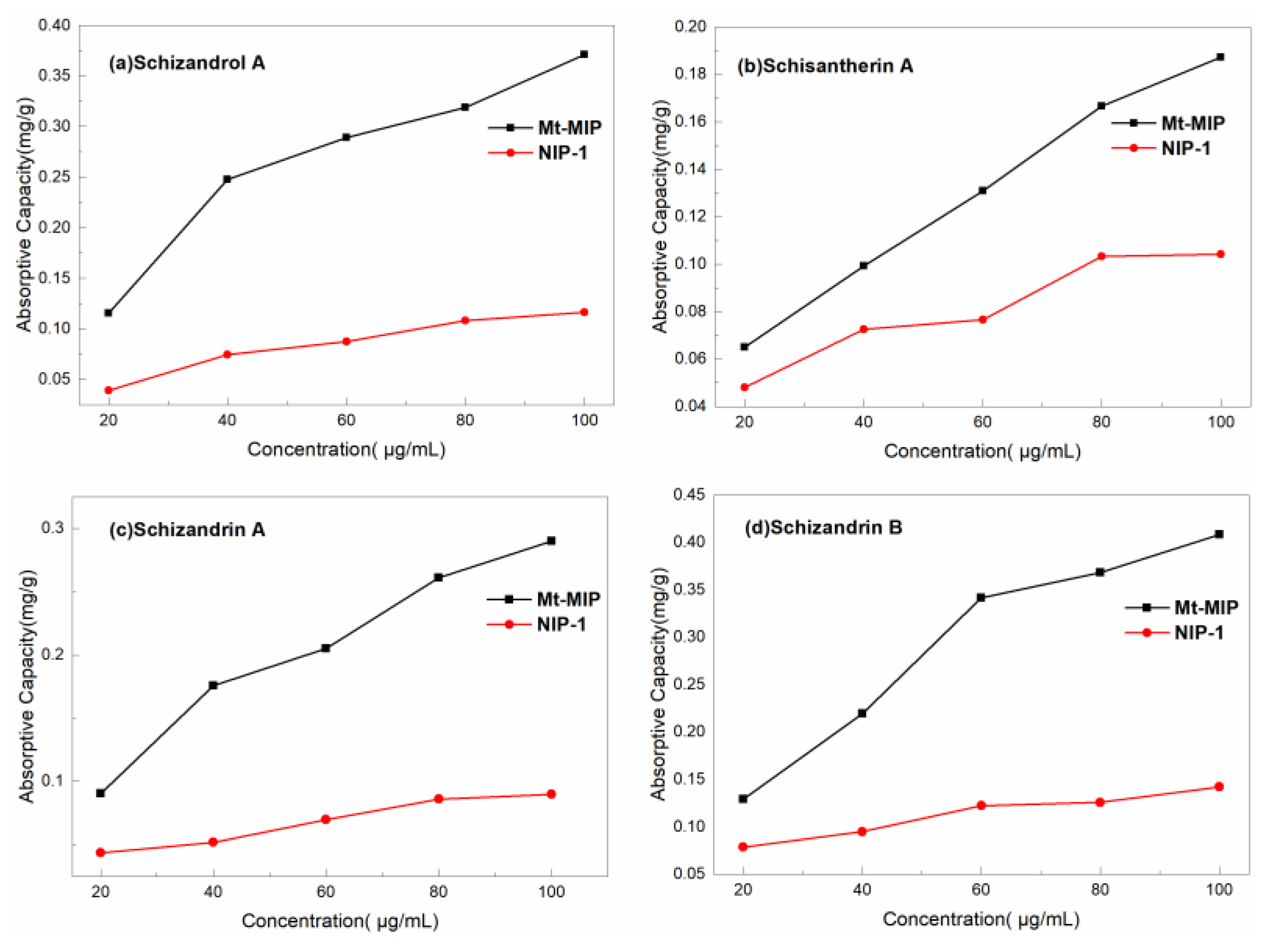
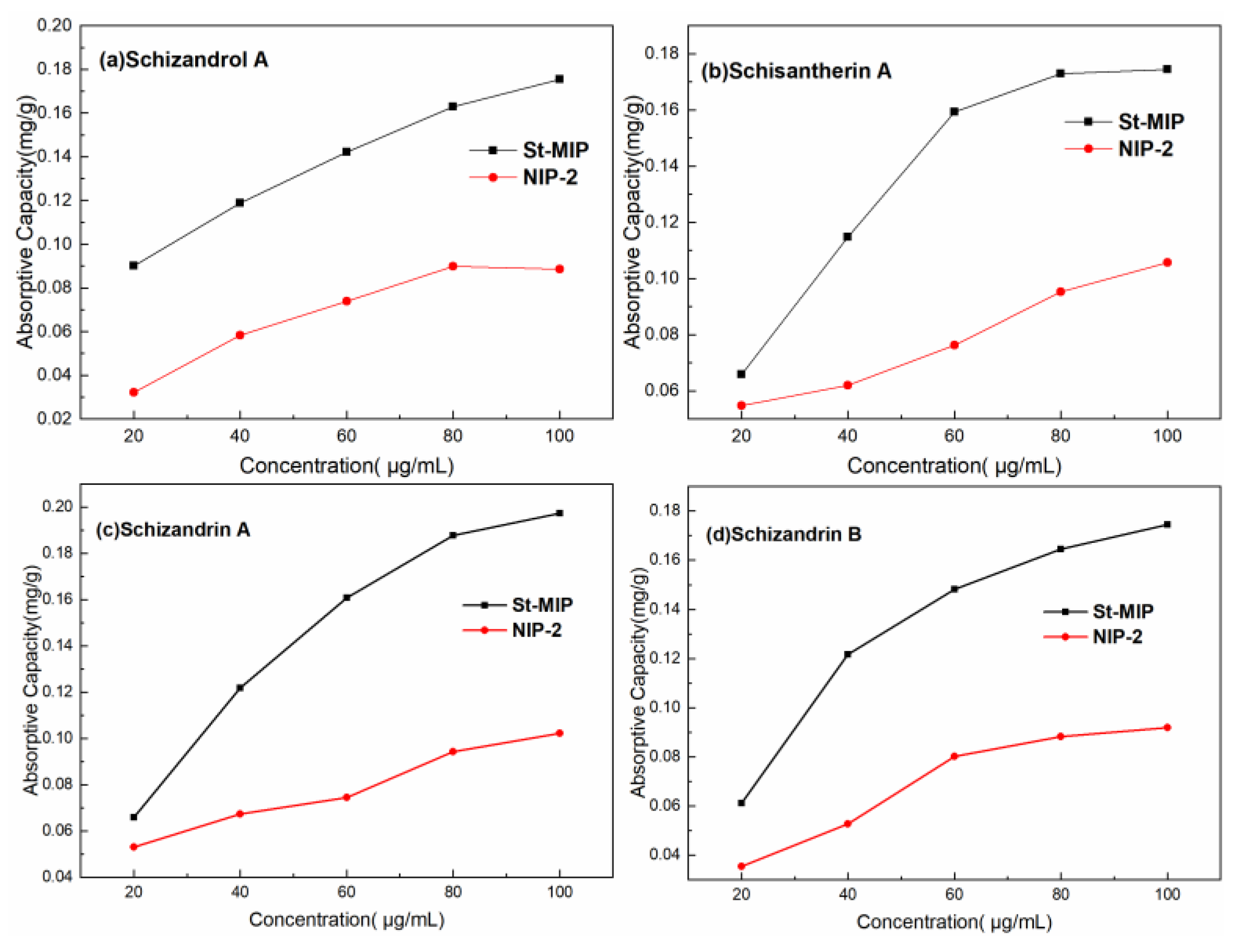
3.5. Adsorption Capacity Evaluation of Mt-MIP and St-MIP
3.6. Selective Adsorption over Mt-MIP and St-MIP
3.7. Re-Usability and Stability Evaluation of Mt-MIP and St-MIP
3.8. Solid-Phase Extraction Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, C.; Liu, T.; Yang, L.; Zu, Y.; Wang, S.; Zhang, R. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal. Chim. Acta 2011, 689, 110–116. [Google Scholar] [CrossRef]
- Ma, C.; Zu, Y.; Yang, L.; Li, J. Two solid-phase recycling method for basic ionic liquid [C4mim]Ac by macroporous resin and ion exchange resin from Schisandra chinensis fruits extract. J. Chromatogr. B 2015, 976–977, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Men, L.; Sun, Y.; Wei, M.; Fan, X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz. (Baill.), a current review. Eur. J. Pharmacol. 2021, 892, 173796. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, T.; Yang, L.; Zu, Y.; Chen, X.; Zhang, L.; Zhang, Y.; Zhao, C. Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Chromatogr. A 2011, 1218, 8573–8580. [Google Scholar] [CrossRef]
- Ma, C.; Yang, L.; Zu, Y.; Wang, N.; Zhang, L.; Zhang, Y.; Chen, X.; Zhao, C. A new approach to catalytic hydrolysis of ester-bound biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill by ion exchange resin. Chem. Eng. Res. Des. 2012, 90, 1189–1196. [Google Scholar] [CrossRef]
- Ma, C.; Liu, T.; Yang, L.; Zu, Y.; Yang, F.; Zhao, C.; Zhang, L.; Zhang, Z. Preparation of high purity biphenyl cyclooctene lignans from Schisandra extract by ion exchange resin catalytic transformation combined with macroporous resin separation. J. Chromatogr. B 2011, 879, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Lafuente González, E.; Guadaño Sánchez, M.; Urriza Arsuaga, I.; Urraca, J.L. Core-Shell Magnetic Imprinted Polymers for the Recognition of FLAG-Tagpeptide. Int. J. Mol. Sci. 2023, 24, 3453. [Google Scholar] [CrossRef] [PubMed]
- Díaz Álvarez, M.; Martín Esteban, A. Molecularly Imprinted Polymer-Quantum Dot Materials in Optical Sensors: An Overview of Their Synthesis and Applications. Biosensors 2021, 11, 79. [Google Scholar] [CrossRef]
