Visible Light-Driven SnIn4S8 Photocatalyst Decorated on Polyurethane-Impregnated Microfiber Non-Woven Fabric for Pollutant Degradation
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Fabrication of Three-Dimensional Microfiber Non-Woven Fabric
2.3. Preparation of SnIn4S8 Photocatalysts
2.4. Preparation of SIS/PNWF Substrates
2.5. Evaluation of Photocatalytic Activity
2.6. Characterization
2.7. Regeneration and Reuse of SIS/PNWF Substrates
3. Results and Discussion
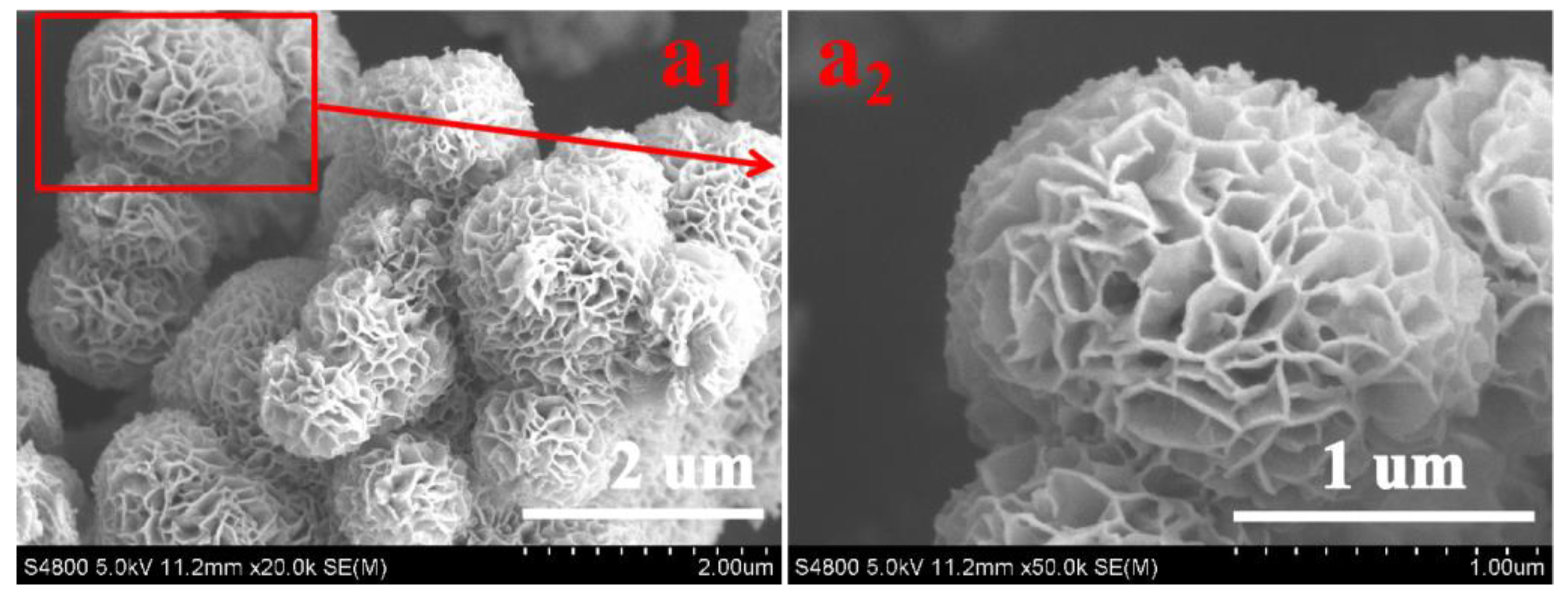
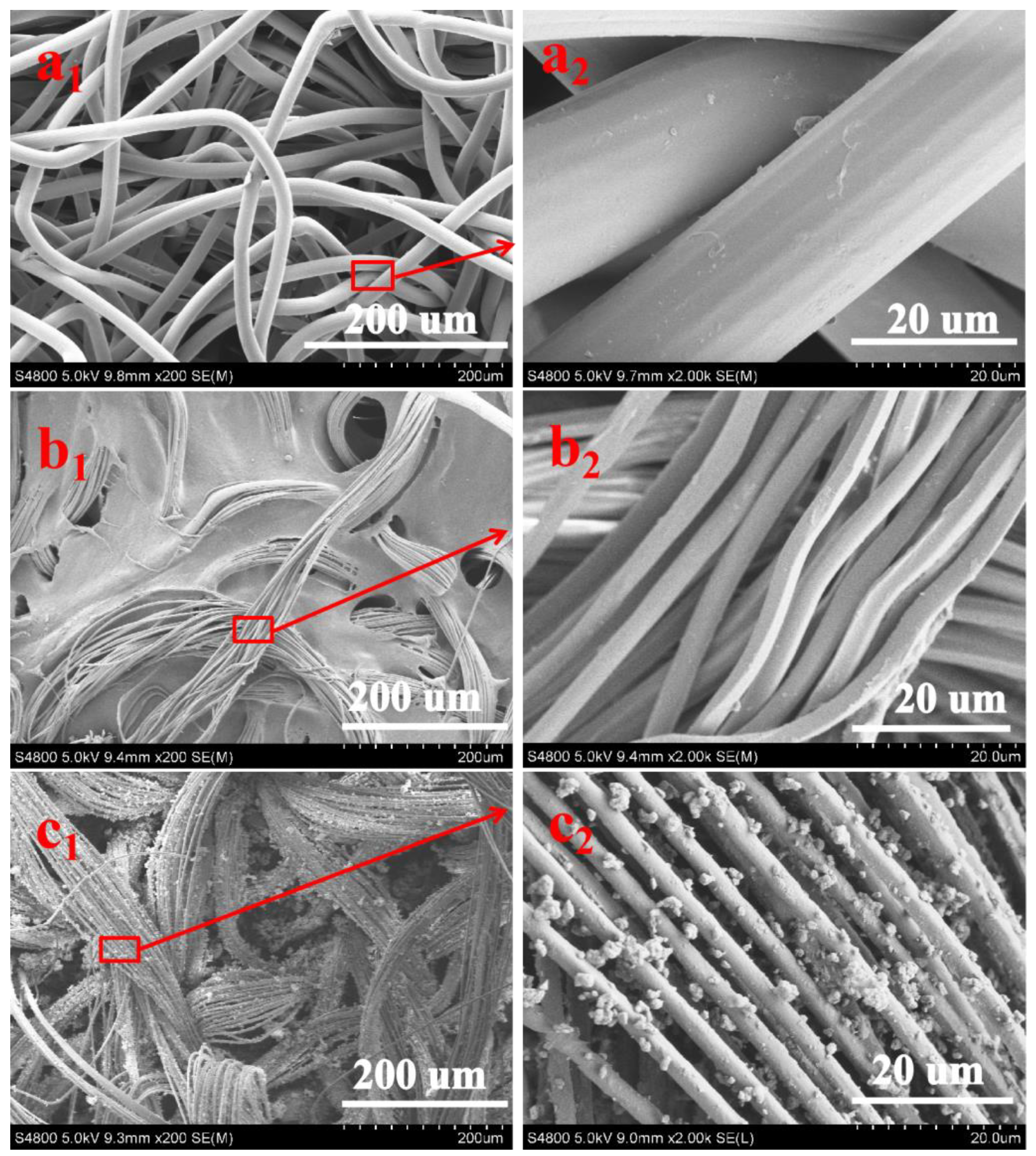
3.1. Morphology
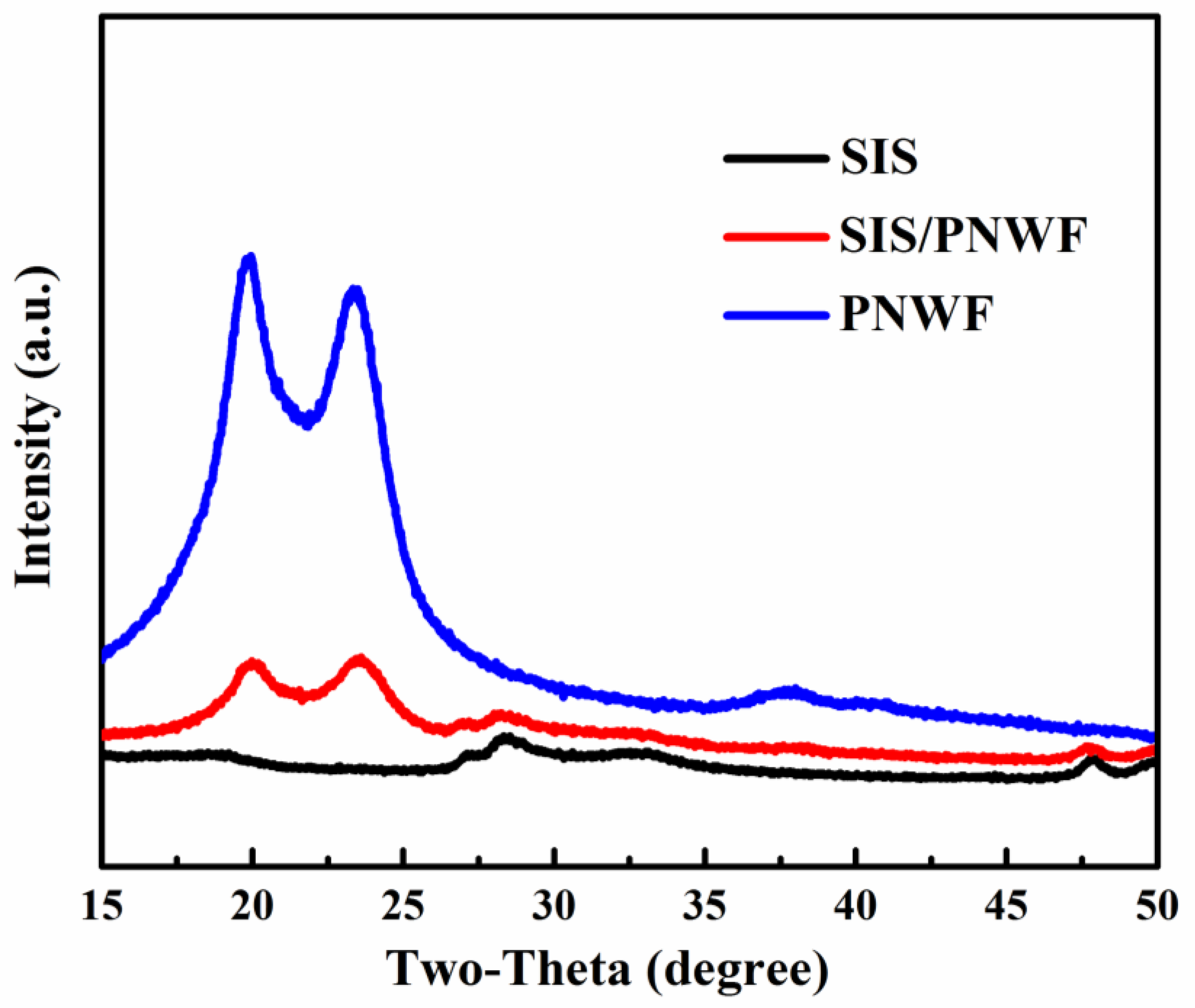
3.2. Crystal Structure
3.3. XPS Analysis
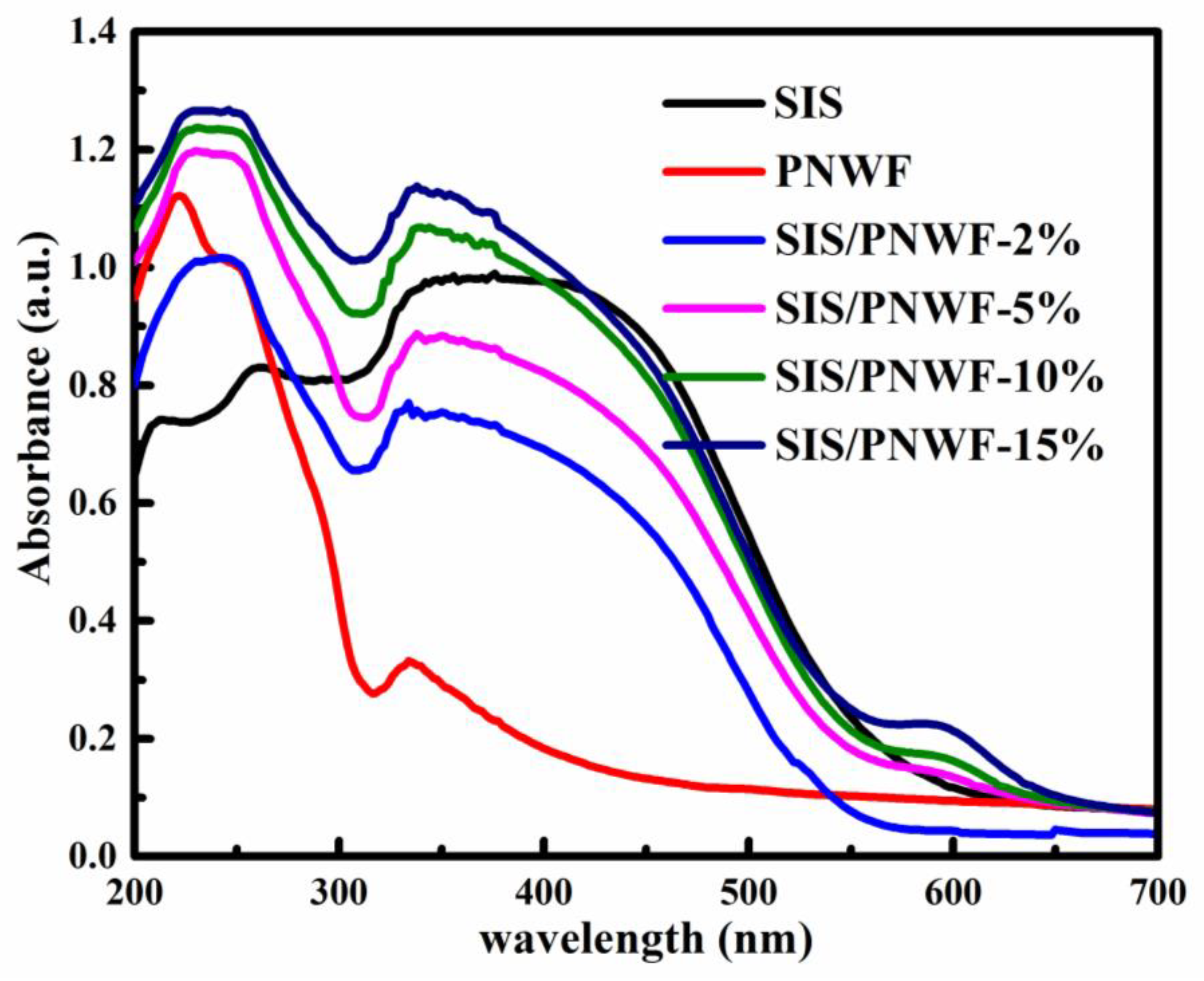
3.4. UV-Vis Diffuse Reflectance Spectra
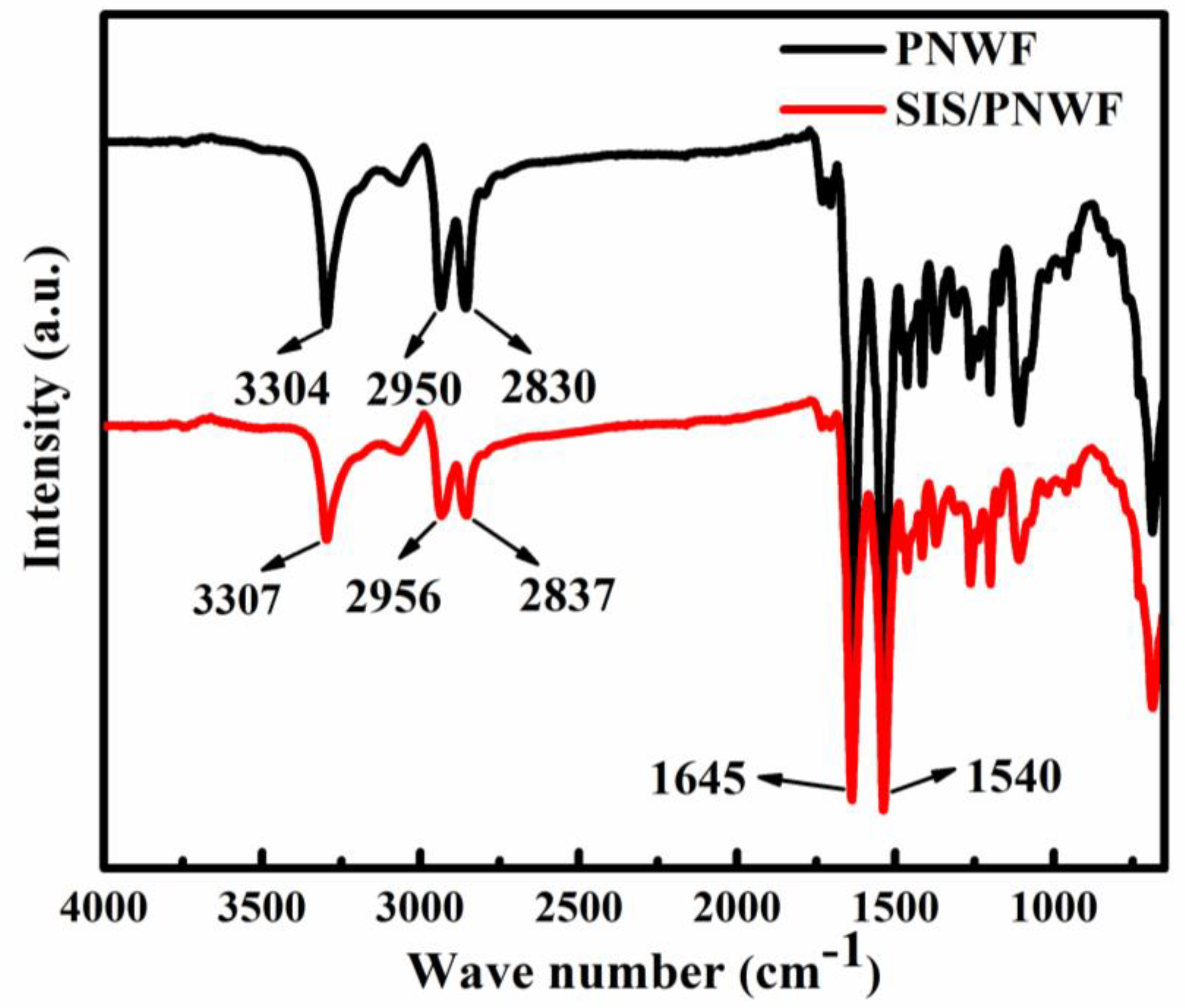
3.5. FT-IR Spectra
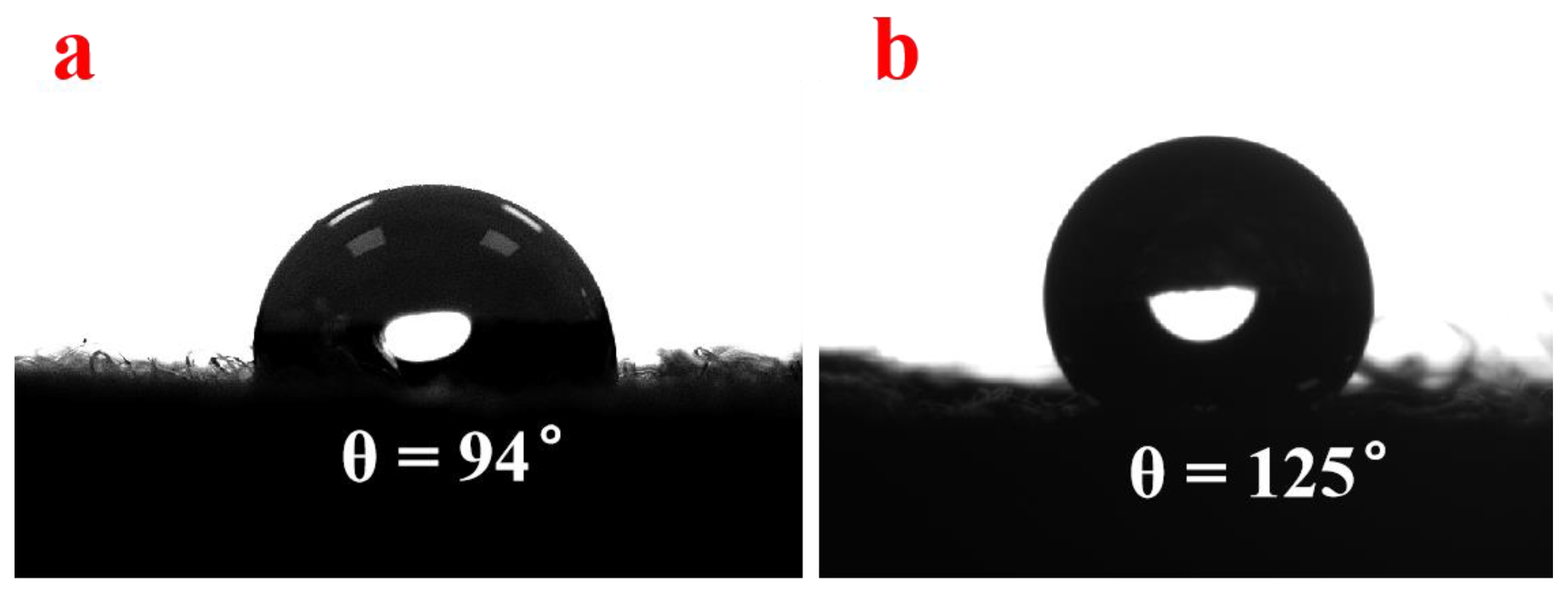
3.6. Wettability
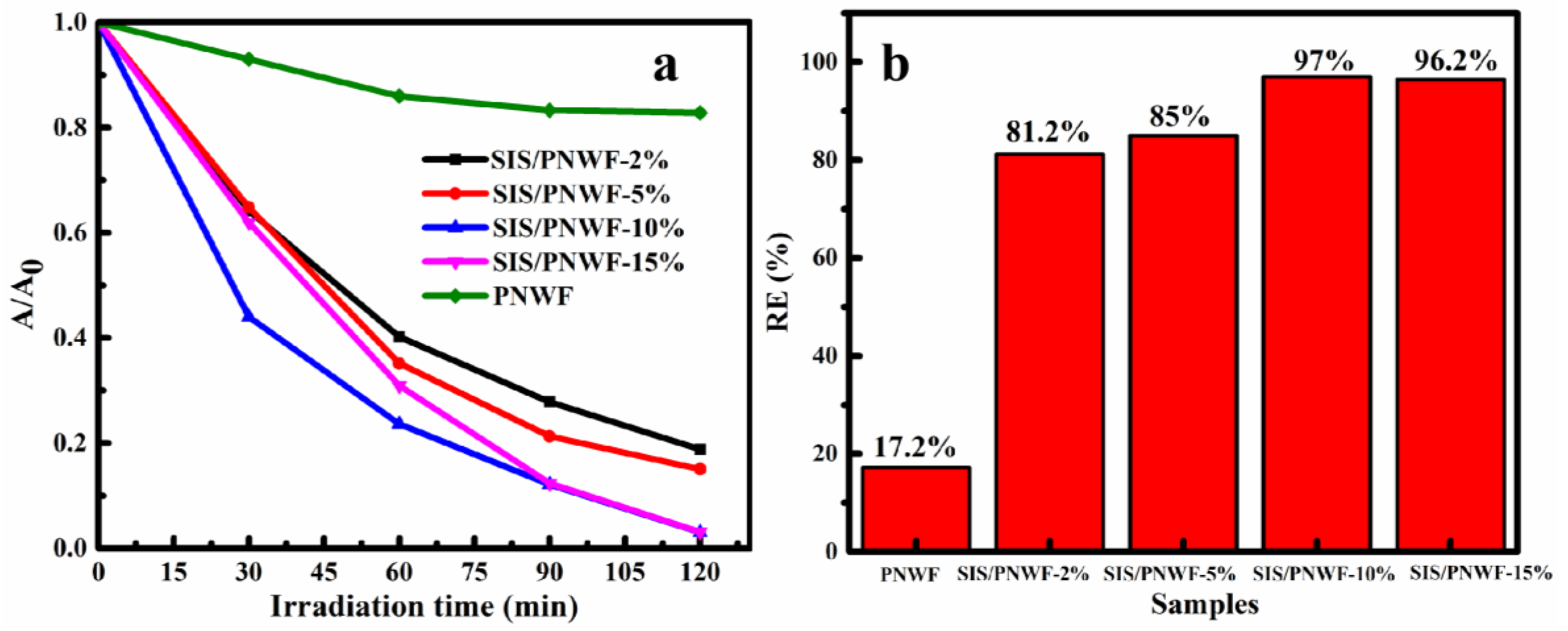
3.7. Photocatalytic Properties of SIS/PNWF Substrates
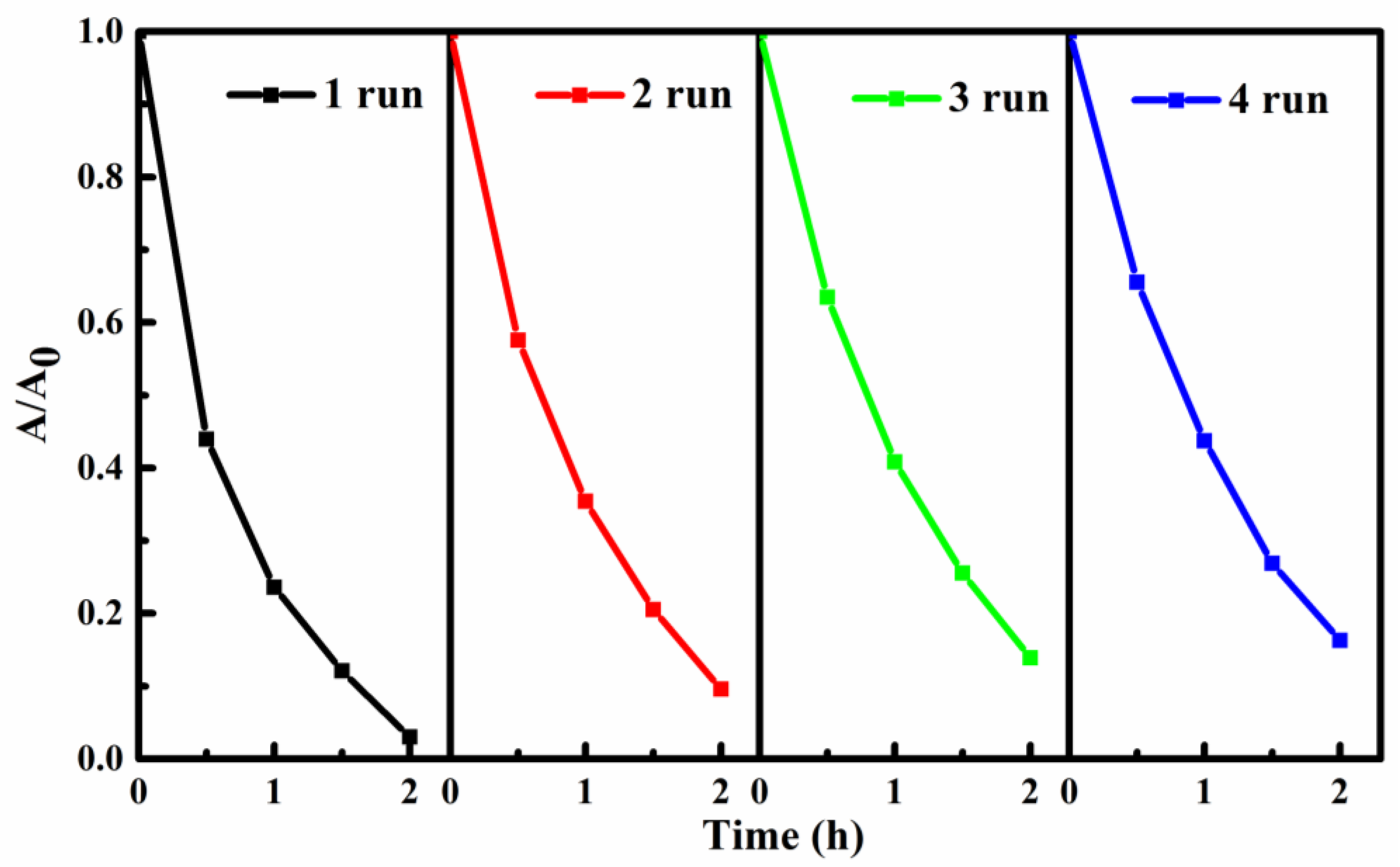
3.8. Reusability and Stability of the SIS/PNWF Substrates
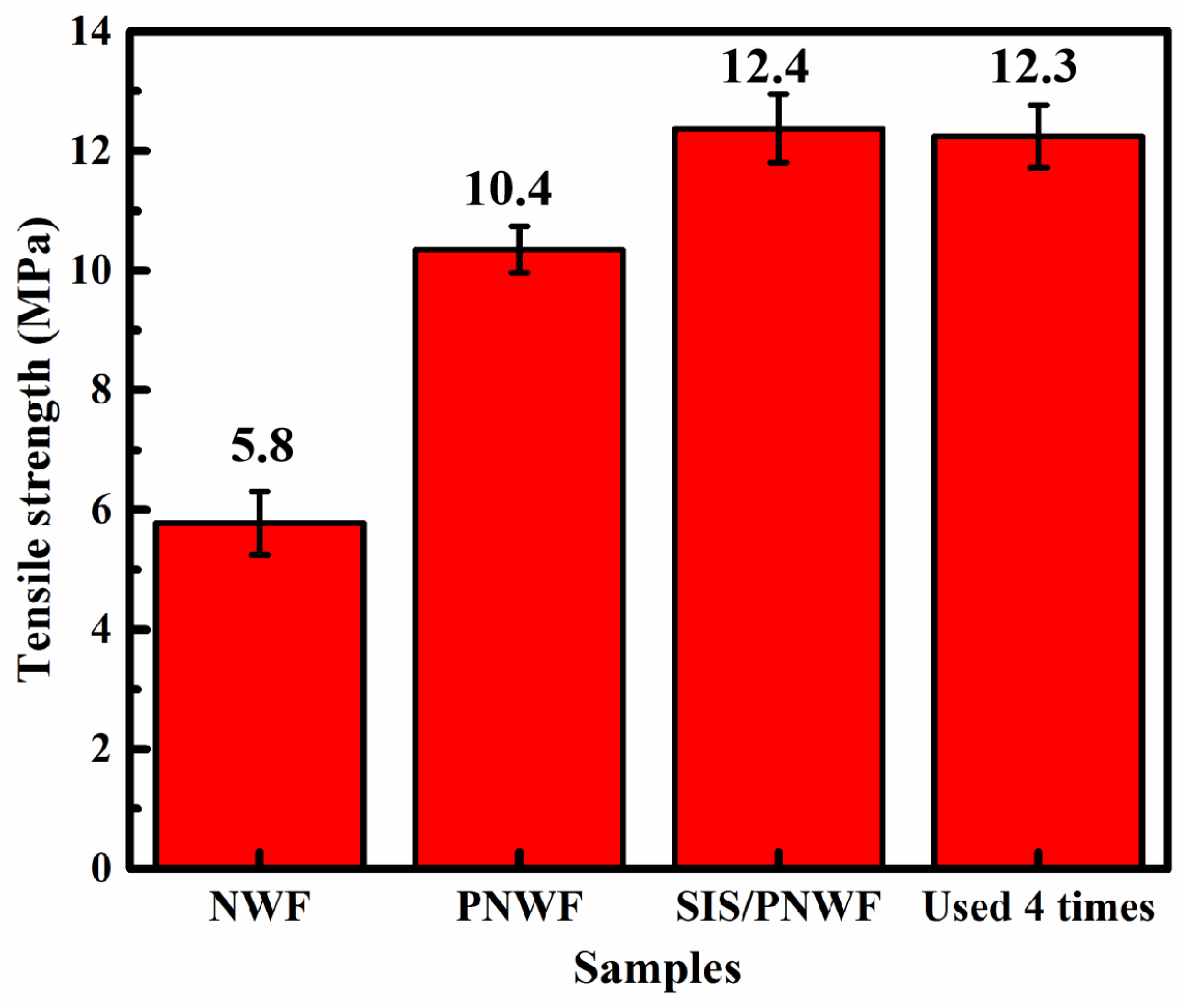
3.9. Tensile Strength of the SIS/PNWF Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D. Applied Catalysis B: Environmental 1D Porous Tubular g-C3N4 Capture Black Phosphorus Quantum Dots as 1D/0D Metal-Free Photocatalysts for Oxytetracycline Hydrochloride Degradation and Hexavalent Chromium Reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Bao, H.; Wu, M.; Meng, X.; Han, H.; Zhang, C.; Sun, W. Application of electrochemical oxidation technology in treating high-salinity organic ammonia-nitrogen wastewater. J. Environ. Chem. Eng. 2023, 11, 110608. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Mang, Z.; Wang, G. An overview of biological mechanisms and strategies for treatingwastewater from printing and dyeing processes. J. Water Process Eng. 2023, 55, 104242. [Google Scholar] [CrossRef]
- Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Grumezescu, A.M.; Purcar, V.; Radițoiu, V.; Fierascu, R.C. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. [Google Scholar] [CrossRef]
- Wei, J.; Yuan, M.; Wang, S.; Wang, X.; An, N.; Lv, G.; Wu, L. Recent advances in metal organic frameworks for the catalytic degradation of organic pollutants. Collagen Leather 2023, 5, 33. [Google Scholar] [CrossRef]
- Anisuzzaman, S.; Joseph, C.; Pang, C.; Affandi, N.; Maruja, S.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering 2022, 6, 58. [Google Scholar] [CrossRef]
- Moyo, S.; Makhanya, B.; Zwane, P. Use of bacterial isolates in the treatment of textile dye wastewater: A review. Heliyon 2022, 8, e09632. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Albaeejan, M.A.; Alshayiqi, A.A.; Aldubeikl, H.K.; Hasan, I. Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles. Nanotechnol. Rev. 2023, 12, 20230143. [Google Scholar] [CrossRef]
- Han, C.; Cheng, C.; Liu, F.; Li, X.L.; Wang, G.; Li, J. Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity. Nanotechnol. Rev. 2023, 12, 20220503. [Google Scholar] [CrossRef]
- Nasar, A.; Mashkoor, F. Application of polyaniline-based adsorbents for dye removal from water and wastewater—A review. Environ. Sci. Pollut. Res. 2019, 26, 5333–5356. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Muneer, M.; Haq, A.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, R.; Rehman, S.U.; Cao, F.; Xu, H.; Ma, X.; Wang, J.W.; Liu, Y.F.; Niu, Y.H.; Jian, X.; Mahmood, N. Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light. Nanotechnol. Rev. 2023, 12, 20220556. [Google Scholar] [CrossRef]
- Deng, F.; Pei, X.; Luo, Y.; Luo, X.; Dionysiou, D.; Wu, S.; Luo, S. Fabrication of Hierarchically Porous Reduced Graphene Oxide/SnIn4S8 Composites by a Low-Temperature Co-Precipitation Strategy and Their Excellent Visible-Light Photocatalytic Mineralization Performance. Catalysts 2016, 6, 113. [Google Scholar] [CrossRef]
