Wastewater Treatment Using Membrane Bioreactor Technologies: Removal of Phenolic Contaminants from Oil and Coal Refineries and Pharmaceutical Industries
Abstract
1. Introduction
2. Phenols and Their Derivatives Posing a Human Health Risk
3. Physicochemical Methods of Phenols Treatment in Wastewater
4. Biodegradation of Phenols and Their Derivatives
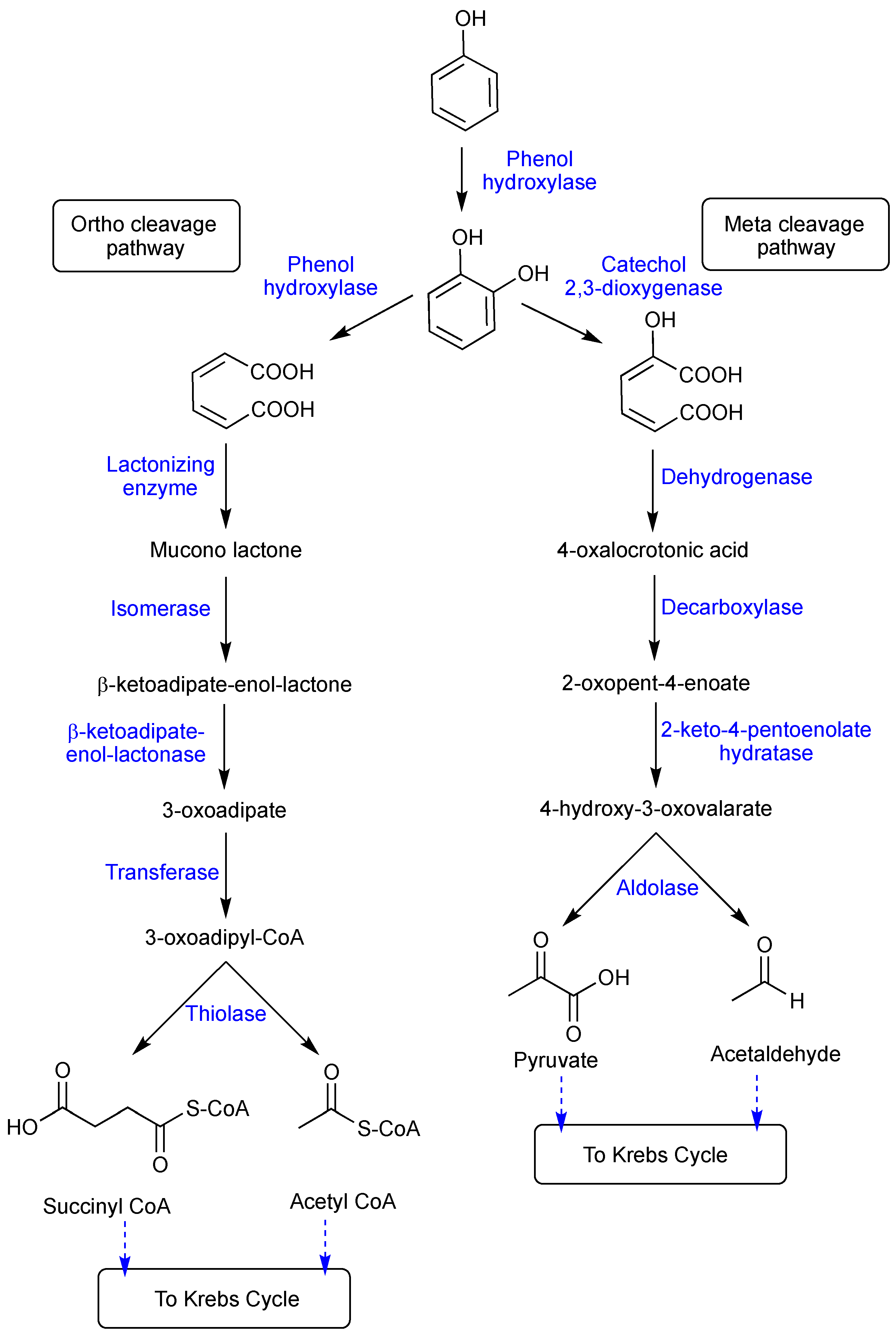
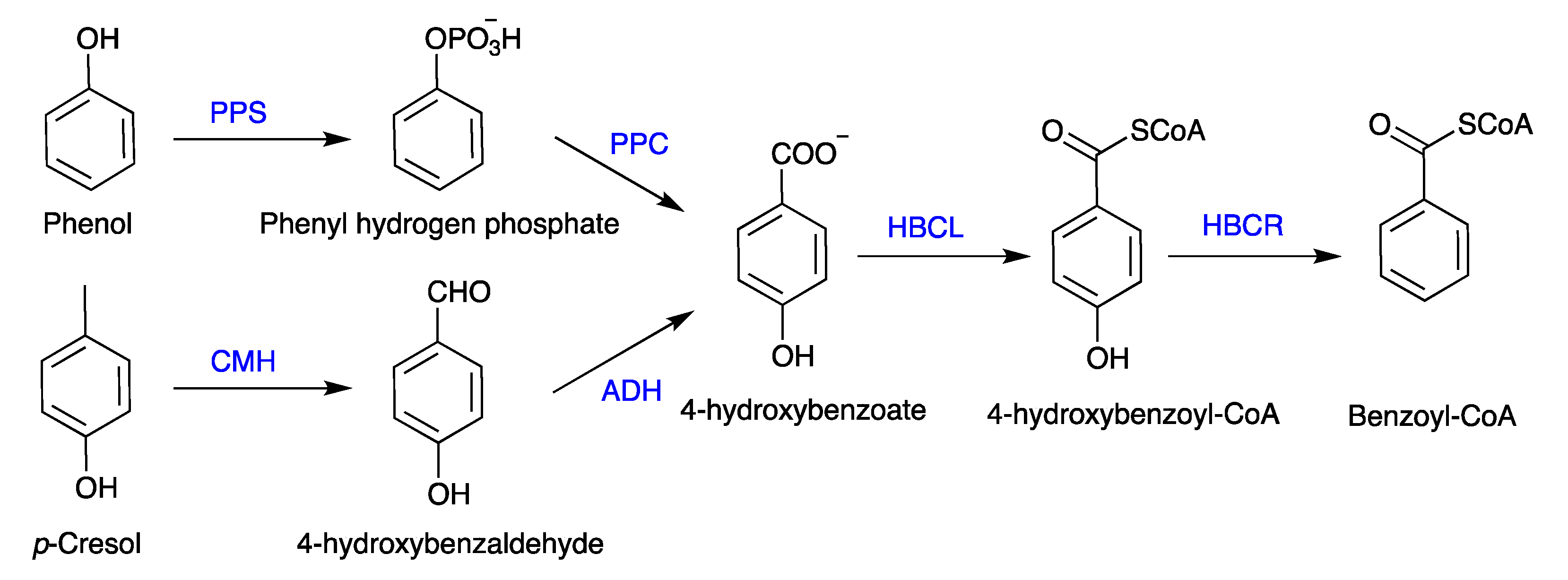
5. Mechanism of Phenol Biodegradation
5.1. Aerobic Biodegradation
5.2. Anaerobic Biodegradation
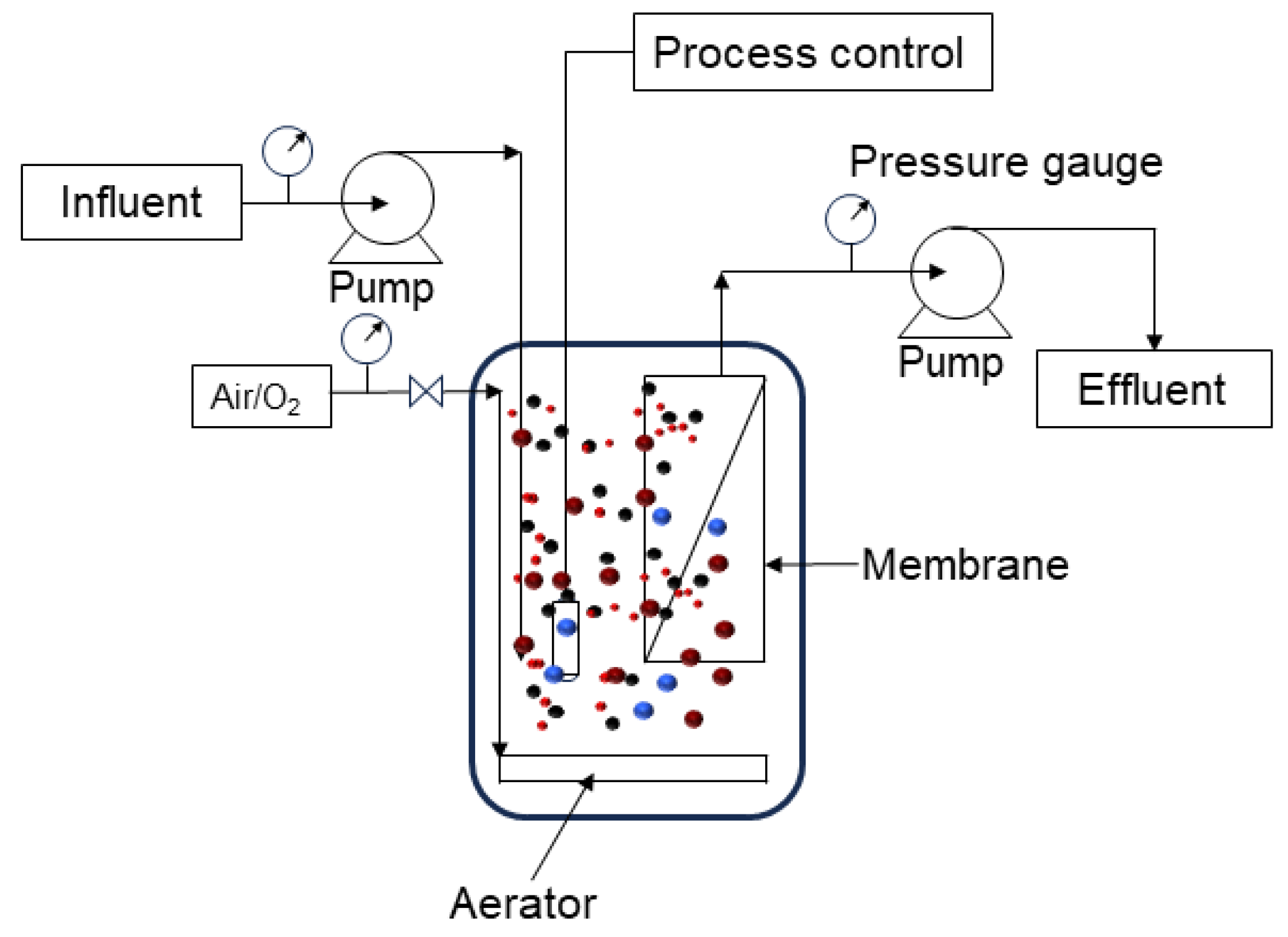
6. Membrane Bioreactors (MBRs)
7. Modified Membrane Bioreactors (MMBRs)
7.1. Capillary Membrane Bioreactor (CMBR)
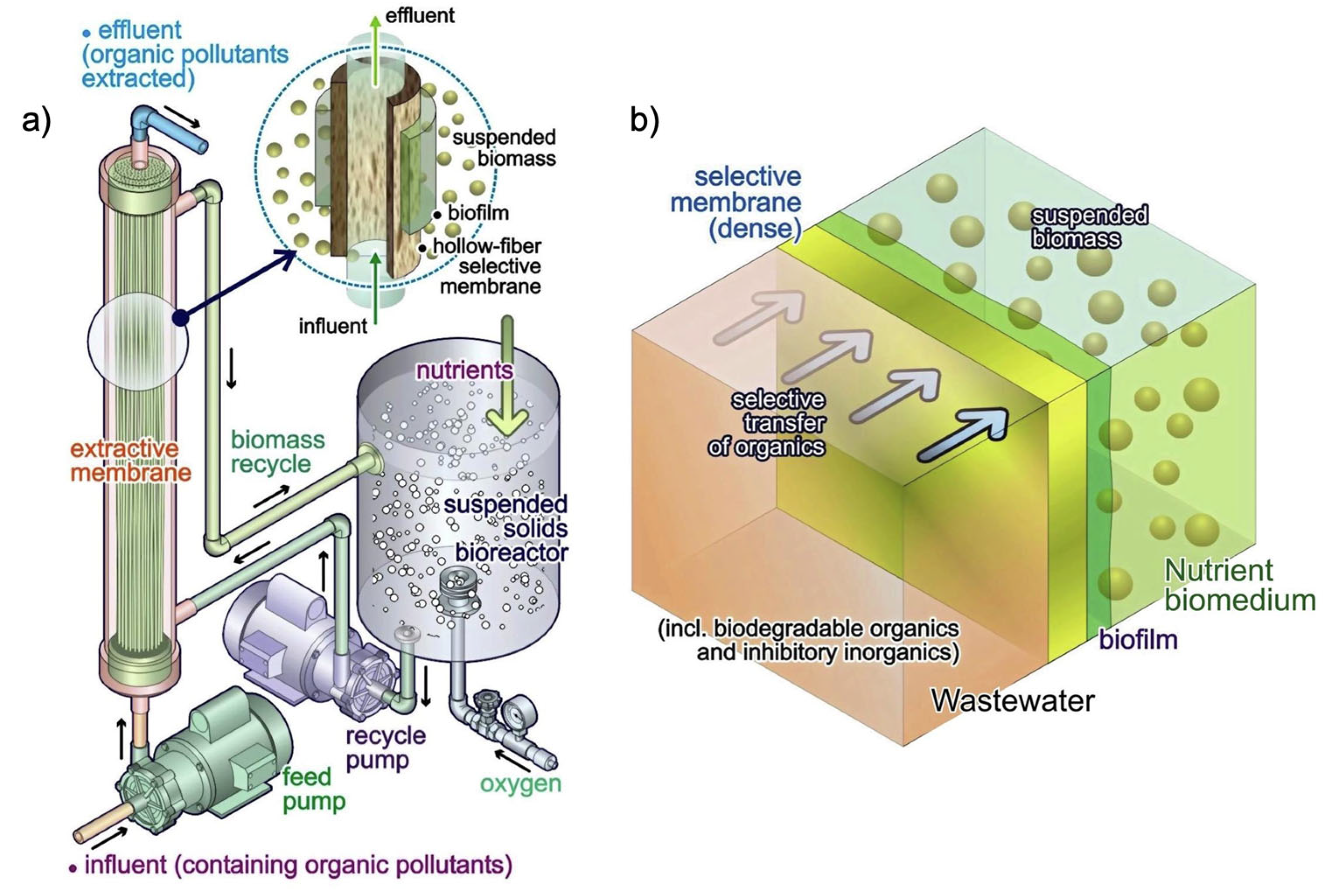
7.2. Extractive Membrane Bioreactor (EMBR)
7.3. Hollow-Fiber Membrane Bioreactor (HFMBR)
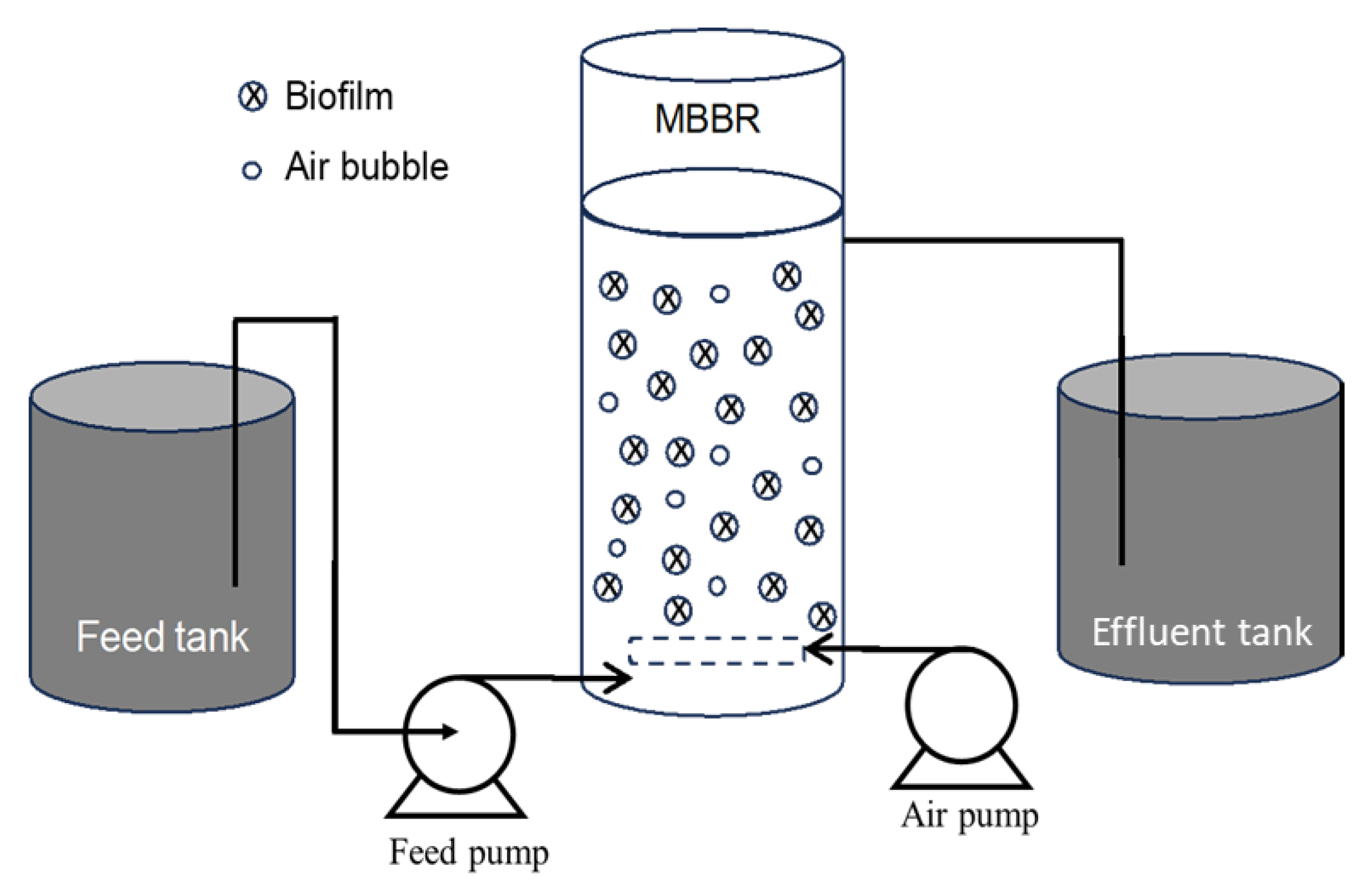
7.4. Moving Bed Biofilm Reactor (MBBR)
8. Cellulose Membranes in MBRs and Wastewater Treatments
9. Techno-Economic Analysis of Wastewater Treatment Using Conventional and MBRs Technologies
10. Conclusions
Funding
Conflicts of Interest
References
- Aljerf, L. Data of thematic analysis of farmer’s use behavior of recycled industrial wastewater. Data Brief 2018, 21, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Hou, S.Y.; Zhao, X.; Liu, Y.; Tillotson, M.R.; Weng, S.L.; Wang, H.; Li, Y.P.; Liu, B.Y.; Feng, K.S.; Zhang, N. Spatial analysis connects excess water pollution discharge, industrial production, and consumption at the sectoral level. Npj Clean Water 2022, 5, 4. [Google Scholar] [CrossRef]
- Haseena, M.; Malik, M.F.; Javed, A.; Arshad, S.; Asif, N.; Zulfiqar, S.; Hanif, J. Water pollution and human health. Environ. Risk Assess. Remediat. 2017, 1, 16–19. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol removal from industrial wastewaters: A short review. Desalin. Water Treat. 2015, 53, 2215–2234. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Veeresh, G.S.; Kumar, P.; Mehrotra, I. Treatment of phenol and cresols in upflow anaerobic sludge blanket (UASB) process: A review. Water Res. 2005, 39, 154–170. [Google Scholar] [CrossRef]
- Vazquez, I.; Rodriguez-Iglesias, J.; Maranon, E.; Castrillon, L.; Alvarez, M. Removal of residual phenols from coke wastewater by adsorption. J. Hazard. Mater. 2007, 147, 395–400. [Google Scholar] [CrossRef]
- Kaur, J.; Maddela, N.R. Microbial bioremediation: A cutting-edge technology for xenobiotic removal. In Advances in the Domain of Environmental Biotechnology: Microbiological Developments in Industries, Wastewater Treatment and Agriculture; Springer: Singapore, 2021; pp. 417–453. [Google Scholar]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: An integrated system design approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Mofrad, M.M.G.; Parseh, I.; Mahdavi, M. Hazardous and industrial wastewaters: From cutting-edge treatment strategies or layouts to micropollutant removal. In Integrated and Hybrid Process Technology for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 233–251. [Google Scholar]
- Tao, J.Y.; Li, C.; Li, J.; Yan, B.B.; Chen, G.Y.; Cheng, Z.J.; Li, W.Q.; Lin, F.W.; Hou, L. Multi-step separation of different chemical groups from the heavy fraction in biomass fast pyrolysis oil. Fuel Process. Technol. 2020, 202, 106366. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J.; Ren, T.; Guo, L.; Yang, Z.; Yang, Q.; Chen, H. Adsorption mechanism of 2,4-dichlorophenoxyacetic acid onto nitric-acid-modified activated carbon fiber. Environ. Technol. 2018, 39, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Peings, V.; Frayret, J.; Pigot, T. Mechanism for the oxidation of phenol by sulfatoferrate(VI): Comparison with various oxidants. J. Environ. Manag. 2015, 157, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Z.X.; Yu, D.W.; Chen, X.F.; Cheng, R.; Min, S.; Wang, J.Q.; Xiao, Q.C.; Wang, J.H. Overview of membrane technology applications for industrial wastewater treatment in China to increase water supply. Resour. Conserv. Recycl. 2015, 105, 1–10. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, C.; Zhang, J.; Han, S.; Peng, Y.; Song, X. Impacts of lipids on the performance of anaerobic membrane bioreactors for food wastewater treatment. J. Membr. Sci. 2023, 666, 121104. [Google Scholar] [CrossRef]
- Nasir, A.M.; Adam, M.R.; Mohamad Kamal, S.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Aziz, F.; Yusof, N.; Bilad, M.R.; Mohamud, R.; et al. A review of the potential of conventional and advanced membrane technology in the removal of pathogens from wastewater. Sep. Purif. Technol. 2022, 286, 120454. [Google Scholar] [CrossRef]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.W.; Kim, K.H.; Yang, J.H. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Narayanan, M.; Ali, S.S.; El-Sheekh, M. A comprehensive review on the potential of microbial enzymes in multipollutant bioremediation: Mechanisms, challenges, and future prospects. J. Environ. Manag. 2023, 334, 117532. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.; Telliard, W. ES&T Special Report: Priority pollutants: I-a perspective view. Environ. Sci. Technol. 2003, 13, 416–423. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Yahaya, A.; Okoh, O.O.; Agunbiade, F.O.; Okoh, A.I. Occurrence of phenolic derivatives in Buffalo River of Eastern Cape South Africa: Exposure risk evaluation. Ecotoxicol. Environ. Saf. 2019, 171, 887–893. [Google Scholar] [CrossRef]
- Chand Meena, M.; Band, R.; Sharma, G. Phenol and its toxicity: A case report. Iran. J. Toxicol. 2015, 8, 1222–1224. [Google Scholar]
- Michalowicz, J.; Wluka, A.; Bukowska, B. A review on environmental occurrence, toxic effects and transformation of man-made bromophenols. Sci. Total Environ. 2022, 811, 152289. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R. Manufacture of dye intermediates, dyes, and their industrial applications. In Handbook of Industrial Chemistry and Biotechnology; Spring: Berlin/Heidelberg, Germany, 2017; pp. 581–676. [Google Scholar]
- Farhan Hanafi, M.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.C.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Annangi, B.; Bonassi, S.; Marcos, R.; Hernandez, A. Biomonitoring of humans exposed to arsenic, chromium, nickel, vanadium, and complex mixtures of metals by using the micronucleus test in lymphocytes. Mutat. Res. Rev. Mutat. Res. 2016, 770, 140–161. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Castro, A.M.; Nogueira, V.; Lopes, I.; Rocha-Santos, T.; Pereira, R. Evaluation of the Potential Toxicity of Effluents from the Textile Industry before and after Treatment. Appl. Sci. 2019, 9, 3804. [Google Scholar] [CrossRef]
