Investigating the Effect of Microwave Induction on the Polymerization Rate of Polycarboxylate Superplasticizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Determination of Kinetic Parameters of Polymerization
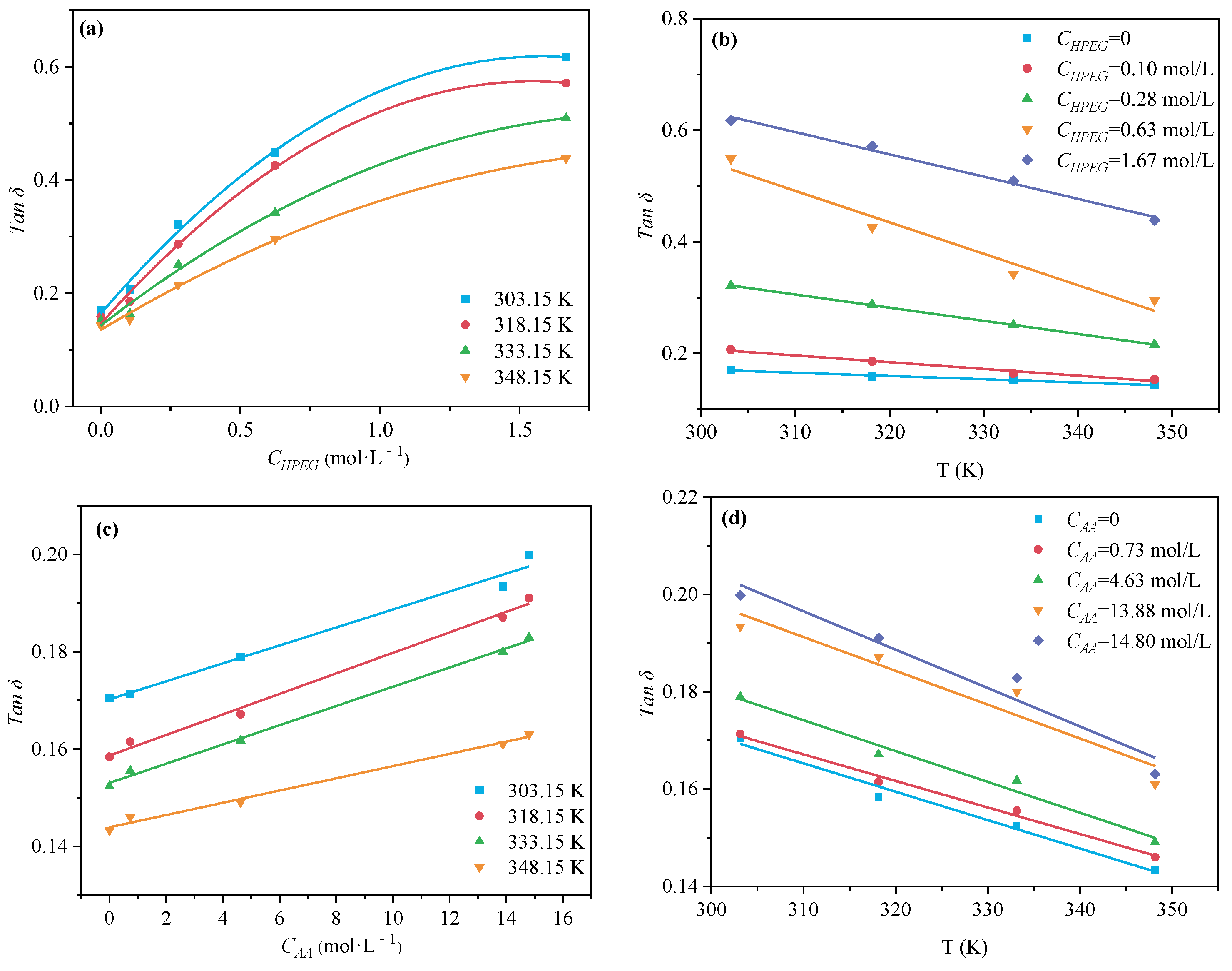
2.3. Determination of Permittivity
2.4. Simulation and Calculation of Electrostatic Potential and Dipole Moment
3. Results and Discussion
3.1. Microscopic Polymerization Kinetics Analysis of PCE under Different Heating Methods
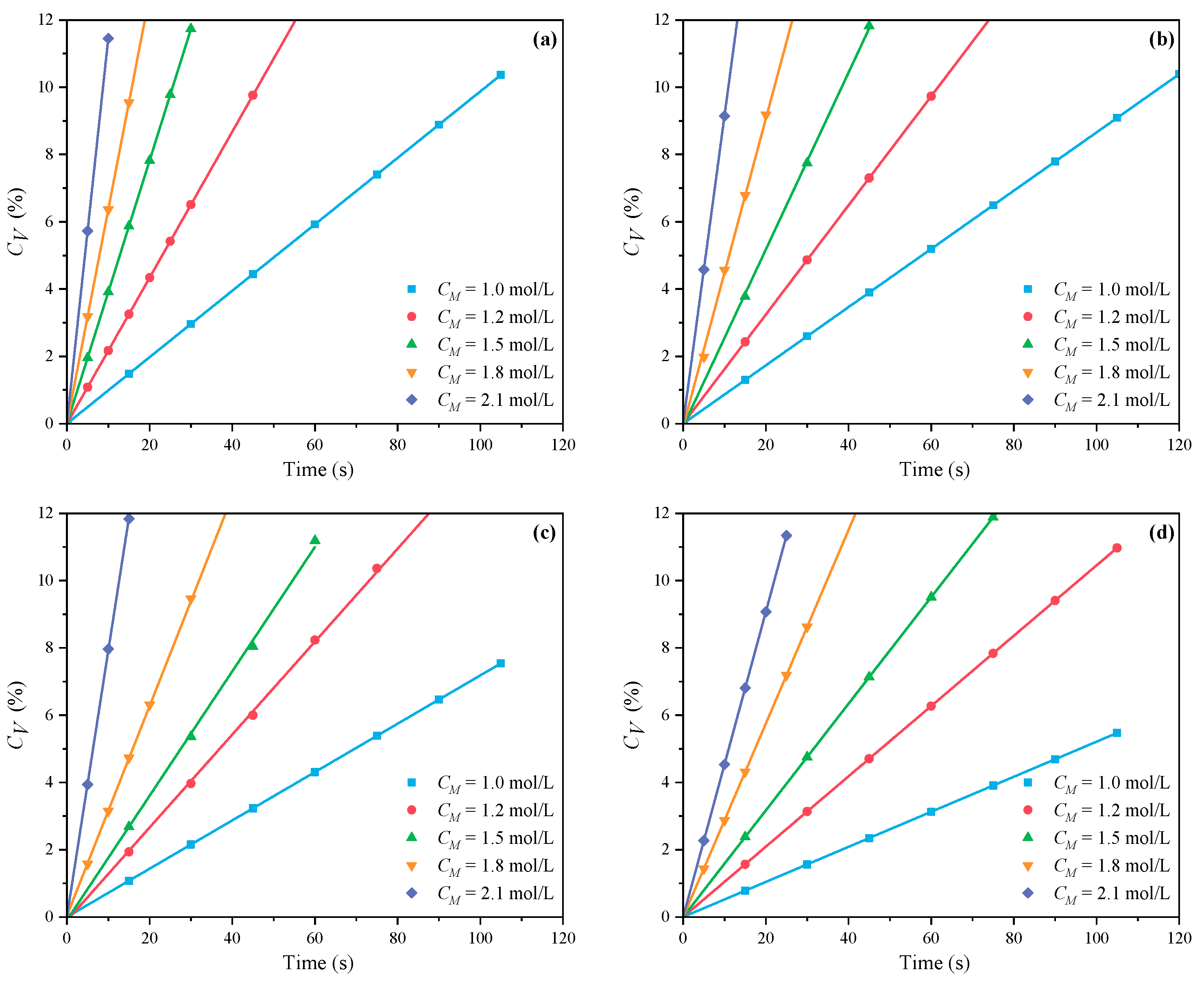
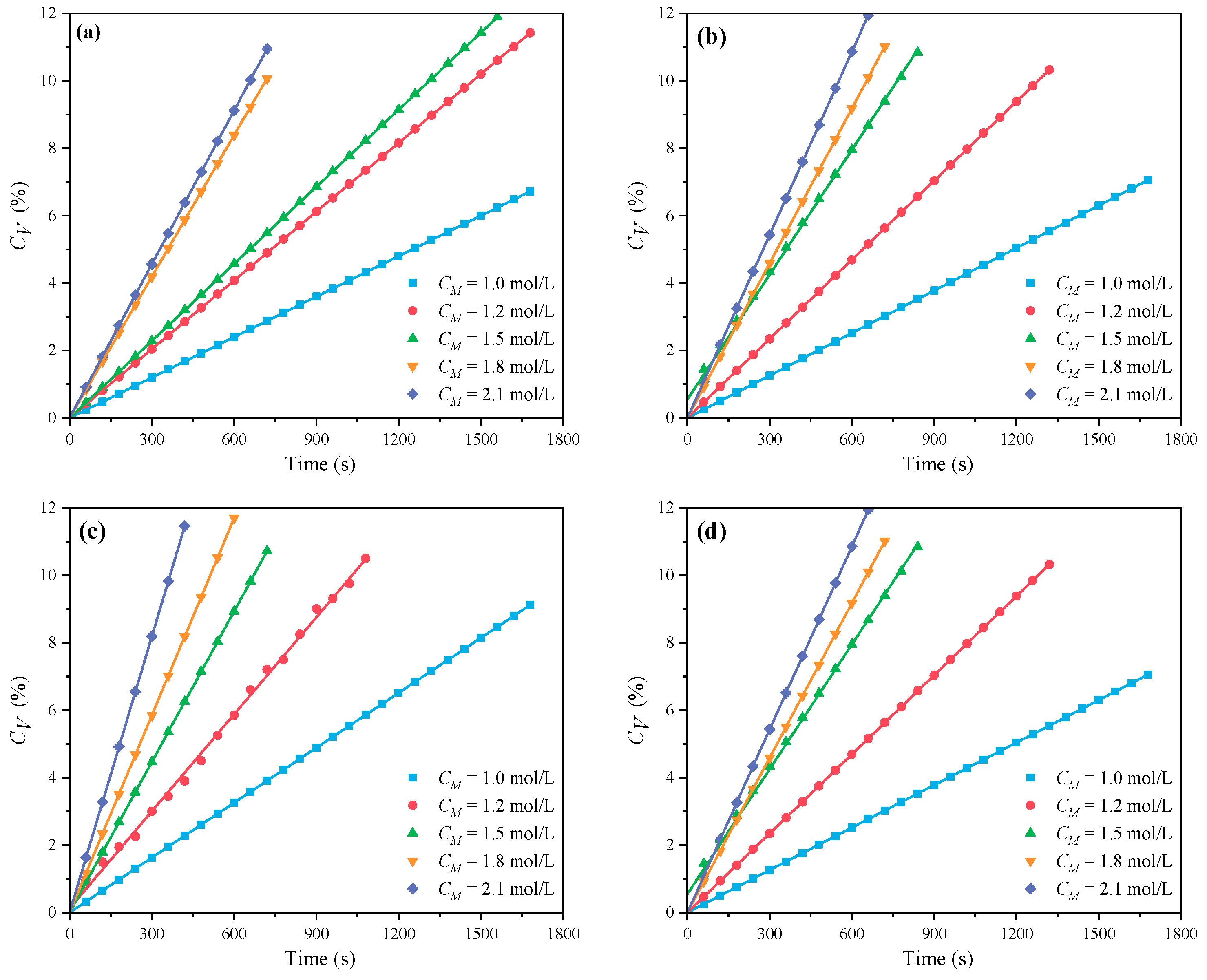
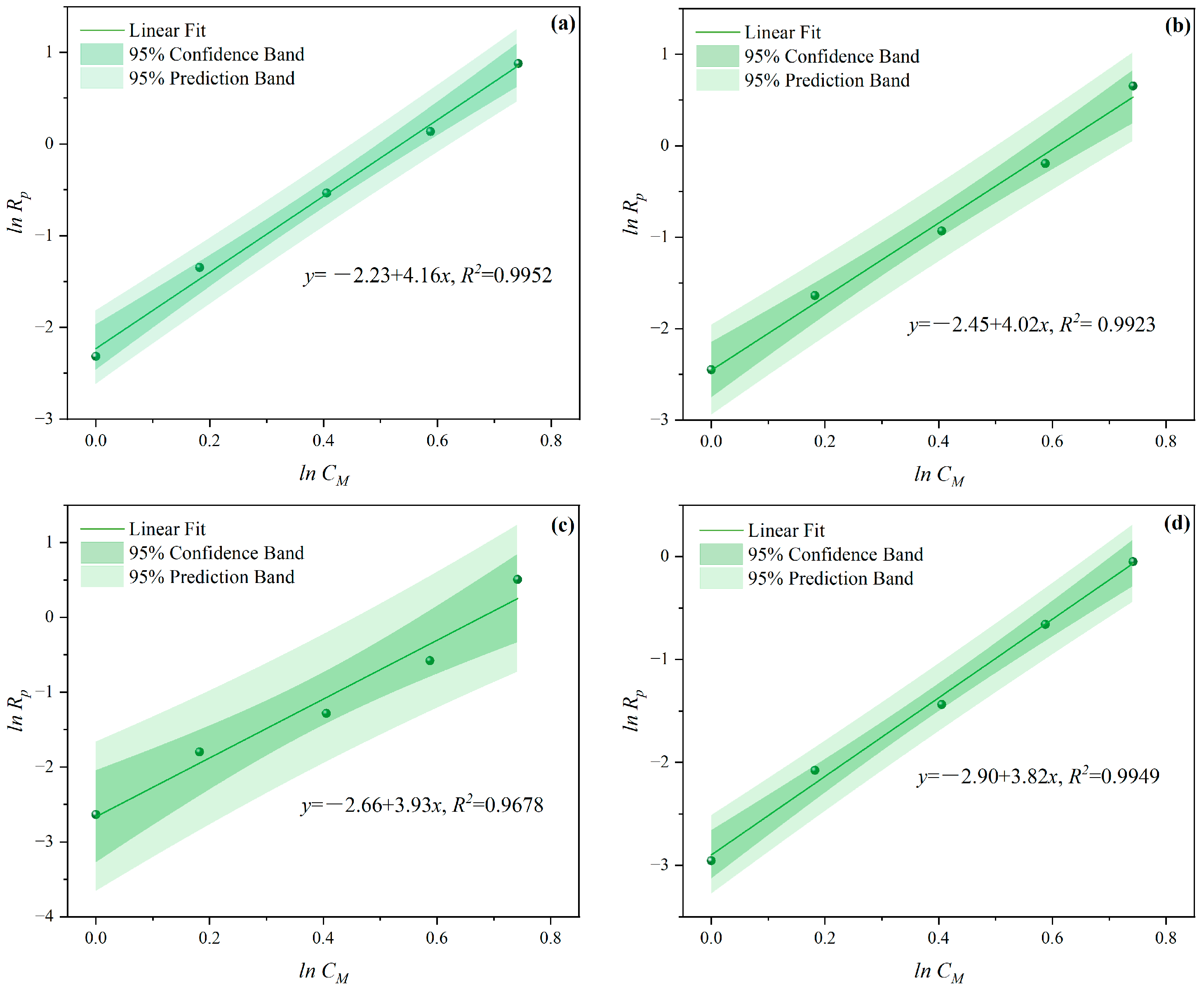
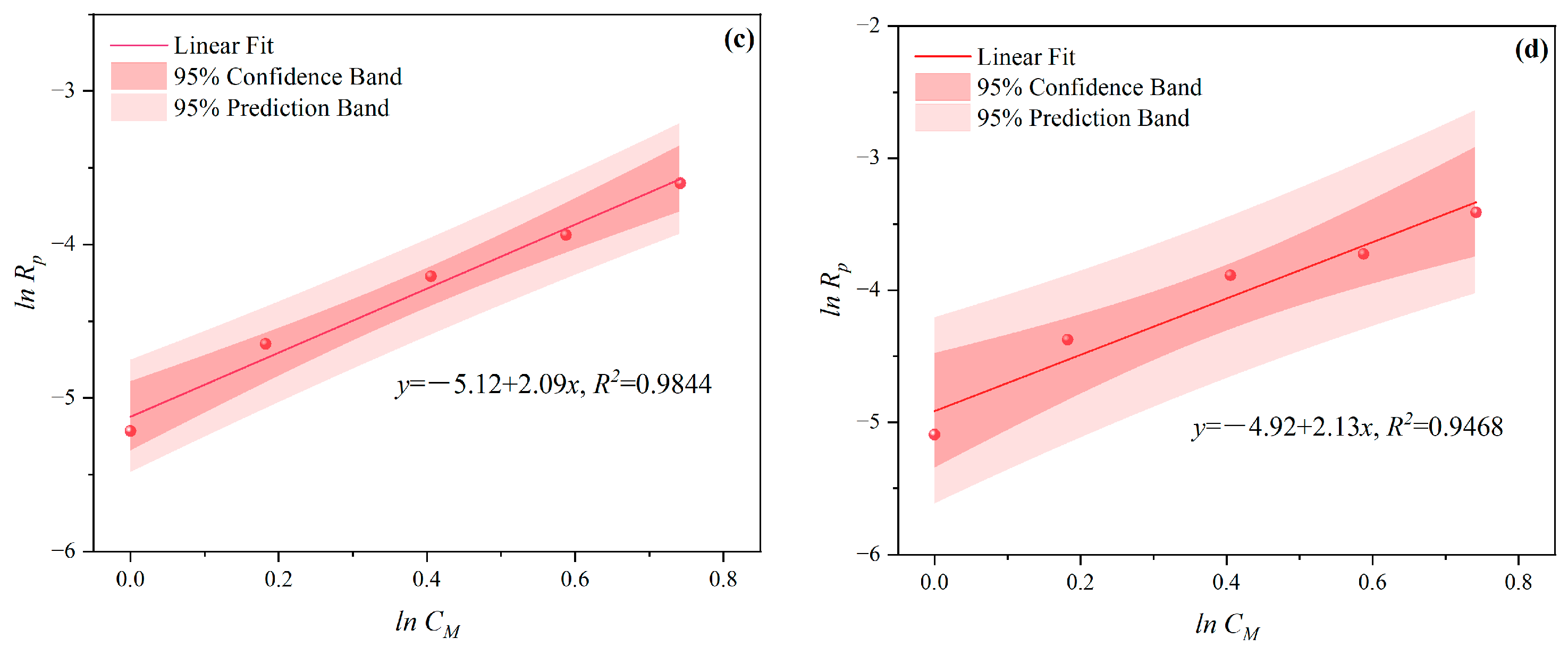
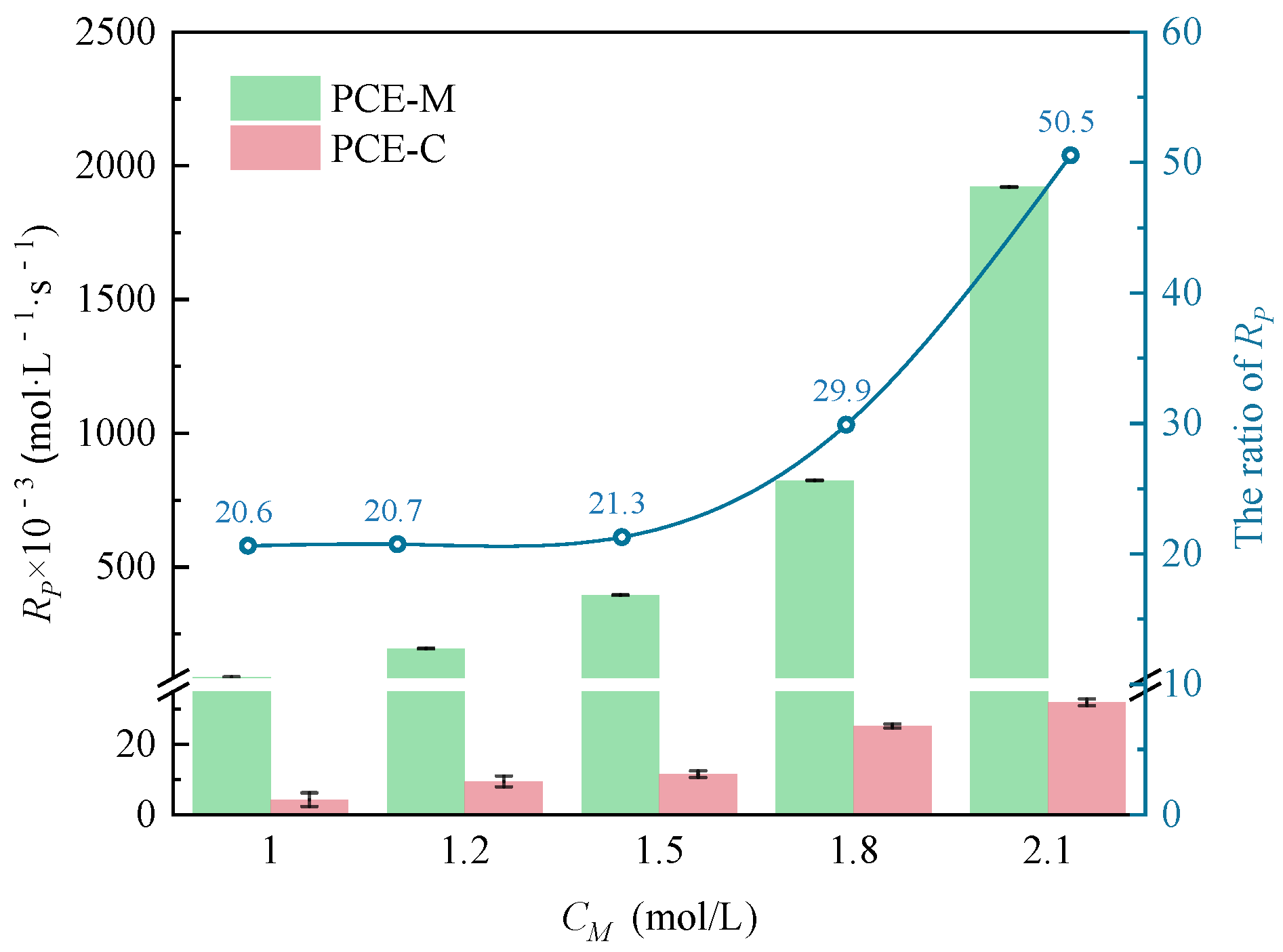
3.1.1. The Effects of CM on CV and Rp
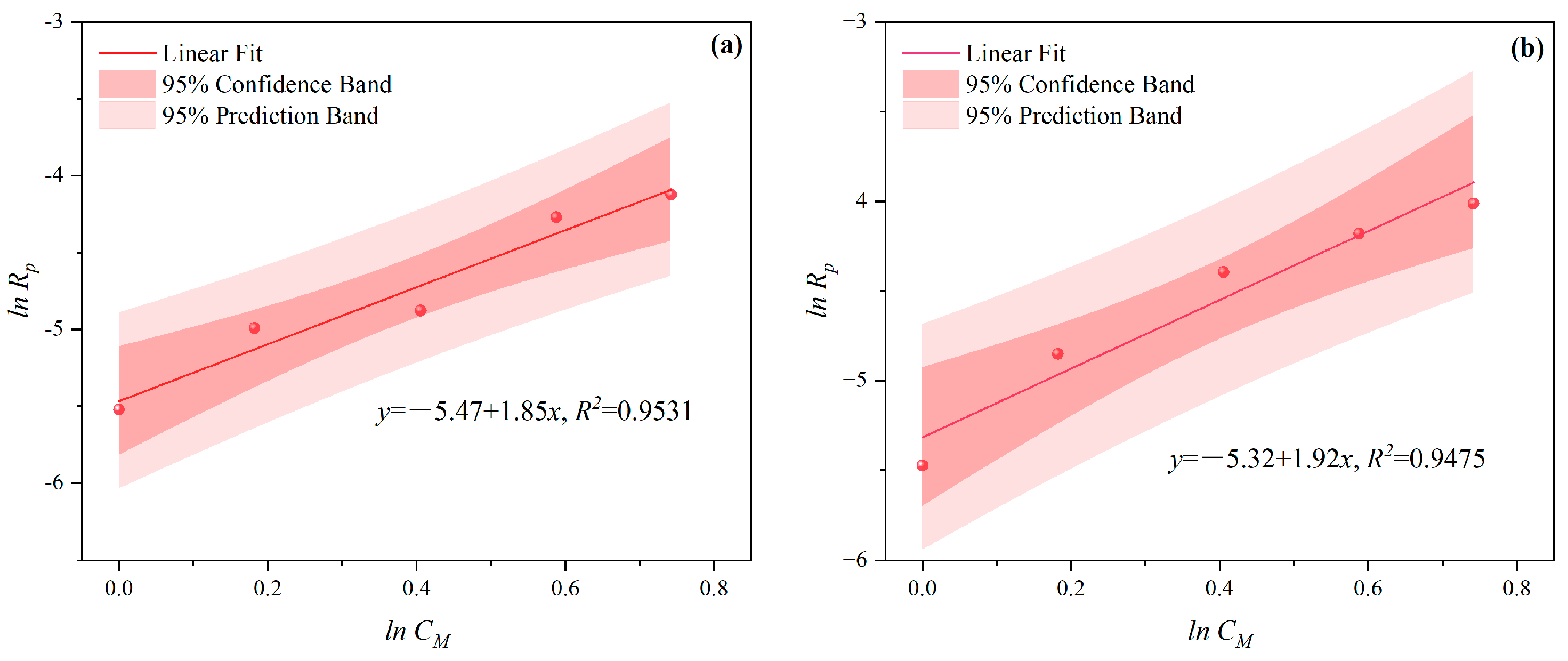
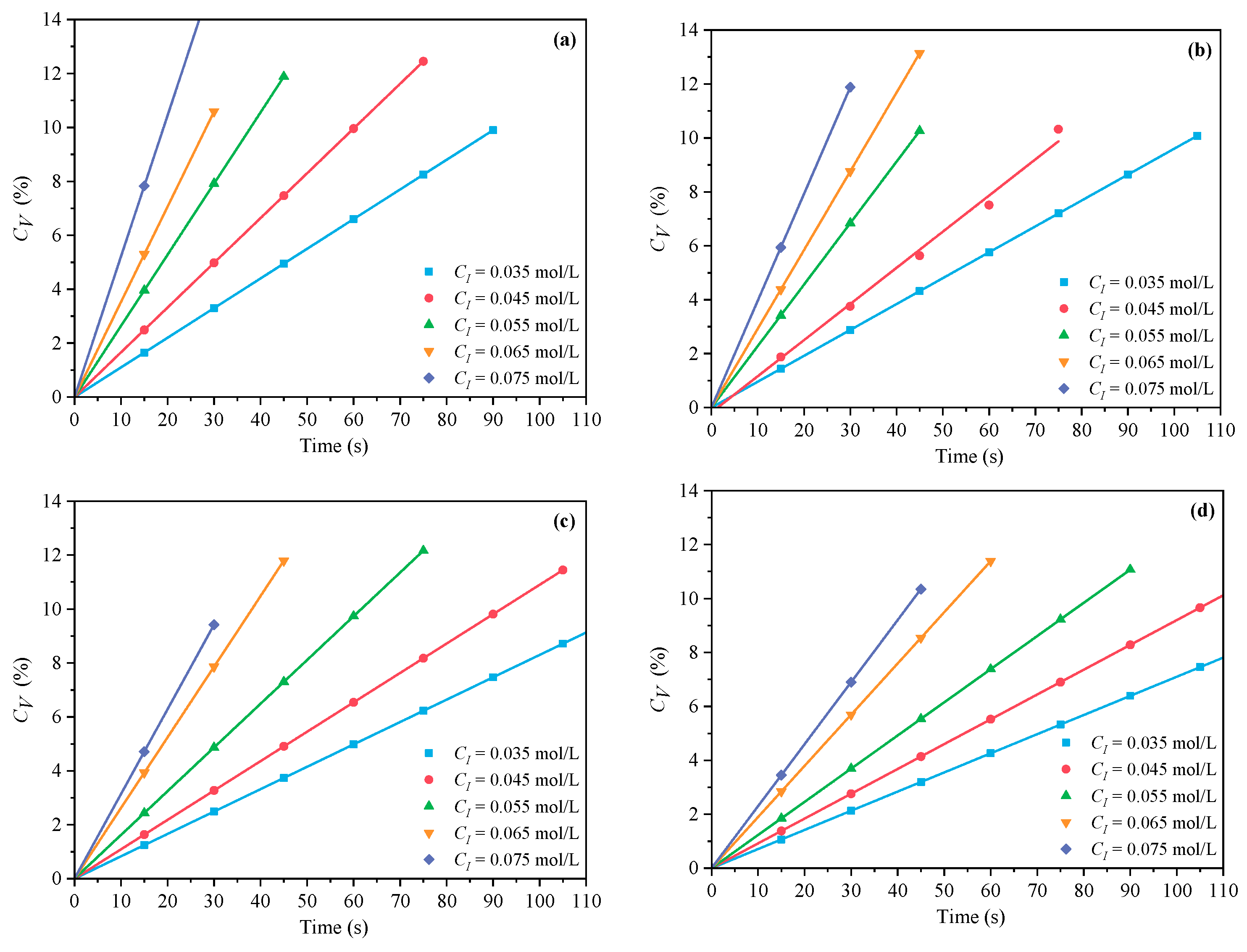
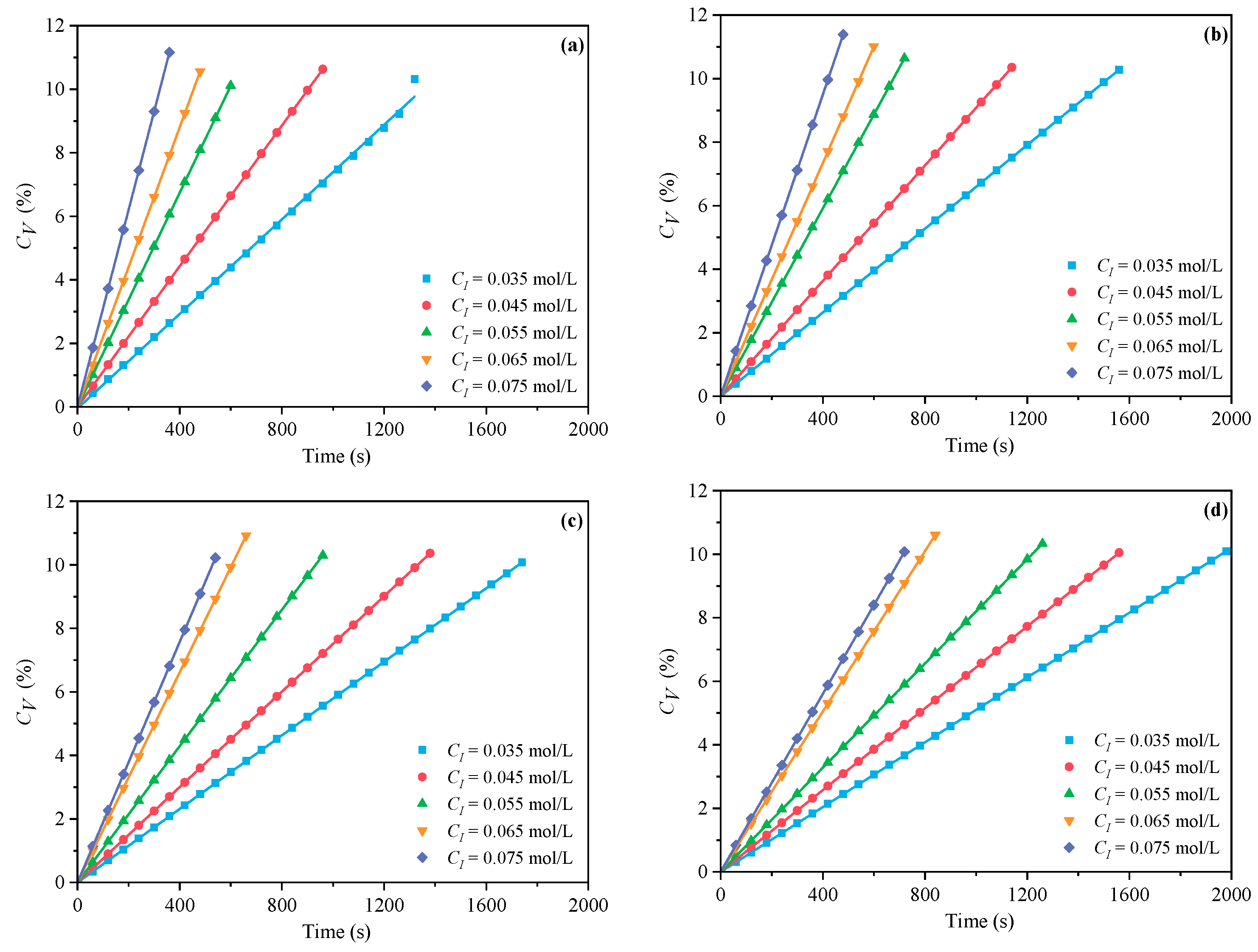
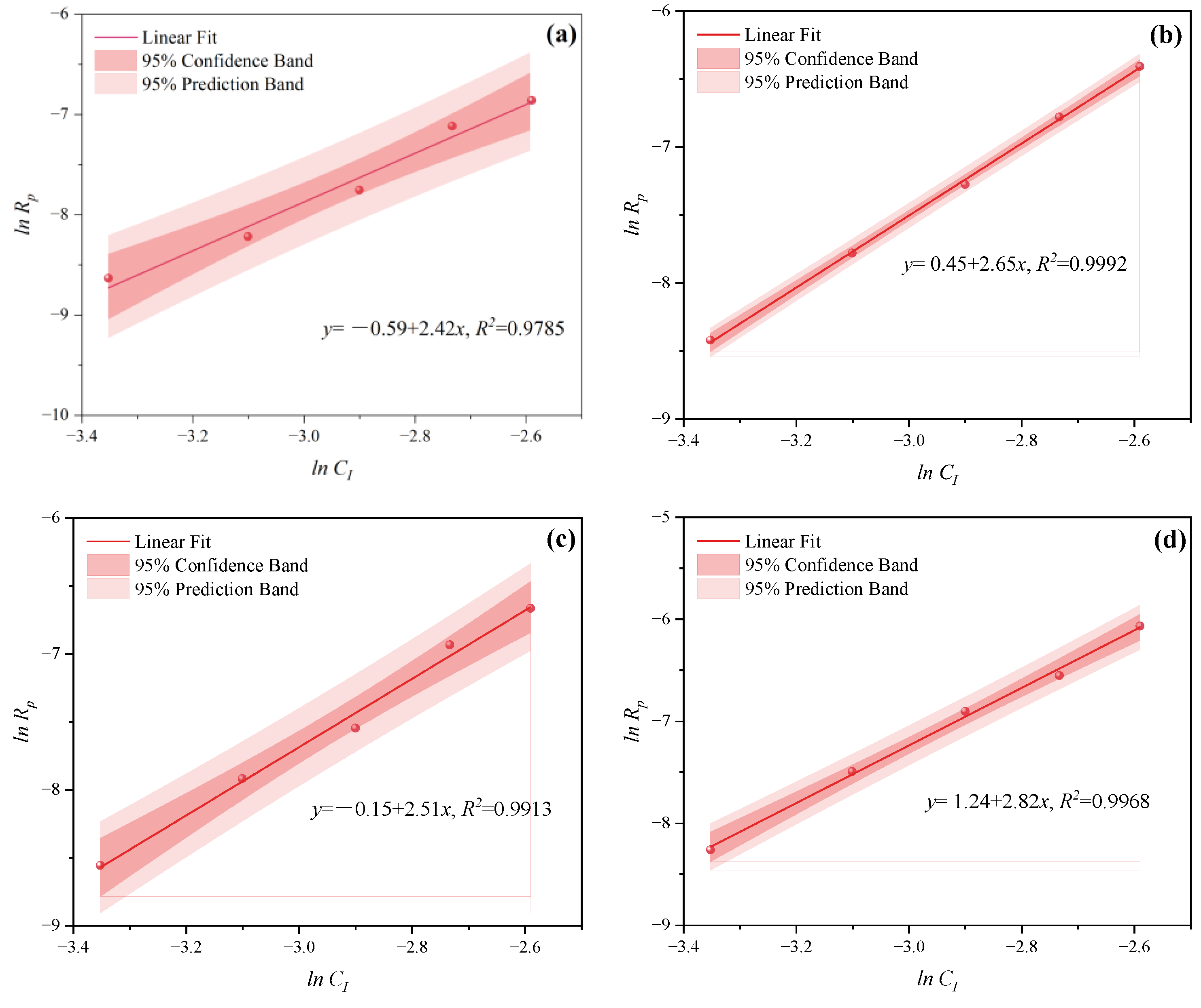
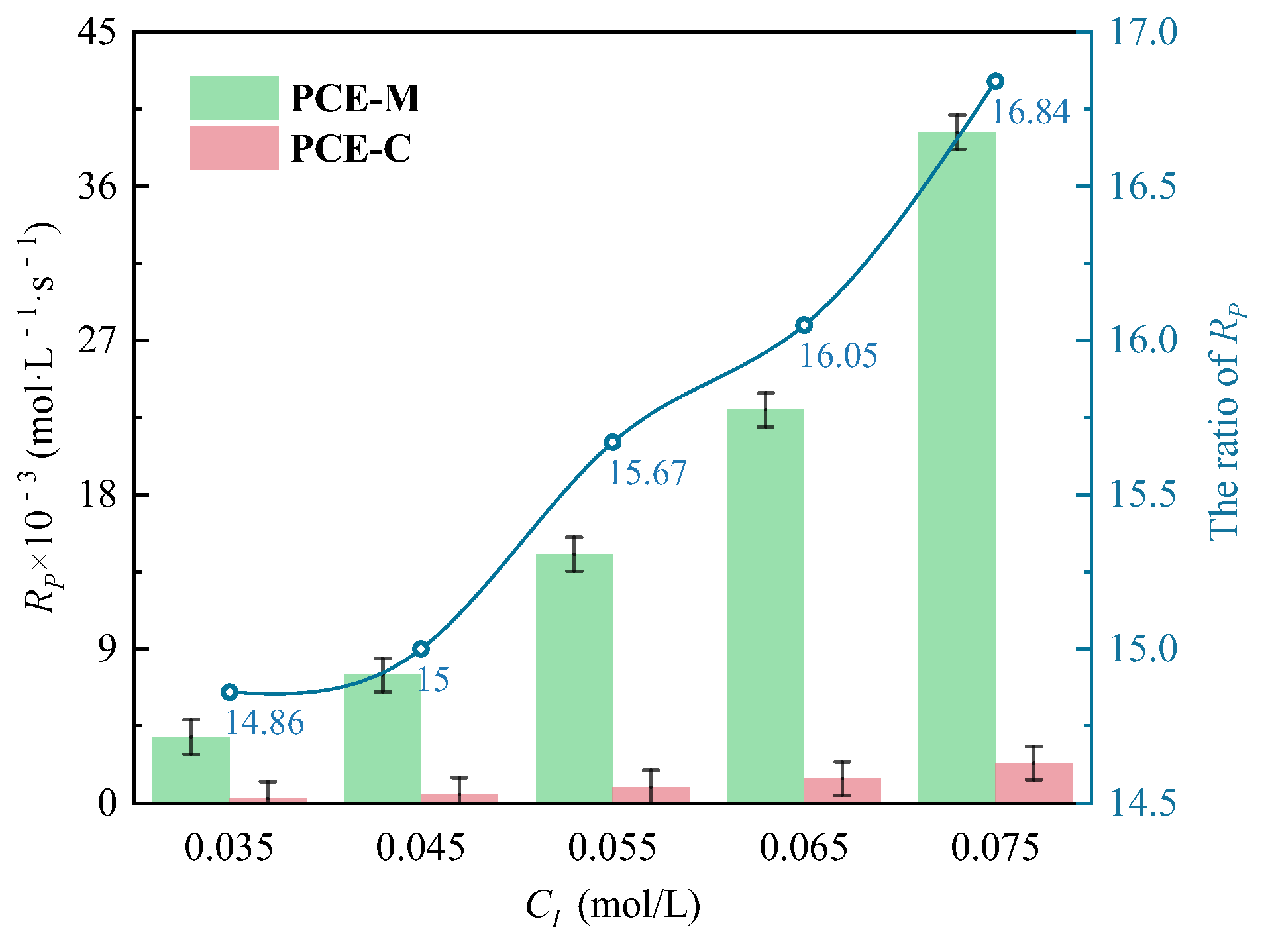
3.1.2. The Effects of CI on CV and Rp
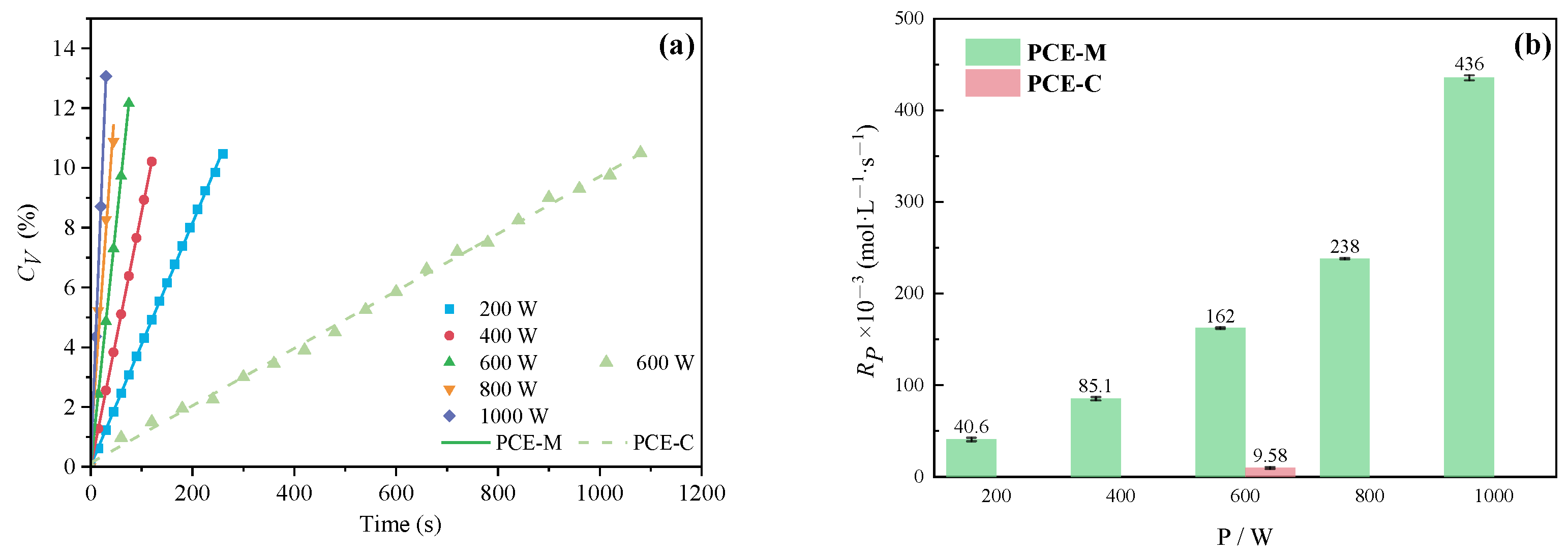
3.1.3. The Effect of Power on CV and Rp
3.1.4. The Effect of Polymerization Temperature on CV and Rp
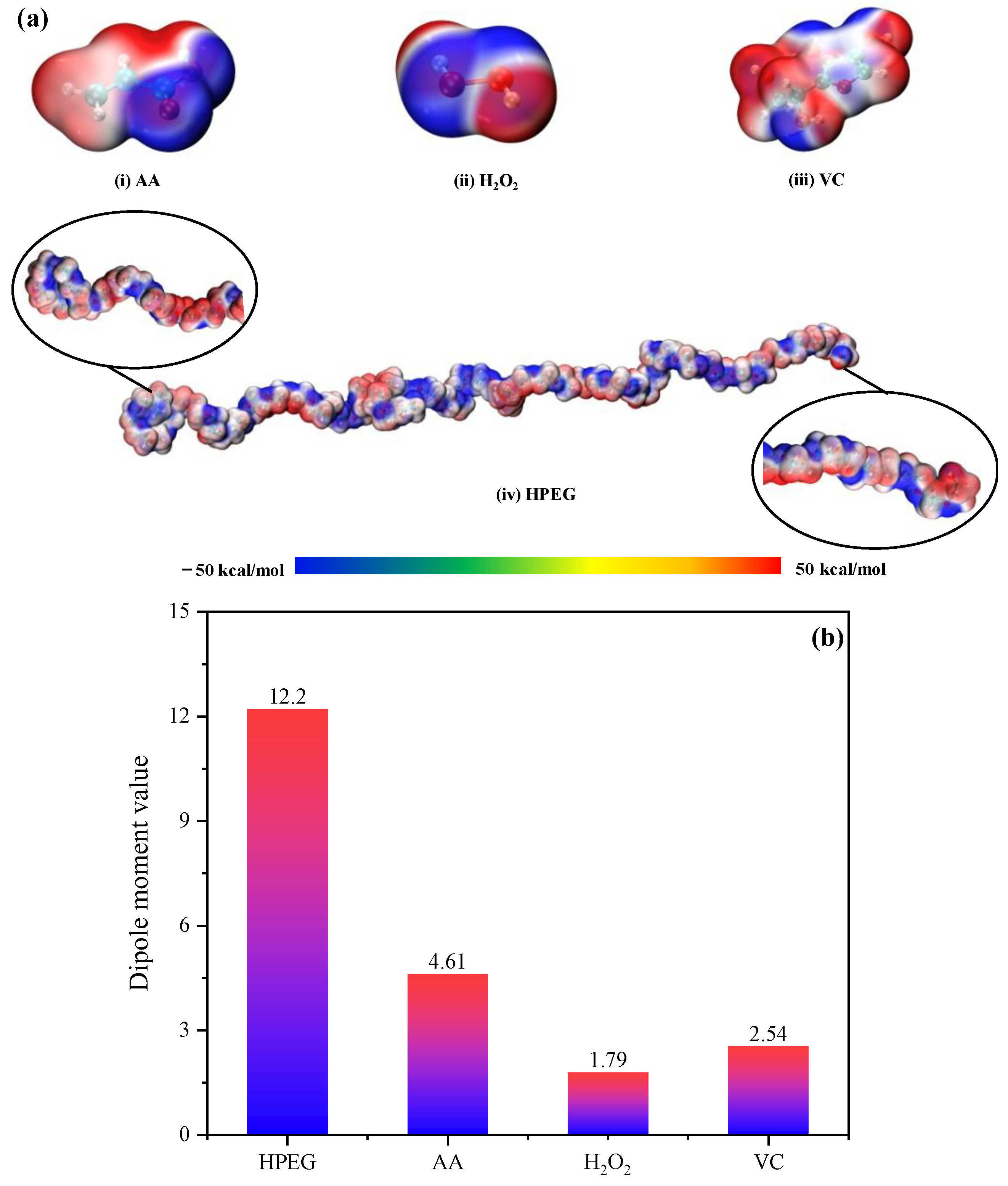
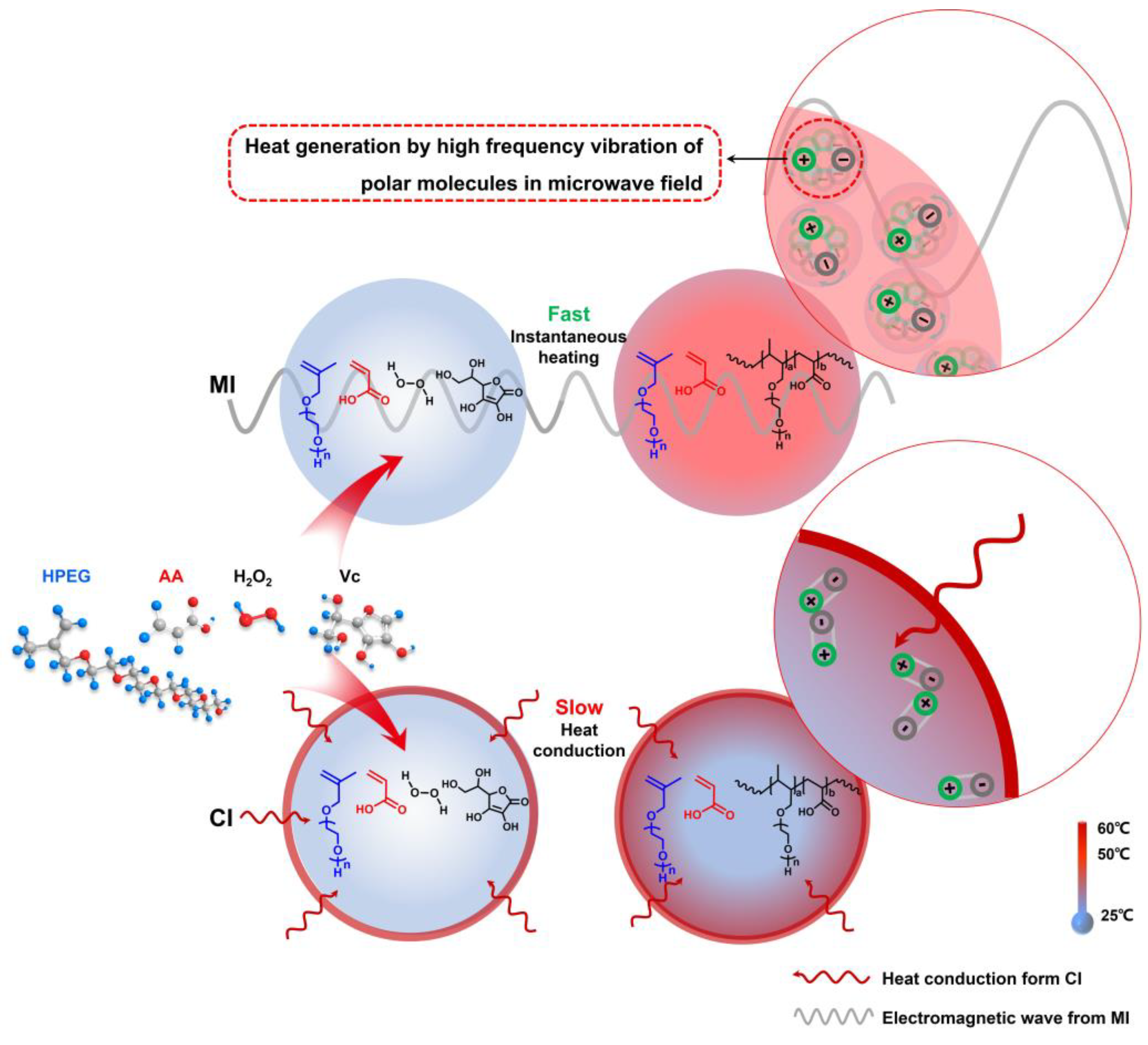
3.2. Mechanism Analysis of Accelerated Rp in PCE Polymerization System: Microwave Thermal Effect
4. Conclusions
- (1)
- Under MI, the influence of total monomer concentration on the polymerization rate Rp was quantified by proportional relationships Rp∝CM4.157, Rp∝CM4.024, Rp∝CM3.928, and Rp∝CM3.816 at nAA:nHPEG = 2.5:1, 3:1, 4:1, and 5:1, respectively. Furthermore, the initiator concentration effects of Rp∝CI3.030, Rp∝CI2.901, Rp∝CI2.848, and Rp∝CI2.655 were also established at nH2O2:nVC = 3.5:1, 4.65:1, 5.85:1, and 7:1, respectively.
