Comprehensive Analysis of Rheological, Mechanical, and Thermal Properties in Poly(lactic acid)/Oxidized Graphite Composites: Exploring the Effect of Heat Treatment on Elastic Modulus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphite Oxidation
2.3. Preparation of Composites
2.4. Film Preparation
2.5. Characterization
2.5.1. Rheological Properties
2.5.2. Dynamic Mechanical Analysis
2.5.3. Thermogravimetric Analysis
2.5.4. Differential Scanning Calorimetry
2.5.5. Electron Microscopy
2.5.6. Fourier Transform Infrared Spectroscopy
2.5.7. Raman Spectroscopy
2.5.8. X-ray Diffraction
2.5.9. X-ray Photoelectron Spectroscopy
2.5.10. Energy-Dispersive Spectra (EDS)
2.5.11. Dynamic Light Scattering (DLS)
3. Results and Discussion
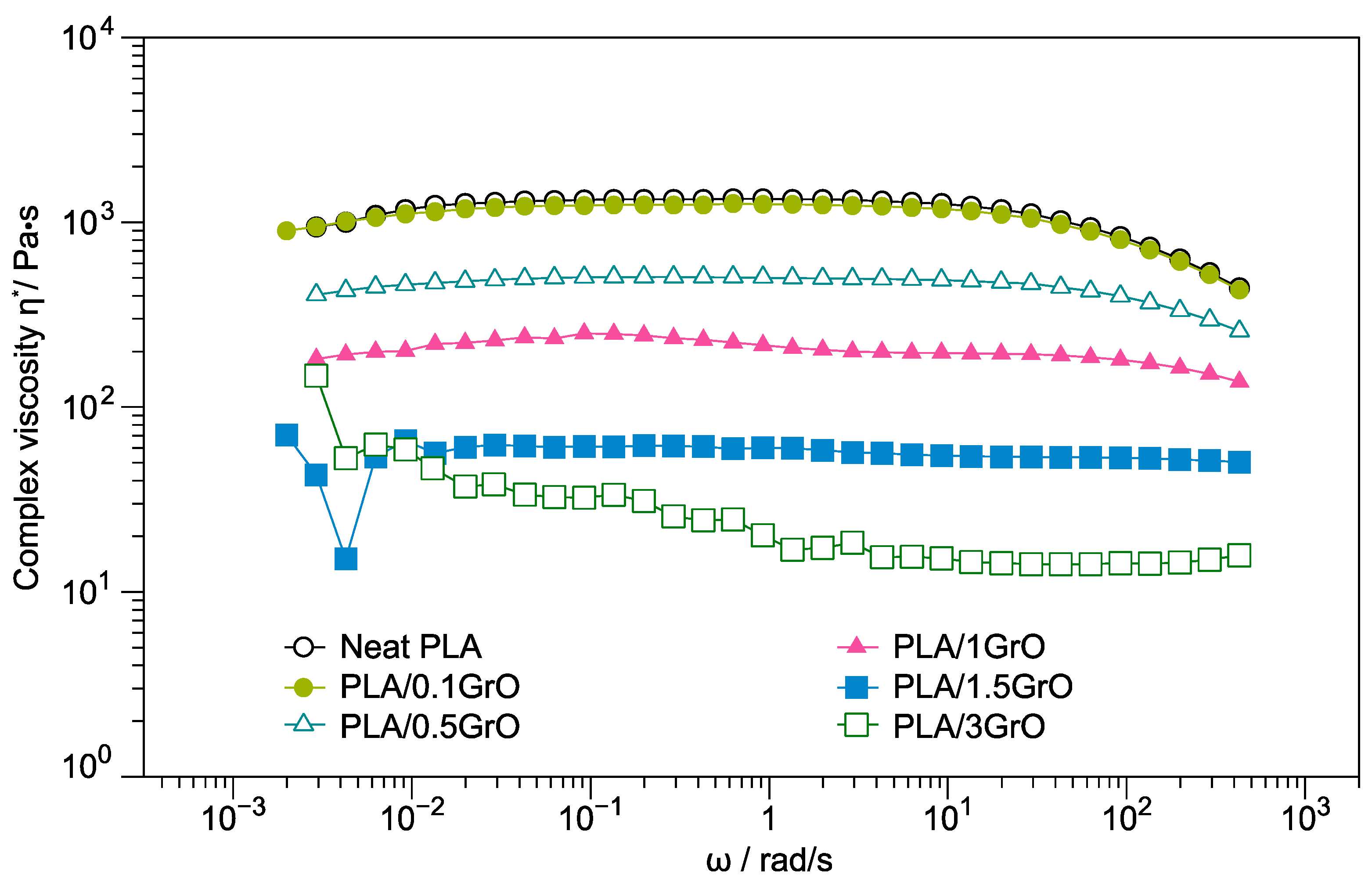
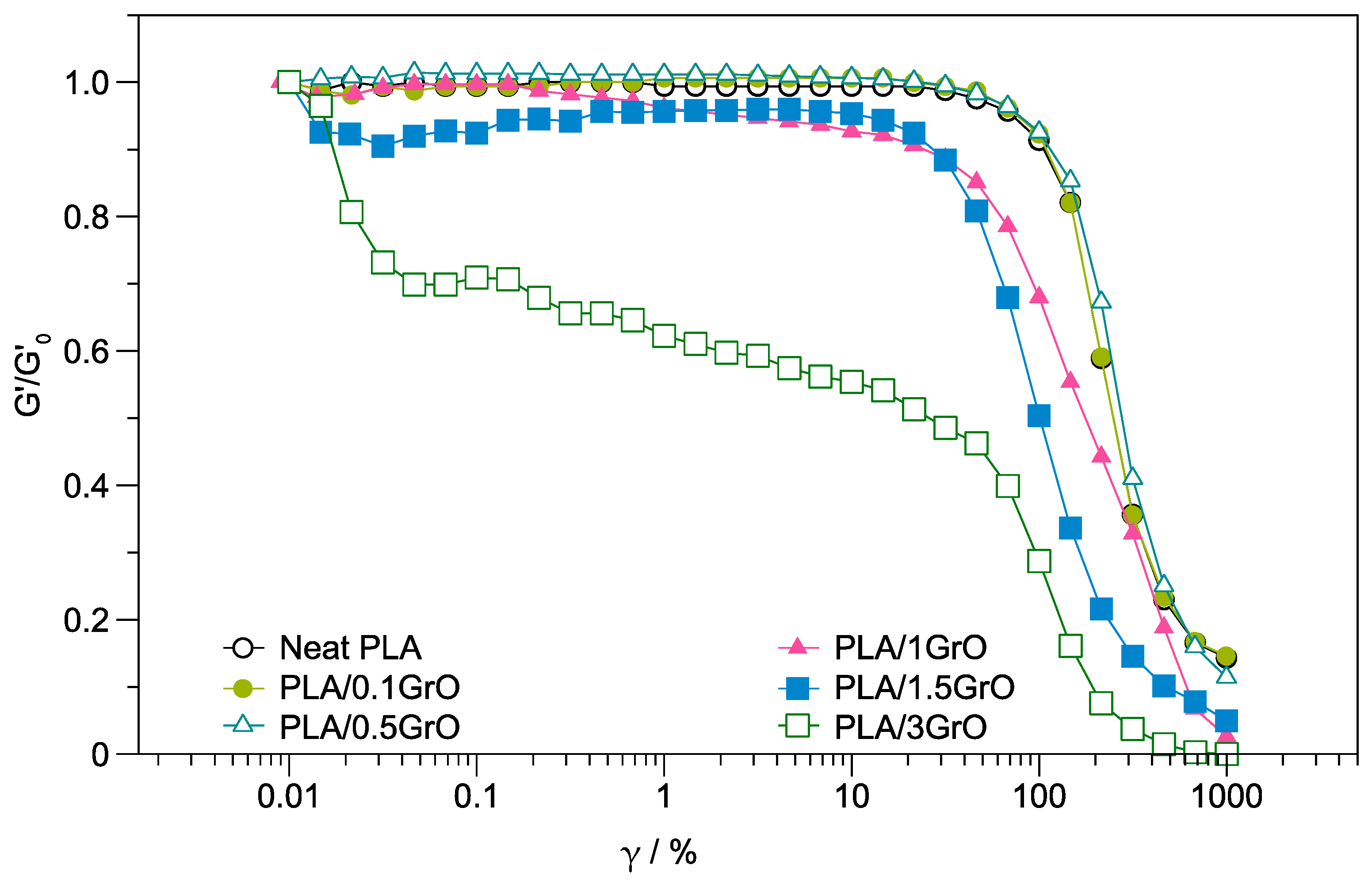
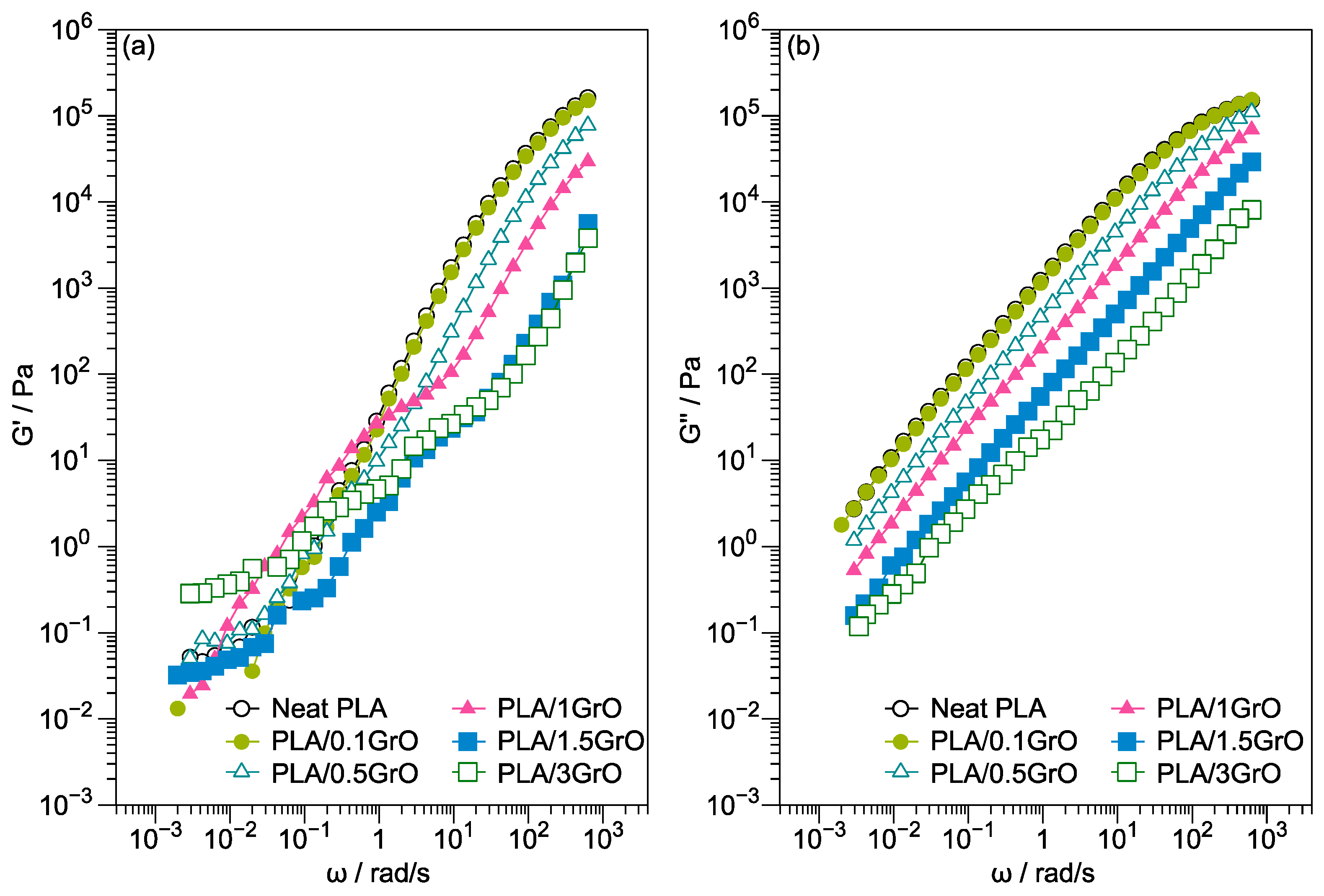
3.1. Rheology
3.2. X-ray Photoelectron Spectroscopy
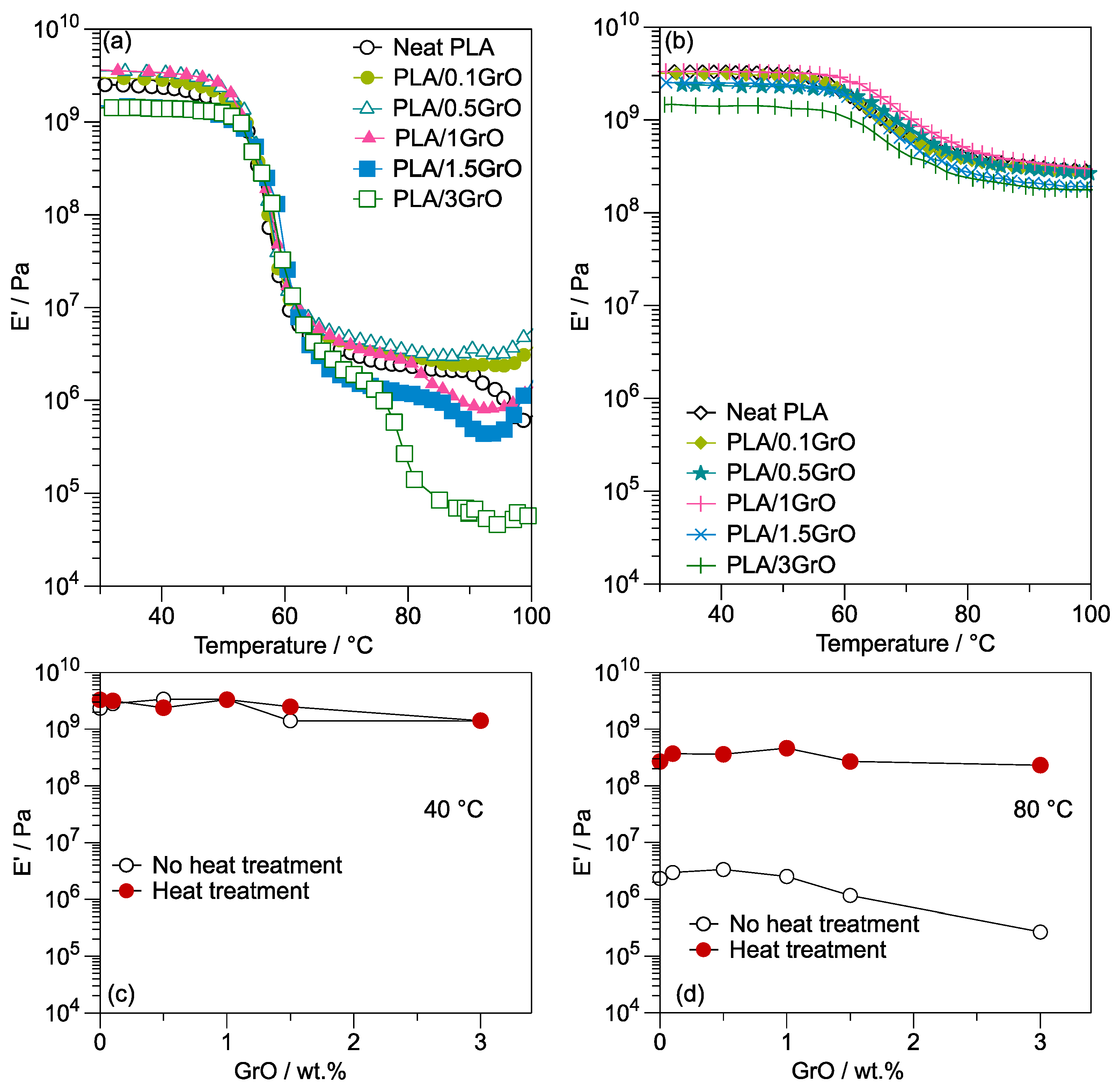
3.3. Dynamic Mechanical Analysis
3.4. Thermogravimetric Analysis
3.5. Differential Scanning Calorimetry
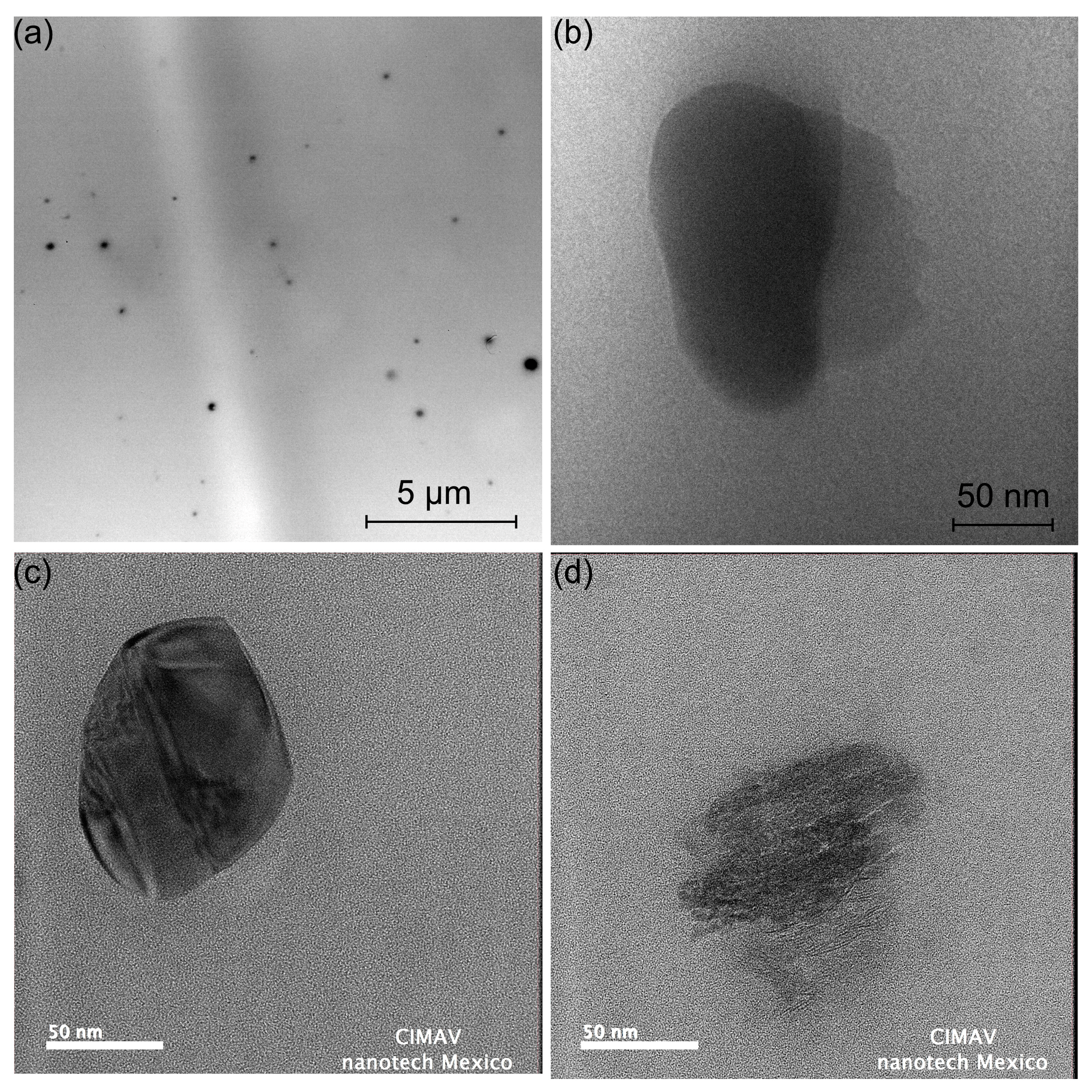
3.6. Transmission Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tayouri, M.I.; Estaji, S.; Mousavi, S.R.; Salkhi Khasraghi, S.; Jahanmardi, R.; Nouranian, S.; Arjmand, M.; Khonakdar, H.A. Degradation of polymer nanocomposites filled with graphene oxide and reduced graphene oxide nanoparticles: A review of current status. Polym. Degrad. Stab. 2022, 206, 110179. [Google Scholar] [CrossRef]
- Mucha, M.; Bialas, S.; Kaczmarek, H. Effect of nanosilver on the photodegradation of poly(lactic acid). J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Mendoza-Duarte, M.E.; Estrada-Moreno, I.A.; García-Casillas, P.E.; Vega-Rios, A. Stiff-Elongated Balance of PLA-Based Polymer Blends. Polymers 2021, 13, 4279. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Duarte, M.E.; Estrada-Moreno, I.A.; López-Martínez, E.I.; Vega-Rios, A. Effect of the Addition of Different Natural Waxes on the Mechanical and Rheological Behavior of PLA—A Comparative Study. Polymers 2023, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Zhu, B.; Liu, H.; Wang, Y.; Song, G.; Liu, C.; Shen, C. Biodegradable poly(lactic acid) nanocomposites reinforced and toughened by carbon nanotubes/clay hybrids. Int. J. Biol. Macromol. 