- Tekin, D.; Kiziltas, H.; Ungan, H. Kinetic Evaluation of ZnO/TiO2 Thin Film Photocatalyst in Photocatalytic Degradation of Orange G. J. Mol. Liq. 2020, 306, 112905. [Google Scholar] [CrossRef]
- Shathy, R.A.; Fahim, S.A.; Sarker, M.; Quddus, M.S.; Moniruzzaman, M.; Masum, S.M.; Molla, M.A.I. Natural Sunlight Driven Photocatalytic Removal of Toxic Textile Dyes in Water Using B-Doped ZnO/TiO2 Nanocomposites. Catalysts 2022, 12, 308. [Google Scholar] [CrossRef]
- Ma, T.; Bai, J.; Li, C. Facile Synthesis of g-C3N4 Wrapping on One-Dimensional Carbon Fiber as a Composite Photocatalyst to Degrade Organic Pollutants. Vacuum 2017, 145, 47–54. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Y.; Fan, J.; Tang, Z. Applied Surface Science Construction of Novel Z-Scheme Flower-like Bi2S3/SnIn4S8 Heterojunctions with Enhanced Visible Light Photodegradation and Bactericidal Activity. Appl. Surf. Sci. 2019, 465, 212–222. [Google Scholar] [CrossRef]
- Chen, C.C.; Shaya, J.; Polychronopoulou, K.; Golovko, V.B.; Tesana, S.; Wang, S.Y.; Lu, C.S. Photocatalytic Degradation of Ethiofencarb by a Visible Light-Driven SnIn4S8 Photocatalyst. Nanomaterials 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Yan, T.; Li, L.; Li, G.; Wang, Y.; Hu, W.; Guan, X. Porous SnIn4S8 Microspheres in a New Polymorph that Promotes Dyes Degradation under Visible Light Irradiation. J. Hazard. Mater. 2011, 186, 272–279. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Wang, M.; Wang, Y.; Zhang, A.; Zhao, X.; Zeng, G.; Deng, F. Bandgap Engineering of Hierarchical Network-like SnIn4S8 Microspheres through Preparation Temperature for Excellent Photocatalytic Performance and High Stability. Green Energy Environ. 2019, 4, 264–269. [Google Scholar] [CrossRef]