- Tišler, T.; Zagorc-Končan, J. Comparative assessment of toxicity of phenol, formaldehyde, and industrial wastewater to aquatic organisms. Water Air Soil Pollut. 1997, 97, 315–322. [Google Scholar] [CrossRef]
- Kahru, A.; Pollumaa, L.; Reiman, R.; Rätsep, A.; Liiders, M.; Maloveryan, A. The toxicity and biodegradability of eight main phenolic compounds characteristic to the oil-shale industry wastewaters: A test battery approach. Environ. Toxicol. Int. J. 2000, 15, 431–442. [Google Scholar] [CrossRef]
- Benosmane, N.; Boutemeur, B.; Hamdi, S.M.; Hamdi, M. Removal of phenol from aqueous solution using polymer inclusion membrane based on mixture of CTA and CA. Appl. Water Sci. 2018, 8, 17. [Google Scholar] [CrossRef]
- Okeowo, I.O.; Balogun, E.O.; Ademola, A.J.; Alade, A.O.; Afolabi, T.J.; Dada, E.O.; Farombi, A.G. Adsorption of Phenol from Wastewater Using Microwave-Assisted Ag-Au Nanoparticle-Modified Mango Seed Shell-Activated Carbon. Int. J. Environ. Res. 2020, 14, 215–233. [Google Scholar] [CrossRef]
- Barlak, M.S.; Degermenci, N.; Cengiz, I.; Özel, H.U.; Yildiz, E. Comparison of phenol removal with ozonation in jet loop reactor and bubble column. J. Environ. Chem. Eng. 2020, 8, 104402. [Google Scholar] [CrossRef]
- Xu, M.; Wu, C.; Zhou, Y. Advancements in the Fenton process for wastewater treatment. Adv. Oxid. Process 2020, 61, 61–77. [Google Scholar]
- Ni, G.H.; Zhao, G.X.; Jiang, Y.M.; Li, J.X.; Meng, Y.D.; Wang, X.K. Steam Plasma Jet Treatment of Phenol in Aqueous Solution at Atmospheric Pressure. Plasma Process. Polym. 2013, 10, 353–363. [Google Scholar] [CrossRef]
- Hamad, H.T. Removal of phenol and inorganic metals from wastewater using activated ceramic. J. King Saud Univ.-Eng. Sci. 2021, 33, 221–226. [Google Scholar] [CrossRef]
- Mandal, A.; Das, S.K. Adsorptive Removal of Phenol by Activated Alumina and Activated Carbon from Coconut Coir and Rice Husk Ash. Water Conserv. Sci. Eng. 2019, 4, 149–161. [Google Scholar] [CrossRef]
- Alkaram, U.F.; Mukhlis, A.A.; Al-Dujaili, A.H. The removal of phenol from aqueous solutions by adsorption using surfactant-modified bentonite and kaolinite. J. Hazard. Mater. 2009, 169, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Khan, M.A.; Xia, M.Z.; Lei, W.; Zhu, S.D.; Wang, F.Y. Efficient preparation and molecular dynamic (MD) simulations of Gemini surfactant modified layered montmorillonite to potentially remove emerging organic contaminants from wastewater. Ceram. Int. 2019, 45, 10782–10791. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Monteoliva-García, A.; Martín-Pascual, J.; Munio, M.M.; García-Mesa, J.J.; Poyatos, J.M. Moving bed biofilm reactor as an alternative wastewater treatment process for nutrient removal and recovery in the circular economy model. Bioresour. Technol. 2020, 299, 122631. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Adio, S.O.; Asif, M.; Dafalla, H. Statistical analysis of phenols adsorption on diethylenetriamine-modified activated carbon. J. Cleaner Prod. 2018, 182, 960–968. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Zhang, F.S.; Zhou, J.; Wu, J.; Alsaedi, A.; Hayat, T.; Li, J.X. Insight into the mechanism of adsorption of phenol and resorcinol on activated carbons with different oxidation degrees. Colloid Surf. A 2019, 563, 22–30. [Google Scholar] [CrossRef]
- Roostaei, N.; Tezel, F.H. Removal of phenol from aqueous solutions by adsorption. J. Environ. Manag. 2004, 70, 157–164. [Google Scholar] [CrossRef]
- Nirmala, G.; Murugesan, T.; Rambabu, K.; Sathiyanarayanan, K.; Show, P.L. Adsorptive removal of phenol using banyan root activated carbon. Chem. Eng. Commun. 2021, 208, 831–842. [Google Scholar] [CrossRef]
- Kulkarni, S.J.; Kaware, J.P. Review on research for removal of phenol from wastewater. Int. J. Sci. Res. Publ. 2013, 3, 1–5. [Google Scholar]
- Kumar, D.P.; Ramesh, D.; Subramanian, P.; Karthikeyan, S.; Surendrakumar, A. Activated carbon production from coconut leaflets through chemical activation: Process optimization using Taguchi approach. Bioresour. Technol. Rep. 2022, 19, 101155. [Google Scholar] [CrossRef]
- Carvajal-Bernal, A.M.; Gómez, F.; Giraldo, L.; Moreno-Piraján, J.C. Chemical modification of activated carbons and its effect on the adsorption of phenolic compounds. Ing. Compet. 2015, 17, 109–119. [Google Scholar]
- Mohammadi, S.Z.; Darijani, Z.; Karimi, M.A. Fast and efficient removal of phenol by magnetic activated carbon-cobalt nanoparticles. J. Alloys Compd. 2020, 832, 154942. [Google Scholar] [CrossRef]
- Aremu, M.O.; Arinkoola, A.O.; Olowonyo, I.A.; Salam, K.K. Improved phenol sequestration from aqueous solution using silver nanoparticle modified Palm Kernel Shell Activated Carbon. Heliyon 2020, 6, e04492. [Google Scholar] [CrossRef]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; da Silva, M.L.C.P.; Alvarez-Mendes, M.O.; Coutinho, A.D.; Thim, G.P. Phenol removal from aqueous solution by activated carbon produced from avocado kernel seeds. Chem. Eng. J. 2011, 174, 49–57. [Google Scholar] [CrossRef]
- Galdino, A.L.; Oliveira, J.C.A.; Magalhaes, M.L.; Lucena, S.M.P.; Liu, D.; Huang, T.; Zhu, L. Prediction of the phenol removal capacity from water by adsorption on activated carbon. Water Sci. Technol. 2021, 84, 135–143. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Lütke, S.F.; Igansi, A.V.; Pegoraro, L.; Dotto, G.L.; Pinto, L.A.A.; Cadaval, T.R.S. Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ. Chem. Eng. 2019, 7, 103396. [Google Scholar] [CrossRef]
- Cheng, W.P.; Gao, W.; Cui, X.Y.; Ma, J.H.; Li, R.F. Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J. Taiwan Inst. Chem. Eng. 2016, 62, 192–198. [Google Scholar] [CrossRef]
- Jiao, T.T.; Li, C.S.; Zhuang, X.L.; Cao, S.S.; Chen, H.N.; Zhang, S.J. The new liquid-liquid extraction method for separation of phenolic compounds from coal tar. Chem. Eng. J. 2015, 266, 148–155. [Google Scholar] [CrossRef]
- Patel, J.; Desai, H. Removal of phenol by liquid-liquid extraction from pharmaceutical wastewater. Mater. Today Proc. 2022, 57, 2396–2399. [Google Scholar] [CrossRef]
- Liu, J.T.; Xie, J.; Ren, Z.Q.; Zhang, W.D. Solvent extraction of phenol with cumene from wastewater. Desalin. Water Treat. 2013, 51, 3826–3831. [Google Scholar] [CrossRef]