2020, 151, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Karimpour-Motlagh, N.; Khonakdar, H.A.; Jafari, S.H.; Panahi-Sarmad, M.; Javadi, A.; Shojaei, S.; Goodarzi, V. An experimental and theoretical mechanistic analysis of thermal degradation of polypropylene/polylactic acid/clay nanocomposites. Polym. Adv. Technol. 2019, 30, 2695–2706. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Cruz, R.; Nisar, M.; Palza, H.; Yazdani-Pedram, M.; Aguilar-Bolados, H.; Quijada, R. Development of bio degradable nanocomposites based on PLA and functionalized graphene oxide. Polym. Test. 2023, 124, 108066. [Google Scholar] [CrossRef]
- Darie-Niţə, R.N.; Vasile, C.; Irimia, A.; Lipşa, R.; Râpə, M. Evaluation of some eco-friendly plasticizers for PLA films processing. J. Appl. Polym. Sci. 2016, 133, 1–11. [Google Scholar] [CrossRef]
- Shao, L.; Xi, Y.; Weng, Y. Recent Advances in PLA-Based Antibacterial Food Packaging and Its Applications. Molecules 2022, 27, 5953. [Google Scholar] [CrossRef] [PubMed]
- Henton, D.E.; Gruber, P.; Lunt, J.; Randall, J. Polylactic Acid Technology. In Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005; pp. 527–578. [Google Scholar] [CrossRef]
- Jeong, J.H.; Choi, M.C.; Nagappan, S.; Lee, W.K.; Ha, C.S. Preparation and properties of poly(lactic acid)/lipophilized graphene oxide nanohybrids. Polym. Int. 2018, 67, 91–99. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Wang, H.; Qiao, Y.; Chen, J.; Cao, S. Strong and ductile poly(lactic acid) nanocomposite films reinforced with alkylated graphene nanosheets. Chem. Eng. J. 2015, 264, 538–546. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Pierre, C.; Diking, D.A.; Ruoff, R.S.; Ramanathan, T.; Catherine Brinson, L.; Torkelson, J.M. Polymer—Graphite nanocomposites: Effective dispersion and major property enhancement via solid-state shear pulverization. Macromolecules 2008, 41, 1905–1908. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Mei, Q.; Liu, B.; Han, G.; Liu, R.; Han, M.Y.; Zhang, Z. Graphene Oxide: From Tunable Structures to Diverse Luminescence Behaviors. Adv. Sci. 2019, 6, 1900855. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.U.; Jung, B.M.; Byun, J.H.; Yi, J.W.; Lee, S.B.; Kim, B.S. Simultaneous enhancement of mechanical, electrical and thermal properties of graphene oxide paper by embedding dopamine. Carbon N. Y. 2013, 65, 296–304. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly(l-lactic acid)/graphene oxide nanocomposites: Influences of graphene oxide loading and crystallization temperature. Thermochim. Acta 2012, 527, 40–46. [Google Scholar] [CrossRef]