- (2)
- Under CI, the total monomer concentration effects on Rp were depicted as Rp∝CM1.856, Rp∝CM1.916, Rp∝CM2.088, and Rp∝CM2.133 at nAA:nHPEG = 2.5:1, 3:1, 4:1, and 5:1, respectively. In addition, the initiator concentration effects of Rp∝CI2.824, Rp∝CI2.650, Rp∝CI2.511, and Rp∝CI2.429 were also established at nH2O2:nVC = 3.5:1, 4.65:1, 5.85:1, and 7:1, respectively.
- (3)
- In polymerization processes, microwaves can enhance reaction rates and shorten reaction time. Compared with the CI method, applying microwave to the polymerization reaction system of PCE reduced the barrier of activation energy from 46.83 kJ·mol−1 to 35.07 kJ·mol−1.
- (4)
- The principal reactants in polymerization were polar molecules that absorbed microwave energy and quickly converted it to heat energy. The temperature gradient that occurs in in the typical heat conduction response system was avoided by this heating mechanism, which considerably enhanced heating efficiency. At the same time, the chance of intermolecular collisions was considerably raised, which improved the polymerization system Rp but also greatly improved the reaction’s conversion rate.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosal, M.; Kumar Chakraborty, A. Superplasticizer Compatibility with Cement Properties—A Study. Mater. Today Proc. 2022, 56, 568–573. [Google Scholar] [CrossRef]