- Zhang, H.; Song, H.; Tian, X.; Wang, Y.; Hao, Y.; Wang, W.; Gao, R.; Yang, W.; Ke, Y.; Tang, Y. Magnetic imprinted nanoparticles with synergistic tailoring of covalent and non-covalent interactions for purification and detection of procyanidin B2. Microchim. Acta 2021, 188, 17. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Tian, X.; Song, H.; Gao, R.; Tang, X.; Zhang, X.; Hao, Y.; Tang, Y. High-efficiency recognition and detection of sulindac in sewage using hydrophilic imprinted resorcinol-formaldehyde resin magnetic nano-spheres as SPE adsorbents combined with HPLC. Chem. Eng. J. 2020, 392, 123716. [Google Scholar] [CrossRef]
- Li, X.; Yang, B.; Xiao, K.; Duan, H.; Wan, J.; Zhao, H. Targeted degradation of refractory organic compounds in wastewaters based on molecular imprinting catalysts. Water Res. 2021, 203, 117541. [Google Scholar] [CrossRef] [PubMed]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, A.; Viter, R.; Ramanavicius, S. Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105. [Google Scholar] [CrossRef]
- Drobysh, M.; Ratautaite, V.; Brazys, E.; Ramanaviciene, A.; Ramanavicius, A. Molecularly imprinted composite-based biosensor for the determination of SARS-CoV-2 nucleocapsid protein. Biosens. Bioelectron. 2024, 251, 116043. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Lorenz, W.; Vanninen, P.; Alizadeh, T.; Cämmerer, M.; Borsdorf, H. Molecularly Imprinted Polymer Materials as Selective Recognition Sorbents for Explosives: A Review. Polymers 2019, 11, 888. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Qi, X.; Wan, X.; Liu, Y.; Chen, Y.; Xu, L. Molecularly imprinted polypyrrole film-coated poly(3,4-ethylenedioxythiophene):polystyrene sulfonate-functionalized black phosphorene for the selective and robust detection of norfloxacin. Mater. Today Chem. 2022, 26, 101043. [Google Scholar] [CrossRef]
- Luliński, P. Molecularly imprinted polymers based drug delivery devices: A way to application in modern pharmacotherapy. A review. Mater. Sci. Eng. C 2017, 76, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, H.; Ma, S.; Bo, C.; Ou, J.; Gong, B. Recent application of molecular imprinting technique in food safety. J. Chromatogr. A 2021, 1657, 462579. [Google Scholar] [CrossRef] [PubMed]
