The Kinetic Study of the Influence of Common Modifiers on the Curing Process of Epoxy Vitrimers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Resin Compositions
2.3. Dissolution of the Cured Compositions and Resin Recovery from the Solution
2.4. Measurements
2.5. Kinetic Computations
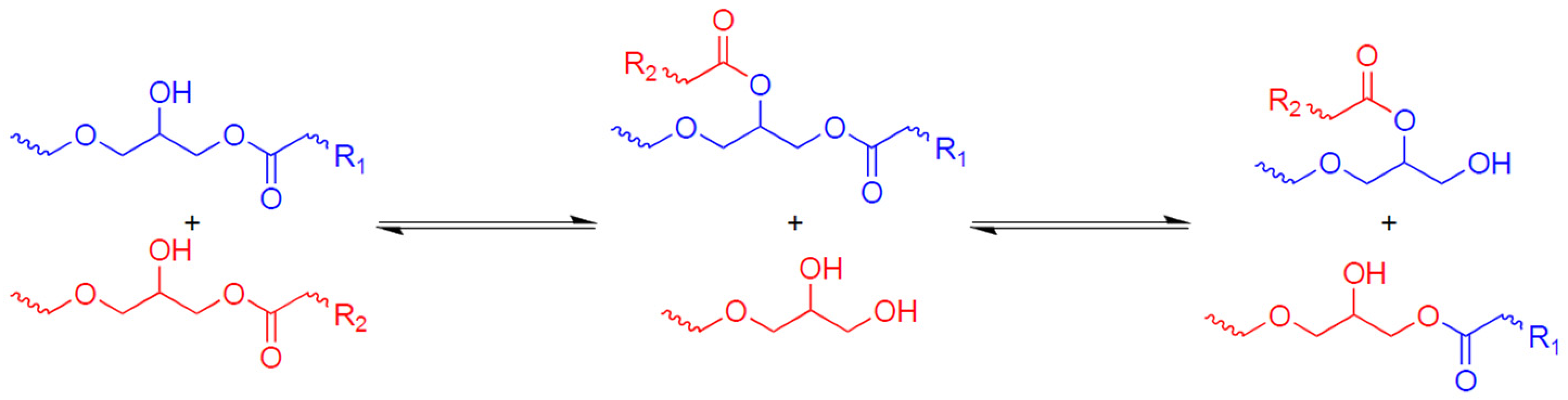
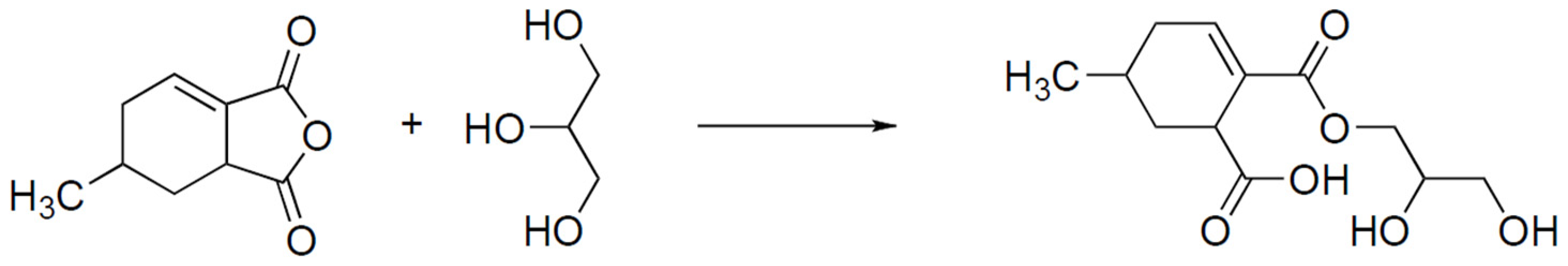
3. Results and Discussion
3.1. Study of Curing Process
3.2. Properties of Cured Resins
3.3. Reprocessibility of Resin Compositions
3.3.1. Solubility of Cured Resin Compositions
3.3.2. Secondary Use of Dissolved Cured Resin Compositions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Holbery, J.; Houston, D. Natural-Fiber-Reinforced Polymer Composites in Automotive Applications. JOM 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Elanchezhian, C.; Ramnath, B.V.; Ramakrishnan, G.; Raghavendra, K.S.; Muralidharan, M.; Kishore, V. Review on Metal Matrix Composites for Marine Applications. Mater. Today Proc. 2018, 5, 1211–1218. [Google Scholar] [CrossRef]
- Eliasson, J.; Sandström, R. Applications of Aluminium Matrix Composites. In Key Engineering Materials; Trans Tech Publications, Ltd.: Bäch, Switzerland, 1995; Volume 104, pp. 3–36. [Google Scholar]
- Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Synthesis and Modifications of Epoxy Resins and Their Composites: A Review. Polym.-Plast. Technol. Eng. 2014, 53, 1723–1758. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chevali, V.S.; Wang, H.; Wang, C.-H. Current Status of Carbon Fibre and Carbon Fibre Composites Recycling. Compos. Part B Eng. 2020, 193, 108053. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite Material Recycling Technology—State-of-the-Art and Sustainable Development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Abdallah, R.; Juaidi, A.; Savaş, M.A.; Çamur, H.; Albatayneh, A.; Abdala, S.; Manzano-Agugliaro, F. Retraction: Abdallah et al. A Critical Review on Recycling Composite Waste Using Pyrolysis for Sustainable Development. Energies 2021, 14, 5748, Energies 2022, 15, 7645. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Jiménez-Suárez, A.; Prolongo, S.G.; Serras-Malillos, A.; Acha, E.; Caballero, B.M. Secondary Raw Materials from Residual Carbon Fiber-Reinforced Composites by an Upgraded Pyrolysis Process. Polymers 2021, 13, 3408. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Winne, J.M.; Prez, F.E.D. Vitrimers: Permanent Organic Networks with Glass-like Fluidity. Chem. Sci. 2015, 7, 30–38. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent Adaptable Networks: Smart, Reconfigurable and Responsive Network Systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed]
- Elling, B.R.; Dichtel, W.R. Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef]
- Winne, J.M.; Leibler, L.; Prez, F.E.D. Dynamic Covalent Chemistry in Polymer Networks: A Mechanistic Perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Yaseen, M.; Huang, K. A Review on the Self-Healing Ability of Epoxy Polymers. J. Appl. Polym. Sci. 2021, 138, 50260. [Google Scholar] [CrossRef]
- Guerre, M.; Taplan, C.; Winne, J.M.; Prez, F.E.D. Vitrimers: Directing Chemical Reactivity to Control Material Properties. Chem. Sci. 2020, 11, 4855–4870. [Google Scholar] [CrossRef]
- Podgórski, M.; Spurgin, N.; Mavila, S.; Bowman, C.N. Mixed Mechanisms of Bond Exchange in Covalent Adaptable Networks: Monitoring the Contribution of Reversible Exchange and Reversible Addition in Thiol–Succinic Anhydride Dynamic Networks. Polym. Chem. 2020, 11, 5365–5376. [Google Scholar] [CrossRef]
- Tian, Q.; Rong, M.Z.; Zhang, M.Q.; Yuan, Y.C. Synthesis and Characterization of Epoxy with Improved Thermal Remendability Based on Diels-Alder Reaction. Polym. Int. 2010, 59, 1339–1345. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Liu, X.; Xu, J.; Wang, D. Facile Fabrication of Fast Recyclable and Multiple Self-Healing Epoxy Materials through Diels-Alder Adduct Cross-Linker. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ji, Y.; Wei, Y. Functional Epoxy Vitrimers and Composites. Prog. Mater. Sci. 2021, 120, 100710. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy Resin with Exchangeable Disulfide Crosslinks to Obtain Reprocessable, Repairable and Recyclable Fiber-Reinforced Thermoset Composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Matxain, J.M.; Ruipérez, F.; Martin, R.; Asua, J.M.; Cabañero, G.; Odriozola, I. Transient Mechanochromism in Epoxy Vitrimer Composites Containing Aromatic Disulfide Crosslinks. J. Mater. Chem. C 2016, 4, 6220–6223. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Guan, Z. Olefin Metathesis for Effective Polymer Healing via Dynamic Exchange of Strong Carbon–Carbon Double Bonds. J. Am. Chem. Soc. 2012, 134, 14226–14231. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Tournilhac, F.; Leibler, L.; Guan, Z. Making Insoluble Polymer Networks Malleable via Olefin Metathesis. J. Am. Chem. Soc. 2012, 134, 8424–8427. [Google Scholar] [CrossRef]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and Recycling of Highly Cross-Linked Ion-Conducting Networks through Transalkylation Exchanges of C–N Bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, B.; Waelkens, J.; Winne, J.M.; Du Prez, F.E. Poly(Thioether) Vitrimers via Transalkylation of Trialkylsulfonium Salts. ACS Macro Lett. 2017, 6, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, O.R.; Chung, J.; Guan, Z. Malleable and Self-Healing Covalent Polymer Networks through Tunable Dynamic Boronic Ester Bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef]
- Cash, J.J.; Kubo, T.; Bapat, A.P.; Sumerlin, B.S. Room-Temperature Self-Healing Polymers Based on Dynamic-Covalent Boronic Esters. Macromolecules 2015, 48, 2098–2106. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation. Angew. Chem. Int. Ed. 2016, 55, 11421–11425. [Google Scholar] [CrossRef] [PubMed]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Pu, W.; Wang, Z.; Lu, X.; Sun, S.; Yu, C.; Xia, H. A Facile Dynamic Crosslinked Healable Poly(Oxime-Urethane) Elastomer with High Elastic Recovery and Recyclability. J. Mater. Chem. A 2018, 6, 18154–18164. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable Photoinduced Self-Healing of Covalently Cross-Linked Polymers through Reshuffling of Trithiocarbonate Units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous Urethane Vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic Covalent Polymer Networks Based on Degenerative Imine Bond Exchange: Tuning the Malleability and Self-Healing Properties by Solvent. Macromolecules 2016, 49, 6277–6284. [Google Scholar] [CrossRef]
- Taynton, P.; Ni, H.; Zhu, C.; Yu, K.; Loob, S.; Jin, Y.; Qi, H.J.; Zhang, W. Repairable Woven Carbon Fiber Composites with Full Recyclability Enabled by Malleable Polyimine Networks. Adv. Mater. 2016, 28, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Q.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Catalyst-Free Dynamic Exchange of Aromatic Schiff Base Bonds and Its Application to Self-Healing and Remolding of Crosslinked Polymers. J. Mater. Chem. A 2015, 3, 19662–19668. [Google Scholar] [CrossRef]
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.; Qi, H.J.; Zhang, W. Heat- or Water-Driven Malleability in a Highly Recyclable Covalent Network Polymer. Adv. Mater. 2014, 26, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Digby, Z.A.; Flum, J.A.; Chakma, P.; Saul, J.M.; Sparks, J.L.; Konkolewicz, D. Dynamic Thiol–Michael Chemistry for Thermoresponsive Rehealable and Malleable Networks. Macromolecules 2016, 49, 6871–6878. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, M.Q.; Rong, M.Z. Application of Alkoxyamine in Self-Healing of Epoxy. J. Mater. Chem. A 2014, 2, 6558–6566. [Google Scholar] [CrossRef]
- Snyder, R.L.; Fortman, D.J.; De Hoe, G.X.; Hillmyer, M.A.; Dichtel, W.R. Reprocessable Acid-Degradable Polycarbonate Vitrimers. Macromolecules 2018, 51, 389–397. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, S.; Hao, C.; Verdi, C.; Liu, W.; Liu, H.; Zhang, J. Glycerol Induced Catalyst-Free Curing of Epoxy and Vitrimer Preparation. Macromol. Rapid Commun. 2019, 40, 1800889. [Google Scholar] [CrossRef]
- Han, J.; Liu, T.; Hao, C.; Zhang, S.; Guo, B.; Zhang, J. A Catalyst-Free Epoxy Vitrimer System Based on Multifunctional Hyperbranched Polymer. Macromolecules 2018, 51, 6789–6799. [Google Scholar] [CrossRef]