- Sathyan, D.; Anand, K.B. Influence of Superplasticizer Family on the Durability Characteristics of Fly Ash Incorporated Cement Concrete. Constr. Build. Mater. 2019, 204, 864–874. [Google Scholar] [CrossRef]
- Vijay, A.; Sajeeb, R. Effect of Superplasticizer on the Characteristics of Stabilized Earth Concrete. Mater. Today Proc. 2022, 65, 455–460. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Wang, J.; Gao, N.; Hu, Z.; Liu, J.; Wang, K. A Novel Shrinkage-Reducing Polycarboxylate Superplasticizer for Cement-Based Materials: Synthesis, Performance and Mechanisms. Constr. Build. Mater. 2022, 321, 126342. [Google Scholar] [CrossRef]
- Jiao, D.; Shi, C.; De Schutter, G. Magneto-Responsive Structural Build-up of Highly Flowable Cementitious Paste in the Presence of PCE Superplasticizer. Constr. Build. Mater. 2022, 327, 126925. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, X.; Kong, X.; Zhou, S. Retardation Effect of PCE Superplasticizers with Different Architectures and Their Impacts on Early Strength of Cement Mortar. Cem. Concr. Compos. 2019, 104, 103369. [Google Scholar] [CrossRef]
- Zhu, W.; Feng, Q.; Luo, Q.; Bai, X.; Chen, K.; Lin, X. Effect of a Specific PCE Superplasticizer on the Initial Dissolution and Early Hydration of Portland Cement. J. Build. Eng. 2022, 46, 103786. [Google Scholar] [CrossRef]
- Liu, X.; Lai, G.; Guan, J.; Qian, S.; Wang, Z.; Cui, S.; Gao, F.; Jiao, Y.; Tao, R. Technical Optimization and Life Cycle Assessment of Environment-Friendly Superplasticizer for Concrete Engineering. Chemosphere 2021, 281, 130955. [Google Scholar] [CrossRef]