- Abburi, K. Adsorption of phenol and p-chlorophenol from their single and bisolute aqueous solutions on Amberlite XAD-16 resin. J. Hazard. Mater. 2003, 105, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Mousset, E.; Frunzo, L.; Esposito, G.; van Hullebusch, E.D.; Oturan, N.; Oturan, M.A. A complete phenol oxidation pathway obtained during electro-Fenton treatment and validated by a kinetic model study. Appl. Catal. B 2016, 180, 189–198. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Durai, H.B.P.; Winzenbacher, R.; Petri, M.; Seitz, W. Drinking water treatment by generated ferrate(VI). Desalination Water Treat. 2015, 55, 731–739. [Google Scholar] [CrossRef]
- Wang, X.S.; Liu, Y.L.; Xu, S.Y.; Zhang, J.; Li, J.; Song, H.; Zhang, Z.X.; Wang, L.; Ma, J. Ferrate Oxidation of Phenolic Compounds in Iodine-Containing Water: Control of Iodinated Aromatic Products. Environ. Sci. Technol. 2020, 54, 1827–1836. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Y.; Jia, L.; Tian, L.; Qi, J.; Ma, J.; Wen, G.; Wang, L. Insight into the oxidation of phenolic pollutants by enhanced permanganate with biochar: The role of high-valent manganese intermediate species. J. Hazard. Mater. 2022, 430, 128460. [Google Scholar] [CrossRef] [PubMed]
- Tasic, Z.; Gupta, V.K.; Antonijevic, M.M. The Mechanism and Kinetics of Degradation of Phenolics in Wastewaters Using Electrochemical Oxidation. Int. J. Electrochem. Sci. 2014, 9, 3473–3490. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Diban, N.; Urtiaga, A. Comparison of microcrystalline and ultrananocrystalline boron doped diamond anodes: Influence on perfluorooctanoic acid electrolysis. Sep. Purif. Technol. 2019, 208, 169–177. [Google Scholar] [CrossRef]
- Calcerrada, A.B.; de la Osa, A.R.; Lopez-Fernandez, E.; Dorado, F.; de Lucas-Consuegra, A. Influence of the carbon support on the Pt-Sn anodic catalyst for the electrochemical reforming of ethanol. Int. J. Hydrogen Energy 2019, 44, 10616–10626. [Google Scholar] [CrossRef]
- Rao, X.F.; Shao, X.L.; Xu, J.; Yi, J.; Qiao, J.L.; Li, Q.Y.; Wang, H.Q.; Chien, M.F.; Inoue, C.; Liu, Y.Y.; et al. Efficient nitrate removal from water using selected cathodes and Ti/PbO anode: Experimental study and -mechanism verification. Sep. Purif. Technol. 2019, 216, 158–165. [Google Scholar] [CrossRef]
- Chen, A.Q.; Xia, S.J.; Ji, Z.G.; Lu, H.W. Insights into the origin of super-high oxygen evolution potential of Cu doped SnO anodes: A theoretical study. Appl. Surf. Sci. 2019, 471, 149–153. [Google Scholar] [CrossRef]
- Chen, G.H. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Sierra, C.; Ruiz-Ruiz, E.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Machuca-Martínez, F.; Hernández-Ramírez, A. Sulfamethoxazole mineralization by solar photo electro-Fenton process in a pilot plant. Catal. Today 2018, 313, 175–181. [Google Scholar] [CrossRef]
- Seibert, D.; Borba, F.H.; Bueno, F.; Inticher, J.J.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Bergamasco, R. Two-stage integrated system photo-electro-Fenton and biological oxidation process assessment of sanitary landfill leachate treatment: An intermediate products study. Chem. Eng. J. 2019, 372, 471–482. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Agrawal, K.; Bhatt, A.; Bhardwaj, N.; Kumar, B.; Verma, P. Integrated Approach for the Treatment of Industrial Effluent by Physico-chemical and Microbiological Process for Sustainable Environment. In Combined Application of Physico-Chemical & Microbiological Processes for Industrial Effluent Treatment Plant; Shah, M., Banerjee, A., Eds.; Springer: Singapore, 2020; pp. 119–143. [Google Scholar]
- Kargi, F.; Eker, S. Kinetics of 2,4-dichlorophenol degradation by Pseudomonas putida CP1 in batch culture. Int. Biodeterior. Biodegrad. 2005, 55, 25–28. [Google Scholar] [CrossRef]
- Mahgoub, S.A.; Qattan, S.Y.A.; Salem, S.S.; Abdelbasit, H.M.; Raafat, M.; Ashkan, M.F.; Al-Quwaie, D.A.; Motwali, E.A.; Alqahtani, F.S.; Abd El-Fattah, H.I. Characterization and Biodegradation of Phenol by Pseudomonas aeruginosa and Klebsiella variicola Strains Isolated from Sewage Sludge and Their Effect on Soybean Seeds Germination. Molecules 2023, 28, 1203. [Google Scholar] [CrossRef]
- Mulla, S.I.; Bharagava, R.N.; Belhaj, D.; Saratale, G.D.; Kumar, A.; Rajasekar, A.; Tallur, P.N.; Edalli, V.A.; Hu, A.; Yu, C.-P. Microbial Degradation of Phenolic Compounds. In Microbes and Enzymes in Soil Health and Bioremediation; Kumar, A., Sharma, S., Eds.; Springer: Singapore, 2019; pp. 305–320. [Google Scholar]
- Topalova, Y.; Dimkov, R.; Todorova, Y.; Daskalova, E.; Petrov, P. Biodegradation of Phenol by Immobilized in Peo-Cryogel Bacillus Laterosporus BT-271 in Sequencing Batch Biofilter. Biotechnol. Biotechnol. Equip. 2011, 25, 2613–2619. [Google Scholar] [CrossRef]
- Zídková, L.; Szőköl, J.; Rucká, L.; Pátek, M.; Nešvera, J. Biodegradation of phenol using recombinant plasmid-carrying Rhodococcus erythropolis strains. Int. Biodeterior. Biodegrad. 2013, 84, 179–184. [Google Scholar] [CrossRef]
- Arutchelvan, V.; Kanakasabai, V.; Nagarajan, S.; Muralikrishnan, V. Isolation and identification of novel high strength phenol degrading bacterial strains from phenol-formaldehyde resin manufacturing industrial wastewater. J. Hazard. Mater. 2005, 127, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, W.; Li, D.; Peng, Y.; Li, Z.; Liu, S. Biodegradation of phenol by bacteria strain Acinetobacter calcoaceticus PA isolated from phenolic wastewater. Int. J. Environ. Res. Public Health 2016, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Qiu, C.; Yang, Q.; Zhang, Y.; Wang, M.; Ye, C.; Guo, M. Analysis of Phenol Biodegradation in Antibiotic and Heavy Metal Resistant Acinetobacter lwoffii NL1. Front. Microbiol. 2021, 12, 725755. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Cydzik-Kwiatkowska, A.; Bernat, K.; Bułkowska, K.; Wojnowska-Baryła, I. Removal of bisphenol A (BPA) in a nitrifying system with immobilized biomass. Bioresour. Technol. 2014, 171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Kumar, S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- Nogina, T.; Fomina, M.; Dumanskaya, T.; Zelena, L.; Khomenko, L.; Mikhalovsky, S.; Podgorskyi, V.; Gadd, G.M. A new Rhodococcus aetherivorans strain isolated from lubricant-contaminated soil as a prospective phenol-biodegrading agent. Appl. Microbiol. Biotechnol. 2020, 104, 3611–3625. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Yang, G.; Du, Y. Immobilized Mutants M1 of Rhodococcus ruber SD3 and Its Application in Phenol Degradation. CN Patent CN103160491A, 15 April 2013. [Google Scholar]