- Delahaye, M.; Winne, J.M.; Du Prez, F.E. Internal Catalysis in Covalent Adaptable Networks: Phthalate Monoester Transesterification As a Versatile Dynamic Cross-Linking Chemistry. J. Am. Chem. Soc. 2019, 141, 15277–15287. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Fan, J.; Huang, J.; Chen, Y. A Robust and Stretchable Cross-Linked Rubber Network with Recyclable and Self-Healable Capabilities Based on Dynamic Covalent Bonds. J. Mater. Chem. A 2019, 7, 4922–4933. [Google Scholar] [CrossRef]
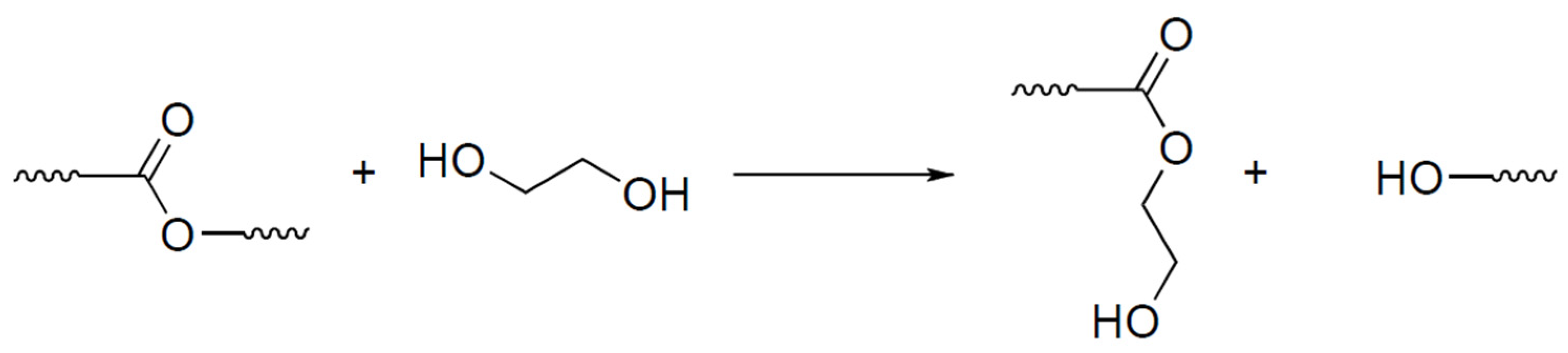
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Influence of Stoichiometry on the Glass Transition and Bond Exchange Reactions in Epoxy Thermoset Polymers. RSC Adv. 2014, 4, 48682–48690. [Google Scholar] [CrossRef]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Reprocessing and Recycling of Thermosetting Polymers Based on Bond Exchange Reactions. RSC Adv. 2014, 4, 10108–10117. [Google Scholar] [CrossRef]
- Lu, L.; Pan, J.; Li, G. Recyclable High-Performance Epoxy Based on Transesterification Reaction. J. Mater. Chem. A 2017, 5, 21505–21513. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zhang, S.; Shao, L.; Fei, M.; Yu, H.; Zhang, J. Catalyst-Free Vitrimer Elastomers Based on a Dimer Acid: Robust Mechanical Performance, Adaptability and Hydrothermal Recyclability. Green Chem. 2020, 22, 870–881. [Google Scholar] [CrossRef]
- Altuna, F.I.; Pettarin, V.; Williams, R.J.J. Self-Healable Polymer Networks Based on the Cross-Linking of Epoxidised Soybean Oil by an Aqueous Citric Acid Solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Hassanein, M.; Hewaidy, I.F. A Simple Method for Preparation of Zinc, Cadmium, Mercuric and Cobalt Acetylacetonates. Z. Anorg. Allg. Chem. 1970, 373, 80–82. [Google Scholar] [CrossRef]