- Jafari, S.M.A.; Khajavi, R.; Goodarzi, V.; Kalaee, M.R.; Khonakdar, H.A. Development of degradable poly(ethylene terephthalate)-based nanocomposites with the aid of polylactic acid and graphenic materials: Thermal, thermo-oxidative and hydrolytic degradation characteristics. J. Appl. Polym. Sci. 2020, 137, 48466. [Google Scholar] [CrossRef]
- Verma, R.; Singh, S.; Dalai, M.K.; Saravanan, M.; Agrawal, V.V.; Srivastava, A.K. Photocatalytic degradation of polypropylene film using TiO2-based nanomaterials under solar irradiation. Mater. Des. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Anzovar, A.; Krzn, A.; Agar, E.Ž. Degradation of PLA/ZnO and PHBV/ZnO composites prepared by melt processing. Arab. J. Chem. 2017, 11, 343–352. [Google Scholar] [CrossRef]
- Kaya, H.; Özdemir, E.; Kaynak, C.; Hacaloglu, J. Effects of nanoparticles on thermal degradation of polylactide/aluminium diethylphosphinate composites. J. Anal. Appl. Pyrolysis 2016, 118, 115–122. [Google Scholar] [CrossRef]
- Luo, Y.B.; Wang, X.L.; Wang, Y.Z. Effect of TiO 2 nanoparticles on the long-term hydrolytic degradation behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721–728. [Google Scholar] [CrossRef]
- Chye, S.; Loo, J.; Ooi, C.P.; Hong, S.; Wee, E.; Chiang, Y.; Boey, F. Effect of isothermal annealing on the hydrolytic degradation rate of poly(lactide-co-glycolide) (PLGA). Biomaterials 2005, 26, 2827–2833. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Frache, A. Crystallization kinetics of poly(lactic acid)-talc composites. Express Polym. Lett. 2011, 5, 849–858. [Google Scholar] [CrossRef]
- Kolstad, J.J. Crystallization kinetics of poly(L-lactide-co-meso-lactide). J. Appl. Polym. Sci. 1996, 62, 1079–1091. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- James, L.W.; Czarnecki, L.; Tanaka, H. Experimental studies of the influence of particle and fiber reinforcement on the rheological properties of polymer melts. Rubber Chem. Technol. 1980, 824, 823–835. [Google Scholar]
- Jyoti, J.; Singh, B.P.; Rajput, S.; Singh, V.N.; Dhakate, S.R. Detailed dynamic rheological studies of multiwall carbon nanotube-reinforced acrylonitrile butadiene styrene composite. J. Mater. Sci. 2016, 51, 2643–2652. [Google Scholar] [CrossRef]