- Afsharara, H.; Asadian, E.; Mostafiz, B.; Banan, K.; Bigdeli, S.A.; Hatamabadi, D.; Keshavarz, A.; Hussain, C.M.; Keçili, R.; Ghorbani-Bidkorpeh, F. Molecularly imprinted polymer-modified carbon paste electrodes (MIP-CPE): A review on sensitive electrochemical sensors for pharmaceutical determinations. TrAC Trends Anal. Chem. 2023, 160, 116949. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Bagheri, A.R.; Guo, X.; Wang, L.; Li, J.; Wang, X.; Li, B.; Chen, L. Strategies of molecular imprinting-based solid-phase extraction prior to chromatographic analysis. TrAC Trends Anal. Chem. 2020, 128, 115923. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Wang, H.; Guo, L.; Zhai, Y.; Zhang, J.; Yang, Y.; Wang, H.; Yin, Z.; Lu, Y. Preparation of molecularly imprinted ordered mesoporous silica for rapid and selective separation of trace bisphenol A from water samples. Appl. Surf. Sci. 2018, 448, 380–388. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.; Huang, J.; Yao, D.; Wang, C.; Zhang, L.; Hou, S.; Chen, L.; Yuan, C. The Multi-Template Molecularly Imprinted Polymer Based on SBA-15 for Selective Separation and Determination of Panax notoginseng Saponins Simultaneously in Biological Samples. Polymers 2017, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Yao, S.; Zhu, P. Selective recognization of dicyandiamide in bovine milk by mesoporous silica SBA-15 supported dicyandiamide imprinted polymer based on surface molecularly imprinting technique. Food Chem. 2018, 240, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, M.; Luliński, P. Molecularly imprinted solid phase extraction—Recent strategies, future prospects and forthcoming challenges in complex sample pretreatment process. TrAC Trends Anal. Chem. 2024, 174, 117695. [Google Scholar] [CrossRef]
- Chen, Q.; Chaihu, L.; Yao, X.; Cao, X.; Bi, W.; Lin, J.; Chen, D.D.Y. Molecular Property-Tailored Soy Protein Extraction Process Using a Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2021, 9, 10083–10092. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; van den Bruinhorst, A.; Kollau, L.J.B.M.; Kroon, M.C.; Binnemans, K. Degradation of Deep-Eutectic Solvents Based on Choline Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2019, 7, 11521–11528. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, R.; Han, H.; Wu, K.; Wang, B.; Liu, Y.; Zhu, Y.; Lu, H.; Liang, B. Preparation strategy and stability of deep eutectic solvents: A case study based on choline chloride-carboxylic acid. J. Clean. Prod. 2022, 345, 131028. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, Z.; Yang, L.; Liu, H. Development and applications of deep eutectic solvent derived functional materials in chromatographic separation. J. Sep. Sci. 2021, 44, 1098–1121. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Huang, Q.; Liu, X.; Zhang, H. Isolation of transferrin by imprinted nanoparticles with magnetic deep eutectic solvents as monomer. Anal. Bioanal. Chem. 2018, 410, 6237–6245. [Google Scholar] [CrossRef]