- ISO 11357-5; Plastics—Differential Scanning Calorimetry (DSC)—Part 5: Determination of Characteristic Reaction-Curve Temperatures and Times, Enthalpy of Reaction and Degree of Conversion. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 11357-2; Plastics—Differential Scanning Calorimetry (DSC)—Part 2: Determination of Glass Transition Temperature and Step Height. International Organization for Standardization: Geneva, Switzerland, 2020.
- ASTM D7028-07; Standard Test Method for Glass Transition Temperature (DMA Tg) of Polymer Matrix Composites by Dynamic Mechanical Analysis (DMA). ASTM Standards: West Conshohocken, PA, USA, 2015.
- ISO 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 2602; Statistical Interpretation of Test Results—Estimation of the Mean—Confidence Interval. International Organization for Standardization: Geneva, Switzerland, 1980.
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dollimore, D. Linear and Nonlinear Procedures in Isoconversional Computations of the Activation Energy of Nonisothermal Reactions in Solids. J. Chem. Inf. Comput. Sci. 1996, 36, 42–45. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the Integral Isoconversional Method to Account for Variation in the Activation Energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Tašner, M.; Vušak, D.; Kekez, I.; Gabud, A.; Pilepić, V.; Mrvoš-Sermek, D.; Matković-Čalogović, D. Zn(II) Halide Coordination Compounds with Imidazole and 2-Methylimidazole. Structural and Computational Characterization of Intermolecular Interactions and Disorder. Heliyon 2022, 8, e11100. [Google Scholar] [CrossRef] [PubMed]
- Vedernikov, A.; Nasonov, Y.; Korotkov, R.; Gusev, S.; Akhatov, I.; Safonov, A. Effects of Additives on the Cure Kinetics of Vinyl Ester Pultrusion Resins. J. Compos. Mater. 2021, 55, 2921–2937. [Google Scholar] [CrossRef]
- Demongeot, A.; Mougnier, S.J.; Okada, S.; Soulié-Ziakovic, C.; Tournilhac, F. Coordination and Catalysis of Zn2+ in Epoxy-Based Vitrimers. Polym. Chem. 2016, 7, 4486–4493. [Google Scholar] [CrossRef]
- Tripathi, M.; Dwivedi, R.; Kumar, D.; Roy, P.K. Thermal Activation of Mendable Epoxy through Inclusion of Microcapsules and Imidazole Complexes. Polym.-Plast. Technol. Eng. 2016, 55, 129–137. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H.J. Recycling of Epoxy Thermoset and Composites via Good Solvent Assisted and Small Molecules Participated Exchange Reactions. ACS Sustain. Chem. Eng. 2018, 6, 9189–9197. [Google Scholar] [CrossRef]