- Lei, L.; Plank, J. Synthesis and Properties of a Vinyl Ether-Based Polycarboxylate Superplasticizer for Concrete Possessing Clay Tolerance. Ind. Eng. Chem. Res. 2014, 53, 1048–1055. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical Admixtures—Chemistry, Applications and Their Impact on Concrete Microstructure and Durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, X.; Xing, F.; Dong, B.; Wang, F. Working Mechanism of Post-Acting Polycarboxylate Superplasticizers Containing Acrylate Segments. J. Appl. Polym. Sci. 2018, 135, 45753. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Ge, C.; Li, Q.; Guo, L.; Shu, X.; Liu, J. Mechanical Behavior and Toughening Mechanism of Polycarboxylate Superplasticizer Modified Graphene Oxide Reinforced Cement Composites. Compos. Part B Eng. 2017, 113, 308–316. [Google Scholar] [CrossRef]
- Balu, A.M.; Dallinger, D.; Obermayer, D.; Campelo, J.M.; Romero, A.A.; Carmona, D.; Balas, F.; Yohida, K.; Gai, P.L.; Vargas, C.; et al. Insights into the Microwave-Assisted Preparation of Supported Iron Oxide Nanoparticles on Silica-Type Mesoporous Materials. Green Chem. 2012, 14, 393–402. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Roccaro, P.; Bonanno, L.; De Guidi, G.; Vagliasindi, F.G.A.; Romano, S. A Review on the Microwave Heating as a Sustainable Technique for Environmental Remediation/Detoxification Applications. Renew. Sustain. Energy Rev. 2018, 95, 147–170. [Google Scholar] [CrossRef]
- Katsuki, H.; Choi, E.-K.; Lee, W.-J.; Hwang, K.-T.; Cho, W.-S.; Huang, W.; Komarneni, S. Ultrafast Microwave-Hydrothermal Synthesis of Hexagonal Plates of Hematite. Mater. Chem. Phys. 2018, 205, 210–216. [Google Scholar] [CrossRef]