- Bhattacharya, A.; Gupta, A.; Kaur, A.; Malik, D. Remediation of Phenol using Microorganisms: Sustainable way to Tackle the Chemical Pollution Menace. Curr. Org. Chem. 2018, 22, 370–385. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Ghareib, M.; Abou-EL-Souod, G. Biodegradation of phenolic and polycyclic aromatic compounds by some algae and cyanobacteria. J. Bioremediat. Biodegrad. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Gu, Y.-J. Biodegradation Kinetic Studies of Phenol and p-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells. Processes 2021, 9, 133. [Google Scholar] [CrossRef]
- Li, F.; Zhao, L.; Jinxu, Y.; Shi, W.; Zhou, S.; Yuan, K.; Sheng, G.D. Removal of dichlorophenol by Chlorella pyrenoidosa through self-regulating mechanism in air-tight test environment. Ecotoxicol. Environ. Saf. 2018, 164, 109–117. [Google Scholar] [CrossRef]
- Wang, R.; Diao, P.; Chen, Q.; Wu, H.; Xu, N.; Duan, S. Identification of novel pathways for biodegradation of bisphenol A by the green alga Desmodesmus sp.WR1, combined with mechanistic analysis at the transcriptome level. Chem. Eng. J. 2017, 321, 424–431. [Google Scholar] [CrossRef]
- Bera, S.; Roy, A.S.; Mohanty, K. Biodegradation of phenol by a native mixed bacterial culture isolated from crude oil contaminated site. Int. Biodeterior. Biodegrad. 2017, 121, 107–113. [Google Scholar] [CrossRef]
- Kietkwanboot, A.; Chaiprapat, S.; Muller, R.; Suttinun, O. Biodegradation of phenolic compounds present in palm oil mill effluent as single and mixed substrates by Trametes hirsuta AK04. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 989–1002. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Duan, W.; Lin, Y.; Zheng, Y. Biodegradation of phenol in saline or hypersaline environments by bacteria: A review. Ecotoxicol. Environ. Saf. 2019, 184, 109658. [Google Scholar] [CrossRef]
- Tian, H.; Xu, X.; Qu, J.; Li, H.; Hu, Y.; Huang, L.; He, W.; Li, B. Biodegradation of phenolic compounds in high saline wastewater by biofilms adhering on aerated membranes. J. Hazard. Mater. 2020, 392, 122463. [Google Scholar] [CrossRef]
- Bai, X.; Nie, M.; Diwu, Z.; Wang, L.; Nie, H.; Wang, Y.; Yin, Q.; Zhang, B. Simultaneous biodegradation of phenolics and petroleum hydrocarbons from semi-coking wastewater: Construction of bacterial consortium and their metabolic division of labor. Bioresour. Technol. 2022, 347, 126377. [Google Scholar] [CrossRef]
- Yan, W.W.; Sun, F.Q.; Liu, J.B.; Zhou, Y. Enhanced anaerobic phenol degradation by conductive materials via EPS and microbial community alteration. Chem. Eng. J. 2018, 352, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Zhu, X.; Zeng, J.; Zhao, Q.; Jiang, X. Extracellular polymeric substances govern the development of biofilm and mass transfer of polycyclic aromatic hydrocarbons for improved biodegradation. Bioresour. Technol. 2015, 193, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Puengprasert, P.; Chalobol, T.; Sinbuathong, N.; Srinophakhun, P.; Thanapimmetha, A.; Liu, C.-G.; Zhao, X.-Q.; Sakdaronnarong, C. A combined cellulosic and starchy ethanol and biomethane production with stillage recycle and respective cost analysis. Renew. Energy 2020, 157, 444–455. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Ittitanakam, A.; Tanubumrungsuk, W.; Chaithong, S.; Thanosawan, S.; Sinbuathong, N.; Jeraputra, C. Potential of lignin as a mediator in combined systems for biomethane and electricity production from ethanol stillage wastewater. Renew. Energy 2015, 76, 242–248. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.K.; Thanosawan, S.; Chaithong, S.; Sinbuathong, N.; Jeraputra, C. Electricity production from ethanol stillage in two-compartment MFC. Fuel 2013, 107, 382–386. [Google Scholar] [CrossRef]
- Puyol, D.; Hulsen, T.; Padrino, B.; Batstone, D.J.; Martinez, F.; Melero, J.A. Exploring the inhibition boundaries of mixed cultures of purple phototrophic bacteria for wastewater treatment in anaerobic conditions. Water Res. 2020, 183, 116057. [Google Scholar] [CrossRef]
- Mahto, K.U.; Mahto, K.U.; Vandana; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial biofilm and extracellular polymeric substances in the treatment of environmental pollutants: Beyond the protective role in survivability. J. Cleaner Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Zeng, W.; Li, F.; Wu, C.; Yu, R.; Wu, X.; Shen, L.; Liu, Y.; Qiu, G.; Li, J. Role of extracellular polymeric substance (EPS) in toxicity response of soil bacteria Bacillus sp. S3 to multiple heavy metals. Bioprocess Biosyst. Eng. 2020, 43, 153–167. [Google Scholar] [CrossRef]
- Kilby, B.A. The bacterial oxidation of phenol to beta-ketoadipic acid. Biochem. J. 1948, 43, v. [Google Scholar]
- Evans, W.C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem. J 1947, 41, 373–382. [Google Scholar] [CrossRef]
- Van Schie, P.M.; Young, L.Y. Biodegradation of phenol: Mechanisms and applications. Bioremediat. J. 2000, 4, 1–18. [Google Scholar] [CrossRef]
- Léonard, D.; Lindley, N.D. Growth of Ralstonia eutropha on inhibitory concentrations of phenol: Diminished growth can be attributed to hydrophobic perturbation of phenol hydroxylase activity. Enzym. Microb. Technol. 1999, 25, 271–277. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, W.; Zhou, H.; Chen, X. Phenol degradation by Sulfobacillus acidophilus TPY via the meta-pathway. Microbiol. Res. 2016, 190, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Nešvera, J.; Rucká, L.; Pátek, M. Chapter Four—Catabolism of Phenol and Its Derivatives in Bacteria: Genes, Their Regulation, and Use in the Biodegradation of Toxic Pollutants. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 93, pp. 107–160. [Google Scholar]
- Chirwa, E.M.; Makgato, S.S.; Tikilili, P.V.; Lutsinge, T.B. Bioremediation of Chlorinated and Aromatic Petrochemical Pollutants in Multiphase Media and Oily Sludge. In Recent Advances in Environmental Management; CRC Press: Boca Raton, FL, USA, 2018; pp. 373–390. [Google Scholar]
- Zhang, H.; Tang, L.; Wang, J.J.; Yu, J.F.; Feng, H.P.; Lu, Y.; Chen, Y.; Liu, Y.N.; Wang, J.J.; Xie, Q.Q. Enhanced surface activation process of persulfate by modified bagasse biochar for degradation of phenol in water and soil: Active sites and electron transfer mechanism. Colloid Surf. A 2020, 599, 124904. [Google Scholar] [CrossRef]