- de Bomfim, A.S.C.; de Oliveira, D.M.; Benini, K.C.C.d.C.; Cioffi, M.O.H.; Voorwald, H.J.C.; Rodrigue, D. Effect of Spent Coffee Grounds on the Crystallinity and Viscoelastic Behavior of Polylactic Acid Composites. Polymers 2023, 15, 2719. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.; Bouza, R.; Pardo, S.G.; Abad, M.J.; Barral, L. Rheological, mechanical and thermal behaviour of wood polymer composites based on recycled polypropylene. J. Polym. Environ. 2010, 18, 318–325. [Google Scholar] [CrossRef]
- Jamshidi, K.; Hyon, S.-H.; Ikada, Y. Thermal characterization of polylactides. Polymer 1988, 29, 2229–2234. [Google Scholar] [CrossRef]
- Shuai, C.; Li, Y.; Yang, W.; Yu, L.; Yang, Y.; Peng, S.; Feng, P. Graphene oxide induces ester bonds hydrolysis of poly-l-lactic acid scaffold to accelerate degradation. Int. J. Bioprinting 2020, 6, 91–104. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, Y.; Ning, J.; Hao, C.; Rock, M.; Amer, M.; Feng, S.; Falahati, M.; Wang, L.J.; Chen, R.K.; et al. No Such Thing as Trash: A 3D-Printable Polymer Composite Composed of Oil-Extracted Spent Coffee Grounds and Polylactic Acid with Enhanced Impact Toughness. ACS Sustain. Chem. Eng. 2019, 7, 15304–15310. [Google Scholar] [CrossRef]
- Bollman, W.; Spreadborough, J. Action of Graphite as a Lubricant. Nature 1960, 186, 29–30. [Google Scholar] [CrossRef]
- Arrigo, R.; Bartoli, M.; Malucelli, G. Poly(lactic Acid)–Biochar Biocomposites: Effect of Processing and Filler Content on Rheological. Polymers 2020, 12, 892. [Google Scholar] [CrossRef]
- Bouakaz, B.S.; Pillin, I.; Habi, A.; Grohens, Y. Synergy between fillers in organomontmorillonite/graphene–PLA nanocomposites. Appl. Clay Sci. 2015, 116, 69–77. [Google Scholar] [CrossRef]
- Cho, J.; Paul, D. Nylon 6 nanocomposites by melt compounding. Polymer 2001, 42, 1083–1094. [Google Scholar] [CrossRef]
- Payne, A.R.; Whittaker, R.E. Low Strain Dynamic Properties of Filled Rubbers. Rubber Chem. Technol. 1971, 44, 440–478. [Google Scholar] [CrossRef]
- Durmus, A.; Kasgoz, A.; Macosko, C.W. Linear low density polyethylene (LLDPE)/clay nanocomposites. Part I: Structural characterization and quantifying clay dispersion by melt rheology. Polymer 2007, 48, 4492–4502. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Eslami, H.; Grmela, M.; Bousmina, M. Linear and nonlinear rheology of polymer/layered silicate nanocomposites. J. Rheol. 2010, 54, 539. [Google Scholar] [CrossRef]