- Deng, F.; Lu, X.; Zhao, L.; Luo, Y.; Pei, X.; Luo, X.; Luo, S. Facile Low-Temperature Co-Precipitation Method to Synthesize Hierarchical Network-like g-C3N4/SnIn4S8 with Superior Photocatalytic Performance. J. Mater. Sci. 2016, 51, 6998–7007. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Zhang, W.; Xiao, Y.; Xu, H.; Liu, Y.; Liu, Z.; Zhang, J.; Jiang, Y. Visible-light-driven double-shell SnIn4S8/TiO2 heterostructure with enhanced photocatalytic activity for MO removal and Cr(VI) cleanup. Appl. Surf. Sci. 2022, 587, 152867. [Google Scholar] [CrossRef]
- Du, Z.; Cui, C.; Zhang, S.; Xiao, H.; Ren, E.; Guo, R.; Jiang, S. Visible-Light-Driven Photocatalytic Degradation of Rhodamine B Using Bi2WO6/GO Deposited on Polyethylene Terephthalate Fabric. J. Leather Sci. Eng. 2020, 2, 16. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Wu, C.; Motora, K. Highly efficient solar-light-driven self-floatable WO2.72@Fe3O4 immobilized cellulose nanofiber aerogel/polypropylene Janus membrane for interfacial photocatalysis. J. Photochem. Photobiol. A Chem. 2023, 438, 114525. [Google Scholar] [CrossRef]
- Lee, J.; Tseng, H.; Wey, M. Excellent dispersion of solar light responsive photocatalyst in the different polymer films for easy recycling and sustainable hydrogen production. Sol. Energy 2022, 231, 949–957. [Google Scholar] [CrossRef]
- Liu, H.; Gu, H.; Li, G.; Li, N. Fabrication of PAN/Ag/ZnO Microporous Membrane and Examination of Visible Light Photocatalytic Performance. Fibers Polym. 2021, 22, 306–313. [Google Scholar] [CrossRef]
- Tran, M.; Fu, C.; Chiang, L.; Hsieh, C.; Liu, S.; Juang, R. Immobilization of TiO2 and TiO2-GO hybrids onto the surface of acrylic acid-grafted polymeric membranes for pollutant removal: Analysis of photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104422. [Google Scholar] [CrossRef]