- Rosana, M.R.; Tao, Y.; Stiegman, A.E.; Dudley, G.B. On the Rational Design of Microwave-Actuated Organic Reactions. Chem. Sci. 2012, 3, 1240–1244. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Cui, L.; Xiong, Q.; Mostafa, E. Microwave-Assisted Fluidized Bed Reactor Pyrolysis of Polypropylene Plastic for Pyrolysis Gas Production towards a Sustainable Development. Appl. Energy 2023, 342, 121099. [Google Scholar] [CrossRef]
- Heffernan, M.A.; O’Reilly, E.J. Rapid Microwave Assisted Synthesis and Characterisation of a Semiconducting Polymer with pKa Tuneable Degradation Properties. Eur. Polym. J. 2019, 114, 206–212. [Google Scholar] [CrossRef]
- Jovanovic, J.; Adnadjevic, B. Comparison of the Kinetics of Conventional and Microwave Methyl Methacrylate Polymerization. J. Appl. Polym. Sci. 2007, 104, 1775–1782. [Google Scholar] [CrossRef]
- Komorowska-Durka, M.; Dimitrakis, G.; Bogdał, D.; Stankiewicz, A.I.; Stefanidis, G.D. A Concise Review on Microwave-Assisted Polycondensation Reactions and Curing of Polycondensation Polymers with Focus on the Effect of Process Conditions. Chem. Eng. J. 2015, 264, 633–644. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Wang, D.; Wang, F.; Fang, K.; Yu, J.; Sheng, B. Syntheses of Polycarboxylate Superplasticizers: Microwave Induction versus Conventional Thermal Induction. Compos. Part B Eng. 2021, 207, 108560. [Google Scholar] [CrossRef]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-Assisted Synthesis—Catalytic Applications in Aqueous