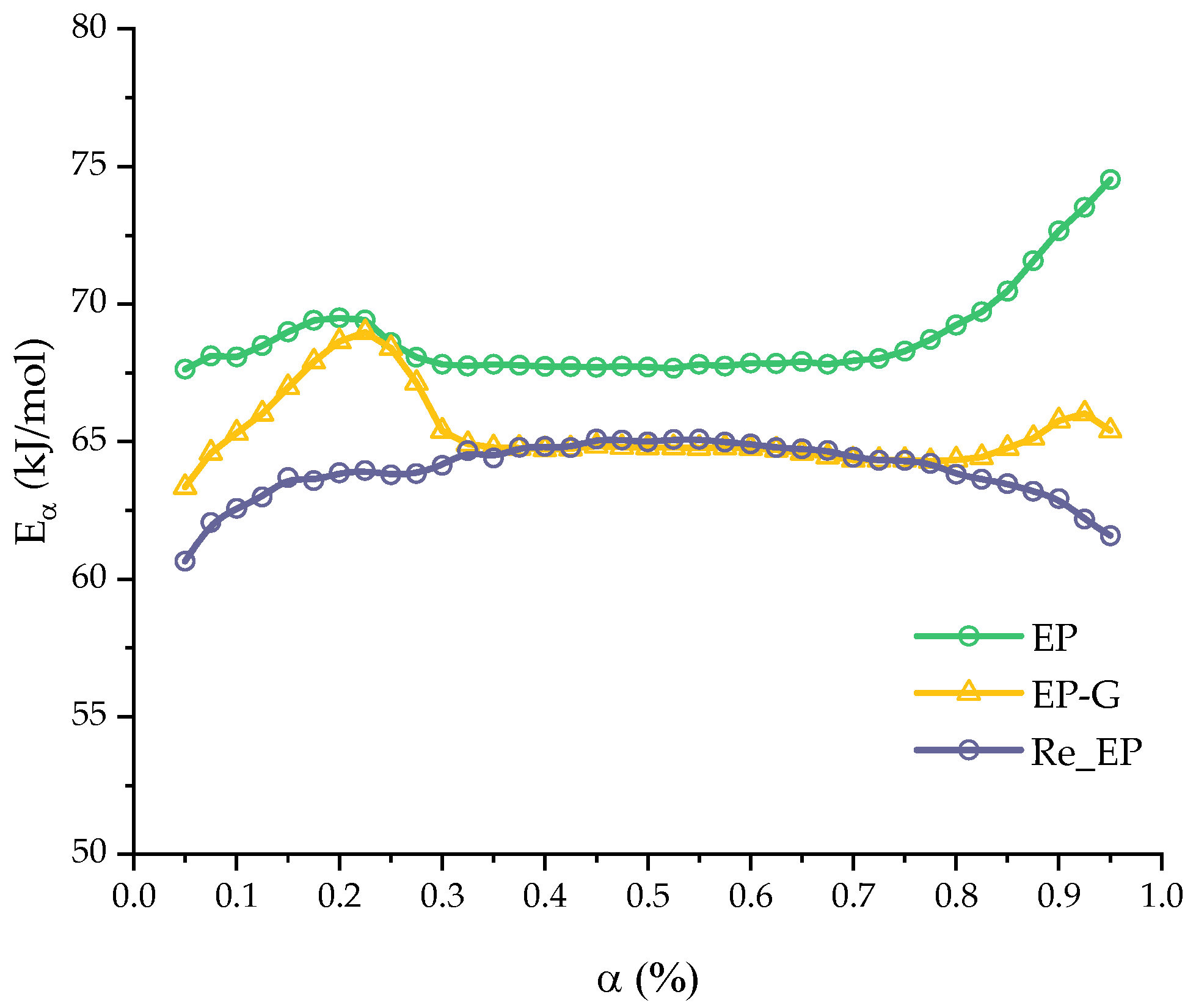
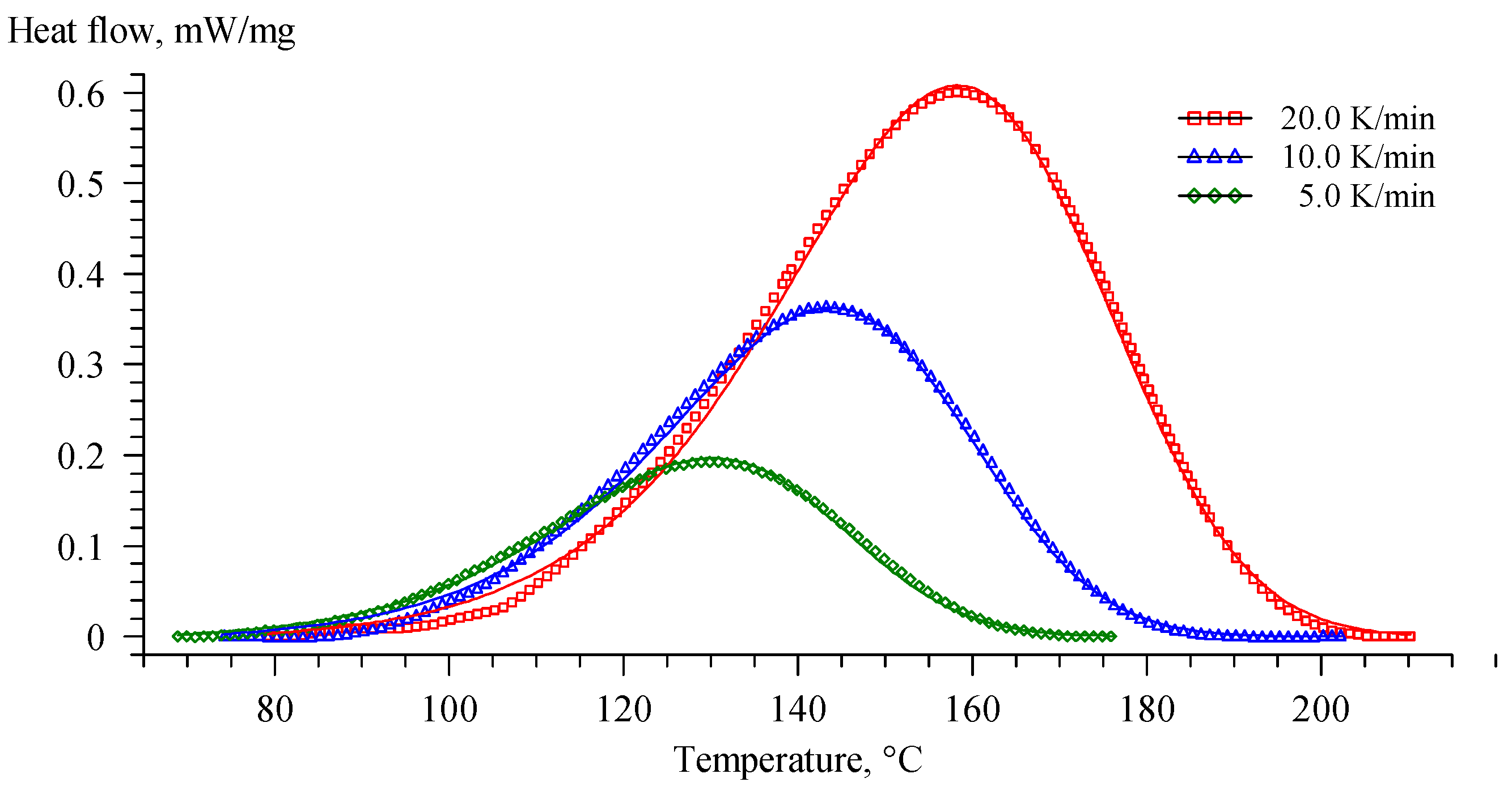
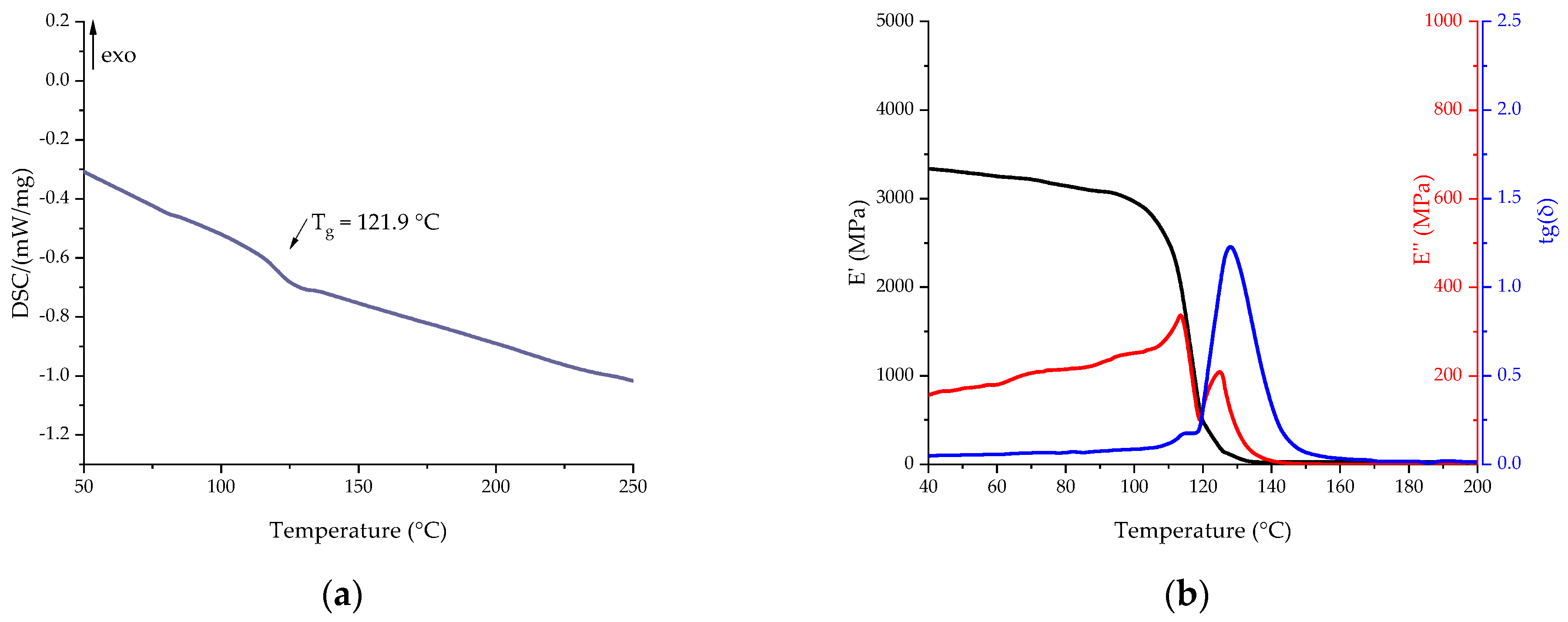
| Component | EP | EP-G | EP-Z | EP-GZ |
|---|---|---|---|---|
| DER 330 | 100 | 100 | 100 | 100 |
| i-MTHPA | 85 | 85 | 85 | 85 |
| Glycerol | - | 10 | - | 10 |
| Zn(AcAc)2 | - | - | 6.73 | 6.73 |
| 4-MIm | 1.85 | 1.85 | 1.85 | 1.85 |
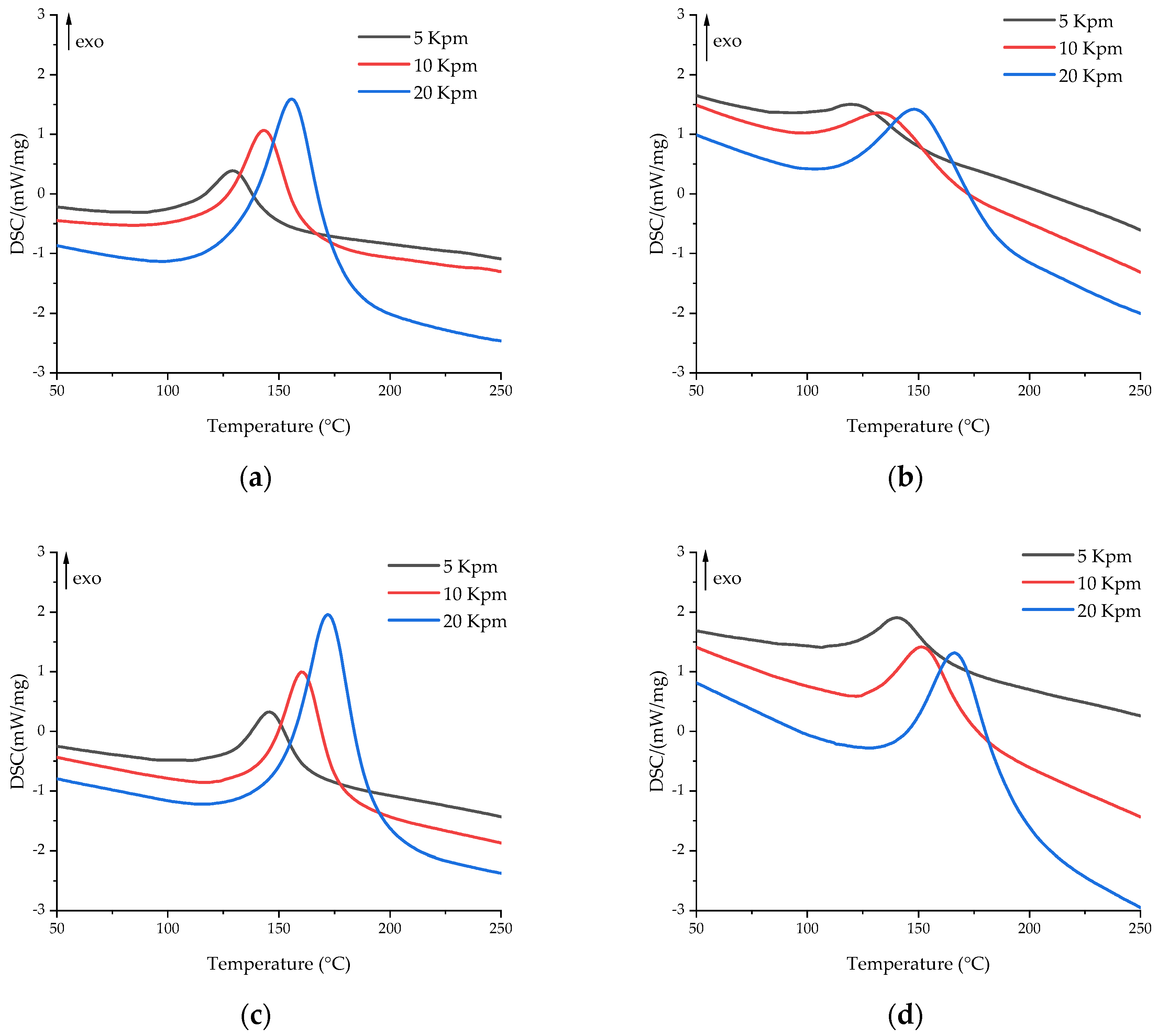
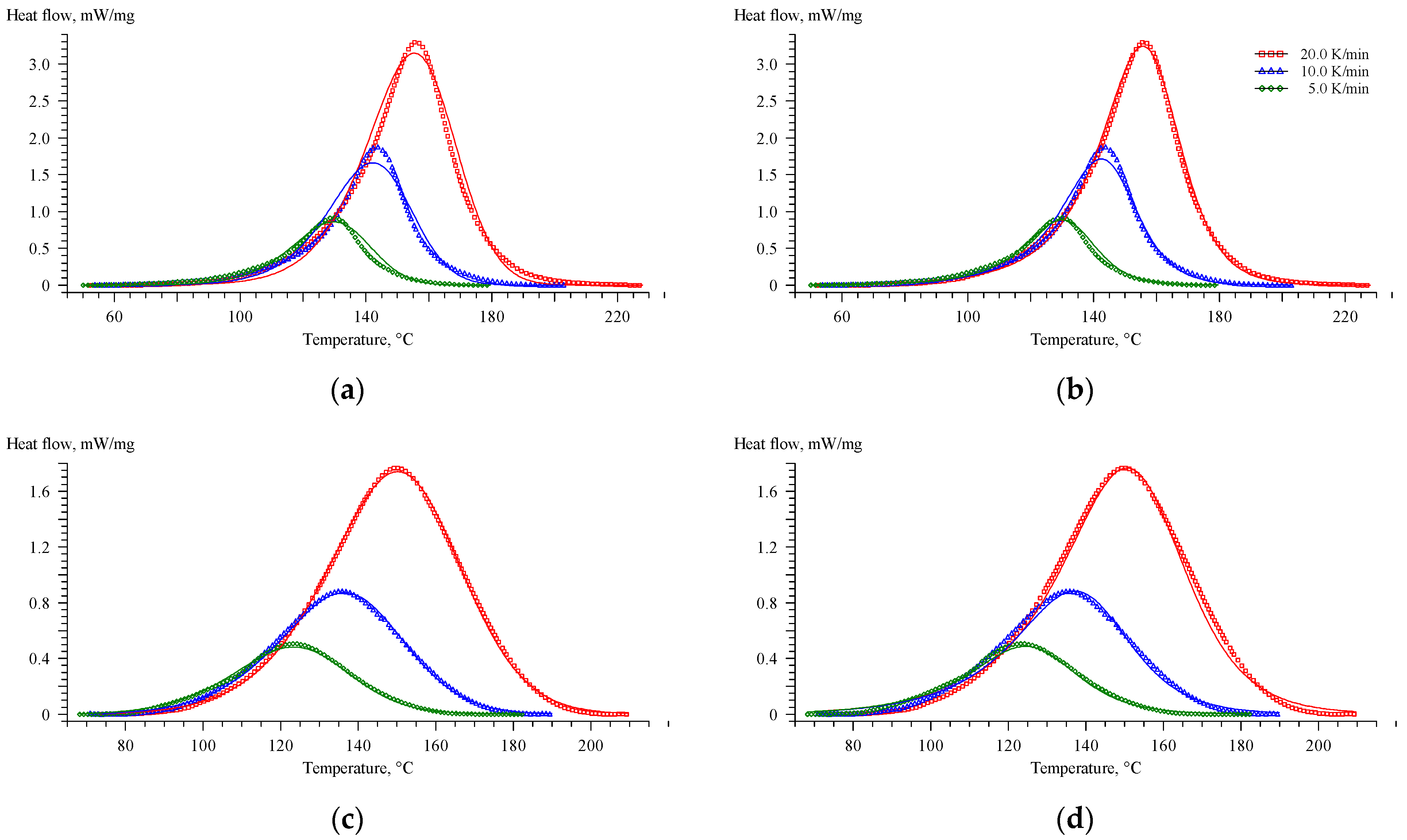
| Formulation Index | Heating Rate, °C/min | Tonset, °C | Tpeak, °C | Tend, °C | ΔH, J/g |
|---|---|---|---|---|---|
| EP | 5 | 108.2 | 129.6 | 146.7 | 326.4 |
| 10 | 122.3 | 143.5 | 160.9 | 347.2 | |
| 20 | 129.1 | 156.0 | 177.2 | 349.0 | |
| EP-G | 5 | 99.9 | 123.4 | 152.5 | 229.0 |
| 10 | 100.6 | 135.5 | 169.1 | 235.3 | |
| 20 | 112.6 | 149.9 | 184.3 | 249.2 | |
| EP-Z | 5 | 127.9 | 146.1 | 161.8 | 336.2 |
| 10 | 142.8 | 160.6 | 176.8 | 348.9 | |
| 20 | 149.7 | 172.3 | 192.1 | 350.4 | |
| EP-GZ | 5 | 120.3 | 141.4 | 161.3 | 244.9 |
| 10 | 127.8 | 152.8 | 175.4 | 249.5 | |
| 20 | 141.3 | 167.5 | 193.9 | 272.4 |
| Formulation Index | and Standard Error, kJ/mol |
|---|---|
| EP | 68.79 ± 0.28 * |
| EP-G | 65.32 ± 0.22 * |
| EP-Z | 75.61 ± 0.17 ** |
| EP-GZ | 72.36 ± 0.27 * |
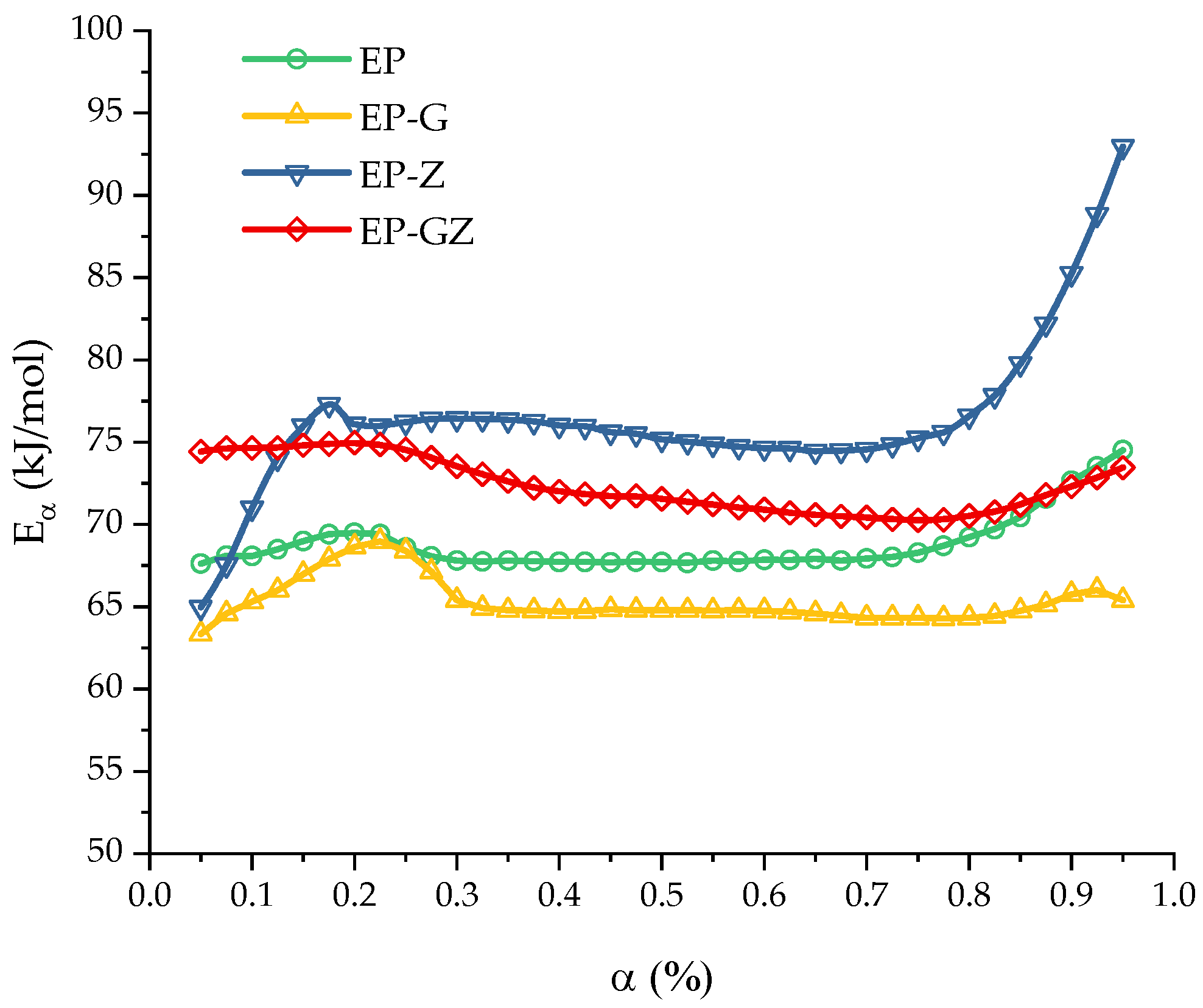
| Formulation Index | , | , | , kJ/mol | R2 | |||
|---|---|---|---|---|---|---|---|
| EP | 6.59 | 7.53 | 71.1 | 1.62 | 1.65 | 8.590 | 0.999 |
| EP-G | - | 6.42 | 65.2 | 1.20 | 0.35 | - | 0.999 |
| EP-Z | 6.95 | 8.05 | 78.5 | 1.54 | 1.30 | 12.417 | 0.997 |
| EP-GZ | 6.26 | 7.25 | 72.4 | 1.64 | 1.01 | 9.772 | 0.999 |
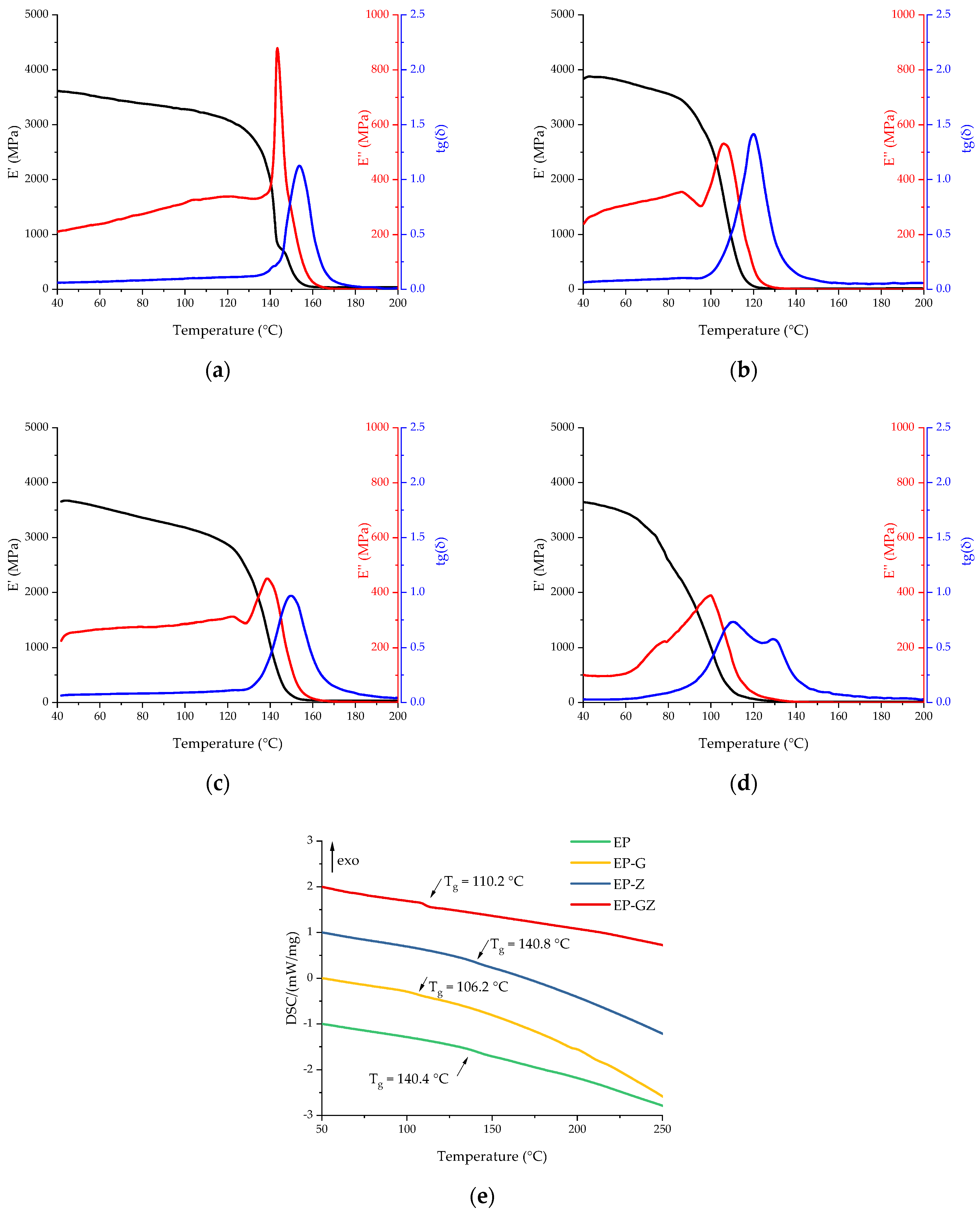
| DSC | DMA | |||
|---|---|---|---|---|
| Formulation Index | Tg Middle, °C | E′ Onset, °C | E″ Peak, °C | Tan (δ) Peak, °C |
| EP | 140.4 | 141.9 | 142.7 | 152.9 |
| EP-G | 106.2 | 106.2 | 106.1 | 120.6 |
| EP-Z | 140.8 | 136.2 | 138.3 | 149.1 |