- Agarry, S.E.; Durojaiye, A.O.; Solomon, B.O. Microbial degradation of phenols: A review. Int. J. Environ. Pollut. 2008, 32, 12–28. [Google Scholar] [CrossRef]
- Breinig, S.; Schiltz, E.; Fuchs, G. Genes involved in anaerobic metabolism of phenol in the bacterium Thauera aromatica. J. Bacteriol. 2000, 182, 5849–5863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, Z.; Wiegel, J. Purification and characterization of an oxygen-sensitive reversible 4-hydroxybenzoate decarboxylase from Clostridium hydroxybenzoicum. Eur. J. Biochem. 1995, 229, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, P.M.; Young, L.Y. Isolation and characterization of phenol-degrading denitrifying bacteria. Appl. Environ. Microbiol. 1998, 64, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.P.; Liu, Y.; Ke, S.Z.; Zhang, T. Anaerobic degradation of phenol in wastewater at ambient temperature. Water Sci. Technol. 2004, 49, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Suarez-Ojeda, M.E.; Carrera, J. Denitritation in an anoxic granular reactor using phenol as sole organic carbon source. Chem. Eng. J. 2016, 288, 289–297. [Google Scholar] [CrossRef]
- Joshi, D.R.; Zhang, Y.; Tian, Z.; Gao, Y.; Yang, M. Performance and microbial community composition in a long-term sequential anaerobic-aerobic bioreactor operation treating coking wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 8191–8202. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Shelvapulle, S.; Reddy, K.R.; Kulkarni, R.V.; Puttaiahgowda, Y.M.; Naveen, S.; Raghu, A.V. Integration of biological pre-treatment methods for increased energy recovery from paper and pulp biosludge. J. Microbiol. Methods 2019, 160, 93–100. [Google Scholar] [CrossRef]
- Skoczko, I.; Puzowski, P.; Szatylowicz, E. Experience from the Implementation and Operation of the Biological Membrane Reactor (MBR) at the Modernized Wastewater Treatment Plant in Wydminy. Water 2020, 12, 3410. [Google Scholar] [CrossRef]
- Hamedi, H.; Ehteshami, M.; Mirbagheri, S.A.; Rasouli, S.A.; Zendehboudi, S. Current Status and Future Prospects of Membrane Bioreactors (MBRs) and Fouling Phenomena: A Systematic Review. Can. J. Chem. Eng. 2019, 97, 32–58. [Google Scholar] [CrossRef]
- Van Houghton, B.D.; Acharya, S.M.; Rosenblum, J.S.; Chakraborty, R.; Tringe, S.G.; Cath, T.Y. Membrane Bioreactor Pretreatment of High-Salinity O&G Produced Water. ACS EST Water 2022, 2, 484–494. [Google Scholar] [CrossRef]
- Chang, H.M.; Chen, S.S.; Hsiao, S.S.; Chang, W.S.; Chien, I.C.; Duong, C.C.; Nguyen, T.X.Q. Water reclamation and microbial community investigation: Treatment of tetramethylammonium hydroxide wastewater through an anaerobic osmotic membrane bioreactor hybrid system. J. Hazard. Mater. 2022, 427, 128200. [Google Scholar] [CrossRef]
- Li, R.-H.; Li, B.; Li, X.-Y. An integrated membrane bioreactor system with iron-dosing and side-stream co-fermentation for enhanced nutrient removal and recovery: System performance and microbial community analysis. Bioresour. Technol. 2018, 260, 248–255. [Google Scholar] [CrossRef]
- Zhao, S.; Yun, H.; Khan, A.; Salama, E.-S.; Redina, M.M.; Liu, P.; Li, X. Two-stage microbial fuel cell (MFC) and membrane bioreactor (MBR) system for enhancing wastewater treatment and resource recovery based on MFC as a biosensor. Environ. Res. 2022, 204, 112089. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. Resource recovery from wastewater by anaerobic membrane bioreactors: Opportunities and challenges. Bioresour. Technol. 2018, 270, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Erkan, H.S.; Turan, N.B.; Engin, G.Ö. Membrane bioreactors for wastewater treatment. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 81, pp. 151–200. [Google Scholar]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Anaerobic membrane bioreactors (AnMBRs) for municipal wastewater treatment—Potential benefits, constraints, and future perspectives: An updated review. Sci. Total Environ. 2022, 802, 149612. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Capodici, M.; Cosenza, A.; Di Trapani, D.; Ekama, G.A. The effect of the solids and hydraulic retention time on moving bed membrane bioreactor performance. J. Cleaner Prod. 2018, 170, 1305–1315. [Google Scholar] [CrossRef]
- Shelly, Y.; Kuc, M.E.; Iasur-Kruh, L.; Azerrad, S.; Kurzbaum, E. A New Isolate Is an Extremely Efficient Biofilm-Formative Denitrifying Bacterium. Front. Environ. Sci. 2020, 8, 556226. [Google Scholar] [CrossRef]
- Luo, D.; Qian, J.; Jin, X.; Zhang, L.; You, K.; Yu, P.F.; Fu, J.X. How phenol stresses anammox for the treatment of ammonia-rich wastewater: Phenomena, microbial community evolution and molecular modeling. Bioresour. Technol. 2022, 347, 126747. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Han, Y.; Ma, W.; Han, H.; Xu, C.; Zhu, H. Simultaneous nitrification and denitrification (SND) bioaugmentation with Pseudomonas sp. HJ3 inoculated for enhancing phenol and nitrogen removal in coal gasification wastewater. Water Sci. Technol. 2019, 80, 1512–1523. [Google Scholar] [CrossRef]
- Joss, A.; Keller, E.; Alder, A.C.; Gobel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Song, I.; Choung, Y.; Park, J. Improved phenol degradation in high-phenol-fed MBR by membrane-driven containment of non-settling biodegradation microbes. Desalin. Water Treat. 2011, 31, 320–325. [Google Scholar] [CrossRef]
- Marrot, B.; Barrios-Martinez, A.; Moulin, P.; Roche, N. Industrial wastewater treatment in a membrane bioreactor: A review. Environ. Prog. 2004, 23, 59–68. [Google Scholar] [CrossRef]
- Liao, B.Q.; Kraemer, J.T.; Bagley, D.M. Anaerobic membrane bioreactors: Applications and research directions. Crit. Rev. Environ. Sci. Technol. 2006, 36, 489–530. [Google Scholar] [CrossRef]
- Abuabdou, S.M.A.; Ahmad, W.; Aun, N.C.; Bashir, M.J.K. A review of anaerobic membrane bioreactors (AnMBR) for the treatment of highly contaminated landfill leachate and biogas production: Effectiveness, limitations and future perspectives. J. Cleaner Prod. 2020, 255, 120215. [Google Scholar] [CrossRef]
- Judd, S. The status of membrane bioreactor technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Vazquez, H.; Jefferson, B.; Judd, S.J. Membrane bioreactors vs conventional biological treatment of landfill leachate: A brief review. J. Chem. Technol. Biotechnol. 2004, 79, 1043–1049. [Google Scholar] [CrossRef]