- Surapong, N.; Santaladchaiyakit, Y.; Burakham, R. A water-compatible magnetic dual-template molecularly imprinted polymer fabricated from a ternary biobased deep eutectic solvent for the selective enrichment of organophosphorus in fruits and vegetables. Food Chem. 2022, 384, 132475. [Google Scholar] [CrossRef]



| Polymers | Molar Ratio a | Functional Monomers | Cross-Linkers |
|---|---|---|---|
| MIP1/NIP1 | 1:3:15 | ChCl-AA | EDGMA |
| MIP2/NIP2 | 1:4:20 | ChCl-AA | EDGMA |
| MIP3/NIP3 | 1:5:25 | ChCl-AA | EDGMA |
| MIP4/NIP4 | 1:6:30 | ChCl-AA | EDGMA |
| Name | Standard Curve Equation | R2 | Linear Detection Range (μg/mL) | Retention Time (min) |
|---|---|---|---|---|
| Schizandrol A | Y = −86.814 + 2.5790 × 104X | 0.9999 | 7.81~1000 | 6.7 |
| Schisantherin A | Y = −145.5 + 2.4182 × 104X | 0.9999 | 7.81~1000 | 13.5 |
| Schizandrin A | Y = −25.482 + 2.9300 × 104X | 0.9999 | 7.81~1000 | 23.0 |
| Schizandrin B | Y = −153.98 + 2.7309 × 104X | 0.9999 | 7.81~1000 | 31.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Sun, L.; Du, Y.; Duan, W.; Li, W.; Luo, S.; Liang, B.; Ma, C.; Pan, G. Magnetic Molecularly Imprinted Polymer Combined with Solid-Phase Extraction for Purification of Schisandra chinensis Lignans. Polymers 2024, 16, 3124. https://doi.org/10.3390/polym16223124
Xu H, Sun L, Du Y, Duan W, Li W, Luo S, Liang B, Ma C, Pan G. Magnetic Molecularly Imprinted Polymer Combined with Solid-Phase Extraction for Purification of Schisandra chinensis Lignans. Polymers. 2024; 16(22):3124. https://doi.org/10.3390/polym16223124
Chicago/Turabian StyleXu, Huijuan, Lihan Sun, Yufei Du, Wenxin Duan, Wei Li, Sha Luo, Bing Liang, Chunhui Ma, and Gaofeng Pan. 2024. "Magnetic Molecularly Imprinted Polymer Combined with Solid-Phase Extraction for Purification of Schisandra chinensis Lignans" Polymers 16, no. 22: 3124. https://doi.org/10.3390/polym16223124
APA StyleXu, H., Sun, L., Du, Y., Duan, W., Li, W., Luo, S., Liang, B., Ma, C., & Pan, G. (2024). Magnetic Molecularly Imprinted Polymer Combined with Solid-Phase Extraction for Purification of Schisandra chinensis Lignans. Polymers, 16(22), 3124. https://doi.org/10.3390/polym16223124