Media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Fang, S.; Wang, M.; Xu, Z.; Zhai, L.; Wang, X.; Xiong, Y. Preparation of an Amphoteric Flocculant Having Both High Polymer Content and Low Viscosity and Its Polymerization Kinetics. Chin. J. Chem. Eng. 2019, 27, 130–135. [Google Scholar] [CrossRef]
- GB/T601-2016; Chemical Reagent-Preparations of Reference Titration Solution. National Standard of the People’s Republic of China: Beijing, China, 2016.
- Mazo, P.; Estenoz, D.; Sponton, M.; Rios, L. Kinetics of the Transesterification of Castor Oil with Maleic Anhydride Using Conventional and Microwave Heating. J. Am. Oil Chem. Soc. 2012, 89, 1355–1361. [Google Scholar] [CrossRef]
- Costa, C.; Alberton, A.L.; Santos, A.F.; Fortuny, M.; Araújo, P.H.H.; Sayer, C.; Pinto, J.C. Kinetic Parameters of the Initiator Decomposition in Microwave and in Conventional Batch Reactors—KPS and V50-Case Studies. Macromol. React. Eng. 2015, 9, 366–373. [Google Scholar] [CrossRef]
- Dudley, G.B.; Richert, R.; Stiegman, A.E. On the Existence of and Mechanism for Microwave-Specific Reaction Rate Enhancement. Chem. Sci. 2015, 6, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Chen, X.; Shen, X.; Zhang, R.; Fu, Z.; Ma, X.; Zhang, X.; Li, B.; Zhang, Q. An Atomic Insight into the Chemical Origin and Variation of the Dielectric Constant in Liquid Electrolytes. Angew. Chem. Int. Ed. 2021, 60, 21473–21478. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, K.; Zeng, X.; Liu, C. Note: Coaxial Apparatus to Measure the Permittivities of Chemical Solutions at Microwave Frequencies. Rev. Sci. Instrum. 