- Krishnamoorti, R.; Ren, J.; Silva, A.S. Shear response of layered silicate nanocomposites. J. Chem. Phys. 2001, 114, 4968–4973. [Google Scholar] [CrossRef]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterisation of melt-blended PLA-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Morphology and properties of polyester/exfoliated graphite nanocomposites. Macromolecules 2008, 41, 3317–3327. [Google Scholar] [CrossRef]
- Vermant, J.; Ceccia, S.; Dolgovskij, M.K.; Maffettone, P.L.; Macosko, C.W. Quantifying dispersion of layered nanocomposites via melt rheology. J. Rheol. 2007, 51, 429–450. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Yang, L.; Chen, R.; Zou, W.; Lin, X.; Qu, J. Preparation, characterization and properties of PLA/TiO 2 nanocomposites based on a novel vane extruder. RSC Adv. 2015, 5, 4639–4647. [Google Scholar] [CrossRef]
- Ferreira, W.H.; Andrade, C.T. The role of graphene on thermally induced shape memory properties of poly(lactic acid) extruded composites. J. Therm. Anal. Calorim. 2021, 143, 3107–3115. [Google Scholar] [CrossRef]
- Erk, K.A.; Rhein, M.; Krafcik, M.J.; Ydstie, S. Demonstrating the Effects of Processing on the Structure and Physical Properties of Plastic Using Disposable PETE Cups. J. Chem. Educ. 2015, 92, 1876–1881. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic Mechanical Analysis Investigations of PLA-Based Renewable Materials: How Are They Useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef] [PubMed]
- Giita Silverajah, V.S.; Ibrahim, N.A.; Md Zin Wan Yunus, W.; Hassan, H.A.; Woei, C.B. A comparative study on the mechanical, thermal and morphological characterization of poly(lactic acid)/epoxidized palm oil blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.; Wolcott, M.P. Comparison of polylactide/nano-sized calcium carbonate and polylactide/montmorillonite composites: Reinforcing effects and toughening mechanisms. Polymer 2007, 48, 7632–7644. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of crystalline forms (α′ and α) of poly(lactic acid) on its mechanical, thermo-mechanical, heat deflection temperature and creep properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of Biodegradable Polylactide/Poly(butylene adipate-co-terephthalate) Blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Naguib, H.E. Study on the morphological, dynamic mechanical and thermal properties of PLA carbon nanofibre composites. Compos. Part B Eng. 2016, 91, 631–639. [Google Scholar] [CrossRef]