- Diez, V.; Ramos, C.; Cabezas, J.L. Treating wastewater with high oil and grease content using an Anaerobic Membrane Bioreactor (AnMBR). Filtration and cleaning assays. Water Sci. Technol. 2012, 65, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Pandya, K.; Brahmbhatt, N. Performance evaluation of effluent treatment plant and hazardous waste management of pharmaceutical industry of Ankleshwar. Adv. Appl. Sci. Res. 2015, 6, 157–161. [Google Scholar]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Huang, X.; Gui, P.; Qian, Y. Effect of sludge retention time on microbial behaviour in a submerged membrane bioreactor. Process Biochem. 2001, 36, 1001–1006. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Piotrowicz, A.; Lagód, G. Modeling of Wastewater Treatment Processes in Membrane Bioreactors Compared to Conventional Activated Sludge Systems. Processes 2019, 7, 285. [Google Scholar] [CrossRef]
- Sun, Y.; Angelotti, B.; Wang, Z.W. Continuous-flow aerobic granulation in plug-flow bioreactors fed with real domestic wastewater. Sci. Total Environ. 2019, 688, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Rezakazemi, M.; Maghami, M.; Mohammadi, T. Wastewaters treatment containing phenol and ammonium using aerobic submerged membrane bioreactor. Chem. Cent. J. 2018, 12, 79. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Liu, L.; Lee, K.; Miao, J.H. Review of Biological Processes in a Membrane Bioreactor (MBR): Effects of Wastewater Characteristics and Operational Parameters on Biodegradation Efficiency When Treating Industrial Oily Wastewater. J. Mar. Sci. Eng. 2022, 10, 1229. [Google Scholar] [CrossRef]
- Ren, L.F.; Ngo, H.H.; Bu, C.N.; Ge, C.H.; Ni, S.Q.; Shao, J.H.; He, Y.L. Novel external extractive membrane bioreactor (EMBR) using electrospun polydimethylsiloxane/polymethyl methacrylate membrane for phenol-laden saline wastewater. Chem. Eng. J. 2020, 383, 123179. [Google Scholar] [CrossRef]
- Zhou, S.; Watanabe, H.; Wei, C.; Wang, D.; Zhou, J.; Tatarazako, N.; Masunaga, S.; Zhang, Y. Reduction in toxicity of coking wastewater to aquatic organisms by vertical tubular biological reactor. Ecotoxicol. Environ. Saf. 2015, 115, 217–222. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Ma, Z.; Luan, Z.; Wang, Y.; Wang, Z.; Wang, L. Removal of phenolic substances from wastewater by algae. A review. Environ. Chem. Lett. 2020, 18, 377–392. [Google Scholar] [CrossRef]
- De Vela, R.J. A review of the factors affecting the performance of anaerobic membrane bioreactor and strategies to control membrane fouling. Rev. Environ. Sci. Bio/Technol. 2021, 20, 607–644. [Google Scholar] [CrossRef]
- Tan, X.; Acquah, I.; Liu, H.; Li, W.; Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 2019, 220, 1150–1162. [Google Scholar] [CrossRef]
- Adeel, M.; Xu, Y.; Ren, L.-F.; Shao, J.; He, Y. Improvement of phenol separation and biodegradation from saline wastewater in extractive membrane bioreactor (EMBR). Bioresour. Technol. Rep. 2022, 17, 100897. [Google Scholar] [CrossRef]
- Mullins, N.R.; Daugulis, A.J. The biological treatment of synthetic fracking fluid in an extractive membrane bioreactor: Selective transport and biodegradation of hydrophobic and hydrophilic contaminants. J. Hazard. Mater. 2019, 371, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Attaway, H.; Gooding, C.H.; Schmidt, M.G. Biodegradation of BTEX vapors in a silicone membrane bioreactor system. J. Ind. Microbiol. Biotechnol. 2001, 26, 316–325. [Google Scholar] [CrossRef]
- Swain, G.; Sonwani, R.K.; Giri, B.S.; Singh, R.S.; Jaiswal, R.P.; Rai, B.N. Collective removal of phenol and ammonia in a moving bed biofilm reactor using modified bio-carriers: Process optimization and kinetic study. Bioresour. Technol. 2020, 306, 123177. [Google Scholar] [CrossRef]
- Nakhli, S.A.; Ahmadizadeh, K.; Fereshtehnejad, M.; Rostami, M.H.; Safari, M.; Borghei, S.M. Biological removal of phenol from saline wastewater using a moving bed biofilm reactor containing acclimated mixed consortia. Springerplus 2014, 3, 112. [Google Scholar] [CrossRef]
- Swain, G.; Lal Maurya, K.; Kumar Sonwani, R.; Sharan Singh, R.; Prakash Jaiswal, R.; Rai, B.N. Effect of mixing intensity on biodegradation of phenol in a moving bed biofilm reactor: Process optimization and external mass transfer study. Bioresour. Technol. 2022, 351, 126921. [Google Scholar] [CrossRef] [PubMed]
- Dosta, J.; Nieto, J.M.; Vila, J.; Grifoll, M.; Mata-Álvarez, J. Phenol removal from hypersaline wastewaters in a Membrane Biological Reactor (MBR): Operation and microbiological characterisation. Bioresour. Technol. 2011, 102, 4013–4020. [Google Scholar] [CrossRef]
- Yasar Arafath, K.A.; Gopinath, S.; Nilavunesan, D.; Sivanesan, S.; Baskaralingam, P. Removal of phenol in coir retting wastewater by membrane bioreactor combined with photo-fenton process using RSM. Mater. Res. Express 2019, 6, 115506. [Google Scholar] [CrossRef]
- Huang, S.; Xu, B.; Ng, T.C.A.; He, M.; Shi, X.; Ng, H.Y. Feasibility of implementing quorum quenching technology to mitigate membrane fouling in MBRs treating phenol-rich pharmaceutical wastewater: Application of Rhodococcus sp. BH4 and quorum quenching consortium. Bioresour. Technol. 2022, 358, 127389. [Google Scholar] [CrossRef]
- Garcia Rea, V.S.; Egerland Bueno, B.; Cerqueda-García, D.; Muñoz Sierra, J.D.; Spanjers, H.; van Lier, J.B. Degradation of p-cresol, resorcinol, and phenol in anaerobic membrane bioreactors under saline conditions. Chem. Eng. J. 2022, 430, 132672. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Liu, H.; Wen, Q. Effects of polyurethane foam carrier addition on anoxic/aerobic membrane bioreactor (A/O-MBR) for coal gasification wastewater (CGW) treatment: Performance and microbial community structure. Sci. Total Environ. 2021, 789, 148037. [Google Scholar] [CrossRef] [PubMed]
- Praveen, P.; Loh, K.C. Two-phase biodegradation of phenol in trioctylphosphine oxide impregnated hollow fiber membrane bioreactor. Biochem. Eng. J. 2013, 79, 274–282. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C. Phenol biodegradation in hybrid hollow-fiber membrane bioreactors. World J. Microbiol. Biotechnol. 2008, 24, 1843–1849. [Google Scholar] [CrossRef]
- Edwards, W.; Bownes, R.; Leukes, W.D.; Jacobs, E.P.; Sanderson, R.; Rose, P.D.; Burton, S.G. A capillary membrane bioreactor using immobilized polyphenol oxidase for the removal of phenols from industrial effluents. Enzym. Microb. Technol. 1999, 24, 209–217. [Google Scholar] [CrossRef]