| EP-GZ | 110.2 | 75.6 | 99.6 | 110.2 |
| Composition | σ, MPa | E, GPa | δ, % |
|---|---|---|---|
| EP | 78.3 ± 5.9 | 3.03 ± 0.13 | 4.40 ± 0.95 |
| EP-G | 58.8 ± 4.6 | 3.12 ± 0.04 | 2.20 ± 0.22 |
| EP-Z | 53.9 ± 13.4 | 3.09 ± 0.06 | 2.09 ± 0.64 |
| EP-GZ | 25.4 ± 5.5 | 3.17 ± 0.08 | 0.81 ± 0.17 |
| Formulation Index | Tg Middle, °C |
|---|---|
| EP | 9.1 |
| EP-G | 34.9 |
| EP-Z | 6.1 |
| EP-GZ | −4.6 |
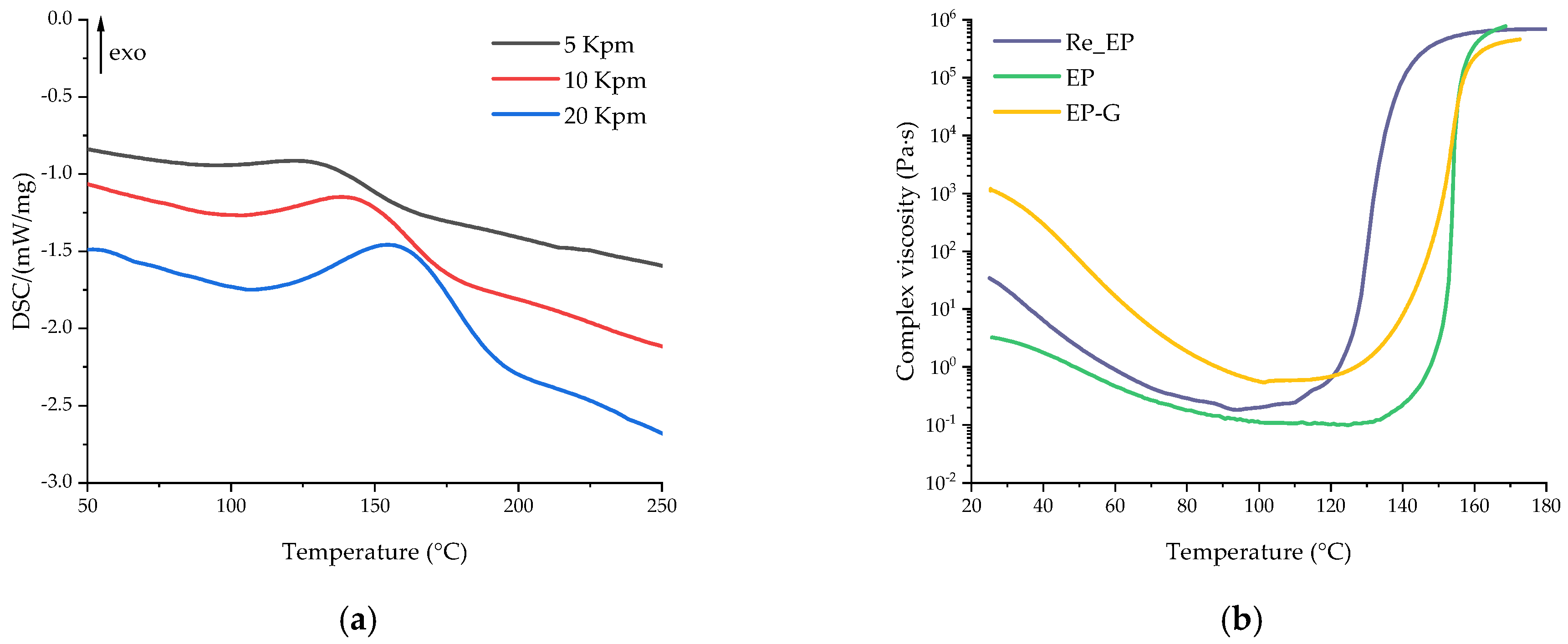
| Formulation Index | Heating Rate, °C/min | Tonset, °C | Tpeak, °C | Tend, °C | ΔH, J/g |
|---|---|---|---|---|---|
| Re_EP | 5 | 94.0 | 131.3 | 161.1 | 80.8 |
| 10 | 106.1 | 143.4 | 173.0 | 85.8 | |
| 20 | 114.5 | 158.5 | 192.2 | 88.0 |
| Formulation Index | and Standard Error, kJ/mol |
|---|---|
| EP | 68.79 ± 0.28 * |
| EP-G | 65.32 ± 0.22 * |
| Re_EP | 63.95 ± 0.18 * |
| Formulation Index | , | , | , kJ/mol | R2 | |||
|---|---|---|---|---|---|---|---|
| Re_EP | - | 6.07 | 64.3 | 1.05 | 0.25 | - | 0.999 |
| Formulation Index | DSC | DMA | ||
|---|---|---|---|---|
| Tg Middle, °C | E′ Onset, °C | E″ Peak, °C | Tan (δ) Peak, °C | |
| Re_EP | 121.9 | 110.2 | 114.3 | 128.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotkov, R.; Shutov, V.; Orlov, A.; Bornosuz, N.; Kulemza, D.; Onuchin, D.; Shcherbina, A.; Gorbunova, I.; Sirotin, I. The Kinetic Study of the Influence of Common Modifiers on the Curing Process of Epoxy Vitrimers. Polymers 2024, 16, 392. https://doi.org/10.3390/polym16030392
Korotkov R, Shutov V, Orlov A, Bornosuz N, Kulemza D, Onuchin D, Shcherbina A, Gorbunova I, Sirotin I. The Kinetic Study of the Influence of Common Modifiers on the Curing Process of Epoxy Vitrimers. Polymers. 2024; 16(3):392. https://doi.org/10.3390/polym16030392
Chicago/Turabian StyleKorotkov, Roman, Vyacheslav Shutov, Alexey Orlov, Natalia Bornosuz, Daria Kulemza, Denis Onuchin, Anna Shcherbina, Irina Gorbunova, and Igor Sirotin. 2024. "The Kinetic Study of the Influence of Common Modifiers on the Curing Process of Epoxy Vitrimers" Polymers 16, no. 3: 392. https://doi.org/10.3390/polym16030392