- Alahiane, S.; Sennaoui, A.; Sakr, F.; Dinne, M.; Qourzal, S.; Assabbane, A. Synchronous role of coupled adsorption-photocatalytic degradation of Direct Red 80 with nanocrystalline TiO2-coated non-woven fibres materials in a static batch photoreactor. Groundw. Sust. Dev. 2020, 11, 100396. [Google Scholar] [CrossRef]
- Parikh, D.V.; Nam, S.; He, Q.L. Evaluation of Three Flame Retardant (FR) Grey Cotton Blend Nonwoven Fabrics Using Micro-Scale Combustion Calorimeter. J. Fire Sci. 2012, 30, 187–200. [Google Scholar] [CrossRef]
- Lu, Z.; Peng, J.; Song, M.; Liu, Y.; Liu, X.; Huo, P.; Dong, H.; Yuan, S.; Ma, Z.; Han, S. Improved recyclability and selectivity of environment-friendly MFA-based heterojunction imprinted photocatalyst for secondary pollution free tetracycline orientation degradation. Chem. Eng. J. 2019, 360, 1262–1276. [Google Scholar] [CrossRef]
- Byun, J.; Landfester, K.; Zhang, K. Conjugated Polymer Hydrogel Photocatalysts with Expandable Photoactive Sites in Water. Chem. Mater. 2019, 31, 3381–3387. [Google Scholar] [CrossRef]
- Stoilova, O.; Manolva, N.; Rashkov, I. Electrospun Poly(Methyl Methacrylate)/TiO2 Composites for Photocatalytic Water Treatment. Polymers 2021, 13, 3923. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, C.; Zhao, X.; Fu, Z. Study on preparation and recovery of magnetic BiOI/rGO/Fe3O4 composite photocatalyst. Results Phys. 2020, 16, 102931. [Google Scholar]
- Zainal, Z.; Hui, L.; Hussein, M.; Abdullah, A.; Hamadneh, I. Characterization of TiO2–Chitosan/Glass photocatalyst for the removal of a monoazo dye via photodegradation–adsorption process. J. Hazard. Mater. 2009, 164, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, W.; Chen, Y.; Li, N.; Zhu, Z.; Wang, G.; Chen, W. Effective elimination of antibiotics over hot-melt adhesive sheath-core polyester fiber supported graphitic carbon nitride under solar irradiation. Chem. Eng. J. 2018, 335, 82–93. [Google Scholar] [CrossRef]