- Luke, A.K.; Burton, S.G. A novel application for Neurospora crassa: Progress from batch culture to a membrane bioreactor for the bioremediation of phenols. Enzyme Microb. Technol. 2001, 29, 348–356. [Google Scholar] [CrossRef]
- Loh, K.-C.; Chung, T.-S.; Ang, W.-F. Immobilized-cell membrane bioreactor for high-strength phenol wastewater. J. Environ. Eng. 2000, 126, 75–79. [Google Scholar] [CrossRef]
- Ntwampe, S.K.O.; Sheldon, M.S. Quantifying growth kinetics of Phanerochaete chrysosporium immobilised on a vertically orientated polysulphone capillary membrane: Biofilm development and substrate consumption. Biochem. Eng. J. 2006, 30, 147–151. [Google Scholar] [CrossRef]
- Edwards, W.; Leukes, W.D.; Rose, P.D.; Burton, S.G. Immobilization of polyphenol oxidase on chitosan-coated polysulphone capillary membranes for improved phenolic effluent bioremediation. Enzym. Microb. Technol. 1999, 25, 769–773. [Google Scholar] [CrossRef]
- Yuan, G.; Tian, Y.; Wang, B.; You, X.; Liao, Y. Mitigation of membrane biofouling via immobilizing Ag-MOFs on composite membrane surface for extractive membrane bioreactor. Water Res. 2021, 209, 117940. [Google Scholar] [CrossRef]
- Gede Wenten, I.; Friatnasary, D.L.; Khoiruddin, K.; Setiadi, T.; Boopathy, R. Extractive membrane bioreactor (EMBR): Recent advances and applications. Bioresour. Technol. 2020, 297, 122424. [Google Scholar] [CrossRef]
- Manetti, M.; Tomei, M.C. Extractive polymeric membrane bioreactors for industrial wastewater treatment: Theory and practice. Process Saf. Environ. Prot. 2022, 162, 169–186. [Google Scholar] [CrossRef]
- Keyvan Hosseini, M.; Liu, L.; Keyvan Hosseini, P.; Bhattacharyya, A.; Lee, K.; Miao, J.; Chen, B. Review of Hollow Fiber (HF) Membrane Filtration Technology for the Treatment of Oily Wastewater: Applications and Challenges. J. Mar. Sci. Eng. 2022, 10, 1313. [Google Scholar] [CrossRef]
- De Bartolo, L. Hollow Fiber Membrane Bioreactor for Cell Growth. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 953–955. [Google Scholar]
- Praveen, P.; Loh, K.-C. Simultaneous extraction and biodegradation of phenol in a hollow fiber supported liquid membrane bioreactor. J. Membr. Sci. 2013, 430, 242–251. [Google Scholar] [CrossRef]
- Cao, L.; Li, Y.; Li, P.; Zhang, X.; Ni, L.; Qi, L.; Wen, H.; Zhang, X.; Zhang, Y. Application of moving bed biofilm reactor-nanofiltration-membrane bioreactor with loose nanofiltration hollow fiber membranes for synthetic roxithromycin-containing wastewater treatment: Long-term performance, membrane fouling and microbial community. Bioresour. Technol. 2022, 360, 127527. [Google Scholar] [CrossRef]
- Kudisi, D.; Lu, X.; Zheng, C.; Wang, Y.; Cai, T.; Li, W.; Hu, L.; Zhang, R.; Zhang, Y.; Zhen, G. Long-term performance, membrane fouling behaviors and microbial community in a hollow fiber anaerobic membrane bioreactor (HF-AnMBR) treating synthetic terephthalic acid-containing wastewater. J. Hazard. Mater. 2022, 424, 127458. [Google Scholar] [CrossRef] [PubMed]
- Keskin, B.; Eryildiz, B.; Pasaoglu, M.E.; Türken, T.; Vatanpour, V.; Koyuncu, I. Fabrication and characterization of different braid-reinforced PVC hollow fiber membranes to use in membrane bioreactor for wastewater treatment. J. Appl. Polym. Sci. 2023, 140, e53794. [Google Scholar] [CrossRef]
- Capodici, M.; Cosenza, A.; Di Bella, G.; Di Trapani, D.; Viviani, G.; Mannina, G. Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kim, I.; Choi, D.C.; Lee, J.; Chae, H.R.; Jang, J.H.; Lee, C.H.; Park, P.K.; Won, Y.J. Preparation and application of patterned hollow-fiber membranes to membrane bioreactor for wastewater treatment. J. Membr. Sci. 2015, 490, 190–196. [Google Scholar] [CrossRef]
- Pan, D.; Shao, S.; Zhong, J.; Wang, M.; Wu, X. Performance and mechanism of simultaneous nitrification-denitrification and denitrifying phosphorus removal in long-term moving bed biofilm reactor (MBBR). Bioresour. Technol. 2022, 348, 126726. [Google Scholar] [CrossRef] [PubMed]
- Raj Deena, S.; Kumar, G.; Vickram, A.S.; Rani Singhania, R.; Dong, C.D.; Rohini, K.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V.K. Efficiency of various biofilm carriers and microbial interactions with substrate in moving bed-biofilm reactor for environmental wastewater treatment. Bioresour. Technol. 2022, 359, 127421. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.; Madan, R.; Hussain, A. Advancement in biological wastewater treatment using hybrid moving bed biofilm reactor (MBBR): A review. Appl. Water Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Wang, S.; Parajuli, S.; Sivalingam, V.; Bakke, R. Biofilm in moving bed biofilm process for wastewater treatment. Bact. Biofilms 2019, 1, 1–15. [Google Scholar]
- Safwat, S. Moving Bed Biofilm Reactors for Wastewater Treatment: A Review of Basic Concepts. Int. J. Res. 2019, 6, 85–90. [Google Scholar]
- Malarat, S.; Khongpun, D.; Limtong, K.; Sinthuwong, N.; Soontornapaluk, P.; Sakdaronnarong, C.; Posoknistakul, P. Preparation of Nanocellulose from Coffee Pulp and Its Potential as a Polymer Reinforcement. ACS Omega 2023, 8, 25122–25133. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Yamamoto, K.; Nghiem, L.D. High retention membrane bioreactors: Challenges and opportunities. Bioresour. Technol. 2014, 167, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Karim, Z.; Charnnok, B.; Poonsawat, T.; Posoknistakul, P.; Laosiripojana, N.; Wu, K.C.; Sakdaronnarong, C. Fabrication and Characterization of Functional Biobased Membranes from Postconsumer Cotton Fabrics and Palm Waste for the Removal of Dyes. Int. J. Mol. Sci. 2023, 24, 6030. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Karim, Z.; Pongchaikul, P.; Posoknistakul, P.; Intra, P.; Laosiripojana, N.; Wu, K.C.W.; Sakdaronnarong, C. Nitrogen and sulfur doped carbon dots coupled cellulose nanofibers: A surface functionalized nanocellulose membranes for air filtration. J. Taiwan Inst. Chem. Eng. 2024, 105324. [Google Scholar] [CrossRef]