- Krishnamachari, P.; Zhang, J.; Lou, J.; Yan, J.; Uitenham, L. Biodegradable poly(Lactic Acid)/clay nanocomposites by melt intercalation: A study of morphological, thermal, and mechanical properties. Int. J. Polym. Anal. Charact. 2009, 14, 336–350. [Google Scholar] [CrossRef]
- Hassan, M.L.; Mathew, A.P.; Hassan, E.A.; Fadel, S.M.; Oksman, K. Improving cellulose/polypropylene nanocomposites properties with chemical modified bagasse nanofibers and maleated polypropylene. J. Reinf. Plast. Compos. 2014, 33, 26–36. [Google Scholar] [CrossRef]
- Domenek, S.; Fernades-Nassar, S.; Ducret, V. Rheology, mechanical properties, barrier properties of poly (lactic acid). Adv. Polym. Sci. 2017, 279, 303–342. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z.; Then, Y.Y.; Loo, Y.Y. Effects of graphene nanoplatelets and reduced graphene oxide on poly(lactic acid) and plasticized poly(lactic acid): A comparative study. Polymers 2014, 6, 2232–2246. [Google Scholar] [CrossRef]
- Wang, P.; Tang, S.W.; Sheng, F.; Cai, J.; Fei, P.; Nawaz, A.; Walayat, N.; Javaid, A.B.; Xiong, H. Crystallization, thermal stability, barrier property, and aging resistance application of multi-functionalized graphene oxide/poly(lactide)/starch nanocomposites. Int. J. Biol. Macromol. 2019, 132, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Valapa, R.B.; Pugazhenthi, G.; Katiyar, V. Effect of graphene content on the properties of poly(lactic acid) nanocomposites. R. Soc. Chem. 2015, 5, 28410–28423. [Google Scholar] [CrossRef]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.A.; Huang, J.; Guo, Y.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Dehnou, K.H.; Norouzi, G.S.; Majidipour, M. A review: Studying the effect of graphene nanoparticles on mechanical, physical and thermal properties of polylactic acid polymer. RSC Adv. 2023, 13, 3976–4006. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric Nanocomposites for Environmental and Industrial Applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef]
- Lu, C.; Lu, Z.; Li, Z.; Leung, C.K.Y. Effect of Graphene Oxide on the Mechanical Behavior of Strain Hardening Cementitious Composites. Constr. Build. Mater. 2016, 120, 457–464. [Google Scholar] [CrossRef]