2017, 88, 046102. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, K.-M.; Yang, X.; Luo, M.; Zhu, H. An Artificial Nerve Network Realization in the Measurement of Material Permittivity. Prog. Electromagn. Res. 2011, 116, 347–361. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Huang, K.-M.; Yang, L. Calculation of Effective Permittivity of Mixtures During the Microwave Heating on the Chemical Reaction. Asian J. Chem. 2009, 21, 5703–5710. [Google Scholar]
- Kong, X.; Shi, Z.; Lu, Z. Synthesis of Novel Polymer Nano-Particles and Their Interaction with Cement. Constr. Build. Mater. 2014, 68, 434–443. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Microwave Pyrolysis of Lignocellulosic Biomass: Heating Performance and Reaction Kinetics. Energy 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Frasso, M.A.; Stiegman, A.E.; Dudley, G.B. Microwave-Specific Acceleration of a Retro-Diels–Alder Reaction. Chem. Commun. 2020, 56, 11247–11250. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Grant, E.H.; Halstead, S.J.B.; Mingos, D.M.P. Dielectric Parameters Relevant to Microwave Dielectric Heating. Chem. Soc. Rev. 1998, 27, 213. [Google Scholar] [CrossRef]
- Mingos, D.M.P.; Baghurst, D.R. Applications of Microwave Dielectric Heating Effects to Synthetic Problems in Chemistry. Chem. Soc. Rev. 1991, 20, 1–47. [Google Scholar] [CrossRef]
- Spasojević, P.; Jovanović, J.; Adnadjevic, B. Unique Effects of Microwave Heating on Polymerization Kinetics of Poly(Methyl Methacrylate) Composites. Mater. Chem. Phys. 2013, 141, 882–890. [Google Scholar] [CrossRef]