- Dao, M.; Nguyen, T.; Le, V.; Hoang, H.; Le, T.; Pham, T.; Nguyen, T.; Akhmadullin, R.; Lee, H.; Tran, H.; et al. Non-woven polyester fabric-supported cuprous oxide/reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue. J. Mater. Sci. 2021, 56, 10353–10366. [Google Scholar] [CrossRef]
- Huo, K.; Wang, J.; Zhuang, T.; Zhao, Y.; Gao, B.; Dou, M.; Wang, X.; Fu, Y.; Wang, D.; Ci, L. Facile fabrication of recyclable and macroscopic D-g-C3N4/sodium alginates/non-woven fabric immobilized photocatalysts with enhanced photocatalytic activity and antibacterial performance. J. Mater. Sci. 2021, 56, 17584–17600. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, X.; Fan, S.; Zhang, Y.; Hu, H.; Huang, Z.; Liang, J.; Qin, Y. Preparation of a stable polyurethane sponge supported Sn-doped ZnO composite via double-template-regulated bionic mineralization for visible-light-driven photocatalytic degradation of tetracycline. J. Environ. Chem. Eng. 2021, 9, 105541. [Google Scholar] [CrossRef]
- Tian, S.Q.; Chen, Y.Y.; Zhu, Y.F.; Fan, H.J. A Fluorescent Polyurethane with Covalently Cross-Linked Rhodamine Derivatives. Polymers 2020, 12, 1989. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Fan, H. Design, Characterization, Dyeing Properties, and Application of Acid-Dyeable Polyurethane in the Manufacture of Microfiber Synthetic Leather. Fibers Polym. 2015, 16, 1970–1980. [Google Scholar] [CrossRef]
- Wen, J.; Sun, Z.; Wang, Z.; Fan, H.; Xiang, J.; Chen, Y.; Yan, J.; Ning, J. Biomimetic Construction of Three-Dimensional Superhydrophobic Microfiber Nonwoven Fabric. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125990. [Google Scholar] [CrossRef]