- Carlsson, J.M. Graphene: Buckle or Break. Nat. Mater. 2007, 6, 801–802. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Zheng, W.-G.; Yan, Q.; Yang, Y.; Wang, J.-W.; Lu, Z.-H.; Ji, G.-Y.; Yu, Z.-Z. Electrically Conductive Polyethylene Terephthalate/Graphene Nanocomposites Prepared by Melt Compounding. Polymer 2010, 51, 1191–1196. [Google Scholar] [CrossRef]
- Boehm, H. Surface Oxides on Carbon and Their Analysis: A Critical Assessment. Carbon N. Y. 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Feng, L.; Gao, G.; Huang, P.; Wang, X.; Zhang, C.; Zhang, J.; Guo, S.; Cui, D. Preparation of Pt Ag Alloy Nanoisland/Graphene Hybrid Composites and Its High Stability and Catalytic Activity in Methanol Electro-Oxidation. Nanoscale Res. Lett. 2011, 6, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-C.; Zhang, F.-J. Preparation and Characterization of Graphene Oxide Reduced From a Mild Chemical Method. Asian J. Chem. 2011, 23, 875–879. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Gonzalez, J.; Valiente, R.; Rodriguez, F. Pressure Dependence of Raman Modes in Graphene Oxide (GO); ImagineNano 2013: Bilbao, Spain, 2013. [Google Scholar]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and Exfoliation of Isocyanate-Treated Graphene Oxide Nanoplatelets. Carbon N. Y. 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Sharma, S.; Kothiyal, N.C. Comparative Effects of Pristine and Ball-Milled Graphene Oxide on Physico-Chemical Characteristics of Cement Mortar Nanocomposites. Constr. Build. Mater. 2016, 115, 256–268. [Google Scholar] [CrossRef]
- Kim, I.H.; Jeong, Y.G. Polylactide/Exfoliated Graphite Nanocomposites with Enhanced Thermal Stability, Mechanical Modulus, and Electrical Conductivity. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Botas, C.; Alvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaria, R.; Romasanta, L.J.; Verdejo, R.; Lopez-Manchado, M.A.; Menendez, R. Graphene Materials with Different Structures Prepared from the Same Graphite by the Hummers and Brodie Methods. Carbon N. Y. 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A Facile Method to Produce Graphene Oxide-g-Poly(L-Lactic-Acid) as an Promising Reinforcement for PLLA Nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Mcallister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud‘homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Tung, T.T.; Castro, M.; Kim, T.Y.; Suh, K.S.; Feller, J.-F. Graphene Quantum Resistive Sensing Skin for the Detection of Alteration Biomarkers. J. Mater. Chem. 2012, 22, 21754. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Chaki, T.K.; Das, N.C. Synthesis and Characterization of Graphene Oxide Filled Ethylene Methyl Acrylate Hybrid Nanocomposites. RSC Adv. 2016, 6, 20781–20790. [Google Scholar] [CrossRef]