- Amini, N.; Dwivedi, S.; Ahmad, W.; Haritos, V.S.; Tanksale, A. Polar Solvents Enhance the Efficiency of Microwave Pre-Treatment of Woody Biomass. Biomass Bioenergy 2021, 155, 106281. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave Chemistry, Recent Advancements, and Eco-Friendly Microwave-Assisted Synthesis of Nanoarchitectures and Their Applications: A Review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Du, W.; Wang, D.; Zhang, Y.; Wang, F.; Zhang, D.; Chen, Y.; Zhai, X.; Liu, Y.; Yi, X. Investigating the Effect of Microwave Induction on the Polymerization Rate of Polycarboxylate Superplasticizers. Polymers 2024, 16, 322. https://doi.org/10.3390/polym16030322
Zhang L, Du W, Wang D, Zhang Y, Wang F, Zhang D, Chen Y, Zhai X, Liu Y, Yi X. Investigating the Effect of Microwave Induction on the Polymerization Rate of Polycarboxylate Superplasticizers. Polymers. 2024; 16(3):322. https://doi.org/10.3390/polym16030322
Chicago/Turabian StyleZhang, Liran, Wenqian Du, Dongmin Wang, Yue Zhang, Fang Wang, Dawang Zhang, Yang Chen, Xinyue Zhai, Yingchun Liu, and Xiao Yi. 2024. "Investigating the Effect of Microwave Induction on the Polymerization Rate of Polycarboxylate Superplasticizers" Polymers 16, no. 3: 322. https://doi.org/10.3390/polym16030322
APA StyleZhang, L., Du, W., Wang, D., Zhang, Y., Wang, F., Zhang, D., Chen, Y., Zhai, X., Liu, Y., & Yi, X. (2024). Investigating the Effect of Microwave Induction on the Polymerization Rate of Polycarboxylate Superplasticizers. Polymers, 16(3), 322. https://doi.org/10.3390/polym16030322