- Li, J.; Cui, M.; Wen, J.; Chen, Y.; Shi, B.; Fan, H.; Xiang, J. Leather-like Hierarchical Porous Composites with Outstanding Electromagnetic Interference Shielding Effectiveness and Durability. Compos. Part B Eng. 2021, 225, 109272. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Gao, Q.; Xiang, J.; Chen, Y.; Yan, J.; Fan, H. A Novel Strategy to Fabricate Nylon 6 Based Flame Retardant Microfiber Nonwoven Fabric with Durability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128482. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. Microwave-Assisted Synthesis of Azo Disperse Dyes for Dyeing Polyester Fabrics: Our Contributions over the Past Decade. Polymers 2022, 14, 1703. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Jen, T.C. Microwave-assisted sol-gel template-free synthesis and characterization of silica nanoparticles obtained from South African coal fly ash. Nanotechnol. Rev. 2022, 11, 3042–3052. [Google Scholar] [CrossRef]
- Chumha, N.; Pudkon, W.; Chachvalvutikul, A.; Luangwanta, T.; Randorn, C.; Inceesungvorn, B.; Ngamjarurojana, A.; Kaowphong, S. Photocatalytic Activity of CuInS2 Nanoparticles Synthesized via a Simple and Rapid Microwave Heating Process. Mater. Res. Express 2020, 7, 015074. [Google Scholar] [CrossRef]
- Li, S.; Xia, X.; Vogt, B.D. Microwave-Enabled Size Control of Iron Oxide Nanoparticles on Reduced Graphene Oxide. Langmuir 2021, 37, 11131–11141. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Gao, Q.; Luo, H.; Fan, H.; Xiang, J.; Chen, Y. Urchin-like SnIn4S8 Photocatalyst Synthesized via Microwave Assisted Method with Durable Photocatalytic Performance under Visible Light. Chem. Phys. Lett. 2023, 817, 140409. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Chen, X.; Hong, W.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. A halogen-free, flame retardant, waterborne polyurethane coating based on the synergistic effect of phosphorus and silicon. Prog. Org. Coat. 2021, 158, 106359. [Google Scholar] [CrossRef]