| Sample | Peak BE (eV) | Bond | At % | C/O | |
|---|---|---|---|---|---|
| GrO | 2.4 | ||||
| C1s | 284.16 | C=C sp2 | 0.0 | ||
| 285.13 | C-C sp3 | 48.81 | |||
| 285.19 | C-O | 33.33 | |||
| 288.21 | C=O | 14.66 | |||
| 289.83 | -COOH | 3.18 | |||
| GrOmb | 2.36 | ||||
| C1s | 284.16 | C=C sp2 | 1.96 | ||
| 284.9 | C-C sp3 | 59.3 | |||
| 286.99 | C-O | 21.55 | |||
| 287.86 | C=O | 17.18 | |||
| 289.83 | -COOH | --- | |||
| Sample | Tg | Tcc | Tm | ΔHcc | ΔHf | χcc |
|---|---|---|---|---|---|---|
| °C | °C | °C | J/g | J/g | % | |
| Neat PLA | 60 | 125 | 145 | 10.4 | 10.5 | 11.1 |
| PLA/0.1GrO | 59 | 122 | 145 | 19.3 | 15.9 | 20.7 |
| PLA/0.5GrO | 59 | 119 | 149 | 20.5 | 18.2 | 22.1 |
| PLA/1GrO | 58 | 120 | 149 | 23.9 | 21.8 | 25.9 |
| PLA/1.5GrO | 58 | 128 | 151 | 14.9 | 13.9 | 16.2 |
| PLA/3GrO | 58 | 111 | 149 | 14.3 | 12.9 | 15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Duarte, M.E.; Vega-Rios, A. Comprehensive Analysis of Rheological, Mechanical, and Thermal Properties in Poly(lactic acid)/Oxidized Graphite Composites: Exploring the Effect of Heat Treatment on Elastic Modulus. Polymers 2024, 16, 431. https://doi.org/10.3390/polym16030431
Mendoza-Duarte ME, Vega-Rios A. Comprehensive Analysis of Rheological, Mechanical, and Thermal Properties in Poly(lactic acid)/Oxidized Graphite Composites: Exploring the Effect of Heat Treatment on Elastic Modulus. Polymers. 2024; 16(3):431. https://doi.org/10.3390/polym16030431
Chicago/Turabian StyleMendoza-Duarte, Mónica Elvira, and Alejandro Vega-Rios. 2024. "Comprehensive Analysis of Rheological, Mechanical, and Thermal Properties in Poly(lactic acid)/Oxidized Graphite Composites: Exploring the Effect of Heat Treatment on Elastic Modulus" Polymers 16, no. 3: 431. https://doi.org/10.3390/polym16030431
APA StyleMendoza-Duarte, M. E., & Vega-Rios, A. (2024). Comprehensive Analysis of Rheological, Mechanical, and Thermal Properties in Poly(lactic acid)/Oxidized Graphite Composites: Exploring the Effect of Heat Treatment on Elastic Modulus. Polymers, 16(3), 431. https://doi.org/10.3390/polym16030431