- Xu, H.; Dai, J.; Fang, K.; Guo, Y.; Chen, W.; Liu, X.; Zhang, L.; Li, R.; Liu, D.; Xie, R. BiOI/PPy/cotton photocatalytic fabric for efficient organic dye contaminant degradation and self-cleaning application. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131862. [Google Scholar] [CrossRef]
- Du, Z.; Cheng, C.; Tan, L.; Lan, J.; Jiang, S.; Zhao, L.; Guo, R. Enhanced Photocatalytic Activity of Bi2WO6/TiO2 Composite Coated Polyester Fabric under Visible Light Irradiation. Appl. Surf. Sci. 2018, 435, 626–634. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Makhlouf, A.; Khalil, K. Novel, Facile, Single-Step Technique of Polymer/TiO2 Nanofiber Composites Membrane for Photodegradation of Methylene Blue. ACS Appl. Mater. Interfaces 2015, 7, 13329–13341. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gao, Q.; Luo, H.; Zhao, J.; Fan, H.; Chen, Y.; Xiang, J. Visible Light-Driven SnIn4S8 Photocatalyst Decorated on Polyurethane-Impregnated Microfiber Non-Woven Fabric for Pollutant Degradation. Polymers 2024, 16, 369. https://doi.org/10.3390/polym16030369
Wang Z, Gao Q, Luo H, Zhao J, Fan H, Chen Y, Xiang J. Visible Light-Driven SnIn4S8 Photocatalyst Decorated on Polyurethane-Impregnated Microfiber Non-Woven Fabric for Pollutant Degradation. Polymers. 2024; 16(3):369. https://doi.org/10.3390/polym16030369
Chicago/Turabian StyleWang, Zhonghui, Qiang Gao, Haihang Luo, Jianming Zhao, Haojun Fan, Yi Chen, and Jun Xiang. 2024. "Visible Light-Driven SnIn4S8 Photocatalyst Decorated on Polyurethane-Impregnated Microfiber Non-Woven Fabric for Pollutant Degradation" Polymers 16, no. 3: 369. https://doi.org/10.3390/polym16030369
APA StyleWang, Z., Gao, Q., Luo, H., Zhao, J., Fan, H., Chen, Y., & Xiang, J. (2024). Visible Light-Driven SnIn4S8 Photocatalyst Decorated on Polyurethane-Impregnated Microfiber Non-Woven Fabric for Pollutant Degradation. Polymers, 16(3), 369. https://doi.org/10.3390/polym16030369