- Yang, M.; Lotfikatouli, S.; Chen, Y.; Li, T.; Ma, H.; Mao, X.; Hsiao, B.S. Nanostructured all-cellulose membranes for efficient ultrafiltration of wastewater. J. Membr. Sci. 2022, 650, 120422. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Guo, Y.; Hu, J.; Lin, S.; Tu, Y.; Chen, L.; Ni, Y.; Huang, L. Recent advances on cellulose-based nanofiltration membranes and their applications in drinking water purification: A review. J. Clean. Prod. 2022, 333, 130171. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 42042. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Joshi, R.; Sebat, N.; Chi, K.; Khan, M.; Johnson, K.I.; Alhamzani, A.G.; Habib, M.A.; Lindstrom, T.; Hsiao, B.S. Low Fouling Nanostructured Cellulose Membranes for Ultrafiltration in Wastewater Treatment. Membranes 2023, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, M.-B.; Zhu, C.-Y.; Ma, M.-Q.; Yang, J.; Wu, J.; Xu, Z.-K. Polyamide Nanofiltration Membranes Incorporated with Cellulose Nanocrystals for Enhanced Water Flux and Chlorine Resistance. ACS Sustain. Chem. Eng. 2019, 7, 12315–12322. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Jacobs, N.V.; Luyt, A.S. Nanofibrous alginate membrane coated with cellulose nanowhiskers for water purification. Cellulose 2018, 25, 417–427. [Google Scholar] [CrossRef]
- Lotfikatouli, S.; Hadi, P.; Yang, M.Y.; Walker, H.W.; Hsiao, B.S.; Gobler, C.; Reichel, M.; Mao, X.W. Enhanced anti-fouling performance in Membrane Bioreactors using a novel cellulose nanofiber-coated membrane. Sep. Purif. Technol. 2021, 275, 119145. [Google Scholar] [CrossRef]
- Etemadi, H.; Yegani, R.; Seyfollahi, M. The effect of amino functionalized and polyethylene glycol grafted nanodiamond on anti-biofouling properties of cellulose acetate membrane in membrane bioreactor systems. Sep. Purif. Technol. 2017, 177, 350–362. [Google Scholar] [CrossRef]
- Dos Santos, L.M.; Livingston, A.G. Membrane-attached biofilms for VOC wastewater treatment. II: Effect of biofilm thickness on performance. Biotechnol. Bioeng. 1995, 47, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef]
- Hao, X.; Li, J.; Van Loosdrecht, M.; Li, T. A sustainability-based evaluation of membrane bioreactors over conventional activated sludge processes. J. Environ. Chem. Eng. 2018, 6, 2597–2605. [Google Scholar] [CrossRef]
- Gabarrón, S.; Ferrero, G.; Dalmau, M.; Comas, J.; Rodriguez-Roda, I. Assessment of energy-saving strategies and operational costs in full-scale membrane bioreactors. J. Environ. Manag. 2014, 134, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Krzeminski, P.; van der Graaf, J.H.J.M.; van Lier, J.B. Specific energy consumption of membrane bioreactor (MBR) for sewage treatment. Water Sci. Technol. 2012, 65, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Pretel, R.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. Economic and environmental sustainability of submerged anaerobic MBR-based (AnMBR-based) technology as compared to aerobic-based technologies for moderate-/high-loaded urban wastewater treatment. J. Environ. Manag. 2016, 166, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; d’Antoni, B.M.; Bongards, M.; Chaparro, A.; Cronrath, A.; Fatone, F.; Lema, J.M.; Mauricio-Iglesias, M.; Soares, A.; Hospido, A. Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl. Energy 2016, 179, 1251–1268. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef]
- Wijmans, J.; Kaschemekat, J.; Davidson, J.; Baker, R. Treatment of organic-contaminated wastewater streams by pervaporation. Environ. Prog. 1990, 9, 262–268. [Google Scholar] [CrossRef]
- Xiao, K.; Xu, Y.; Liang, S.; Lei, T.; Sun, J.; Wen, X.; Zhang, H.; Chen, C.; Huang, X. Engineering application of membrane bioreactor for wastewater treatment in China: Current state and future prospect. Front. Environ. Sci. Eng. 2014, 8, 805–819. [Google Scholar] [CrossRef]
- Guo, T.; Englehardt, J.; Wu, T. Review of cost versus scale: Water and wastewater treatment and reuse processes. Water Sci. Technol. 2014, 69, 223–234. [Google Scholar] [CrossRef]
- Jalab, R.; Awad, A.M.; Nasser, M.S.; Minier-Matar, J.; Adham, S.; Judd, S.J. An empirical determination of the whole-life cost of FO-based open-loop wastewater reclamation technologies. Water Res. 2019, 163, 114879. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.J. Membrane technology costs and me. Water Res. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- DeCarolis, J.; Adham, S.; Pearce, W.; Hirani, Z.; Lacy, S.; Stephenson, R. Cost trends of MBR systems for municipal wastewater treatment. Proc. Water Environ. Fed. 2007, 2007, 3407–3418. [Google Scholar] [CrossRef]
- Makisha, N.; Saveliev, O.; Katella, S. Cost aspects of membrane bioreactors for wastewater treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 177, 012037. [Google Scholar] [CrossRef]
- Humeau, P.; Hourlier, F.; Bulteau, G.; Massé, A.; Jaouen, P.; Gérente, C.; Faur, C.; Le Cloirec, P. Estimated costs of implementation of membrane processes for on-site greywater recycling. Water Sci. Technol. 2011, 63, 2949–2956. [Google Scholar] [CrossRef]
- Iglesias, R.; Simón, P.; Moragas, L.; Arce, A.; Rodriguez-Roda, I. Cost comparison of full-scale water reclamation technologies with an emphasis on membrane bioreactors. Water Sci. Technol. 2017, 75, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sancho, F.; Molinos-Senante, M.; Sala-Garrido, R. Economic valuation of environmental benefits from wastewater treatment processes: An empirical approach for Spain. Sci. Total Environ. 2010, 408, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Xiao, K.; Zhang, J.; Zhang, X.; Wang, X.; Liang, S.; Sun, J.; Meng, F.; Huang, X. Cost-benefit analysis and technical efficiency evaluation of full-scale membrane bioreactors for wastewater treatment using economic approaches. J. Clean. Prod. 2021, 301, 126984. [Google Scholar] [CrossRef]



| Type of Contaminants | Initial Concentration | Microorganisms | Contaminant Removal Efficiency (%) | Time | References |
|---|---|---|---|---|---|
| 2,4-dichlorophenol | 51 mg L−1 | Pseudomonas putida | 35 | 8 days | [82] |
| Phenol | 1000 mg L−1 | Pseudomonas aeruginosa, Klebsiella pneumoniae, Klebsiella variicola | 71.70–74.67 | 3 days | [83] |
| Phenol | 300 mg L−1 | Bacillus laterosporus BT-271 | 100 | 15 days | [85] |
| Phenol | NA | Rhodococcus erythropolis (pSRKBphe-cat) Rhodococcus erythropolis (pSRKphe) | 100 87 | 14 days | [86] |
| Phenol | 2500 mg L−1 | Pseudomonas cepacia | 100 | 96 h | [87] |
| Phenol | 1750 mg L−1 | Bacillus brevis | 100 | 132 h | [87] |
| Phenol | 800 mg L−1 | Acinetobacter calcoaceticus | 91.6 | 48 h | [88] |
| Phenol | 1.1 g L−1 | Acinetobacter lwoffii NL1 | 0.5 g L−1 | 12 h | [89] |
| Bisphenol A | 2.5 to 10.0 mg L−1 | Consortia of immobilized microorganisms | 87.1–92.9 | NA | [90] |
| Phenol | 1000 mg L−1 | Pseudomonas putida (MTCC1194) | 100 | 162 h | [91] |
| Catechol | 500 mg L−1 | Pseudomonas putida (MTCC1194) | 100 | 92 h | [91] |
| Phenol | 500 mg L−1 | Rhodococcus aetherivorans UCMAc-603 | 35.7 mg L−1 h−1 | NA | [92] |
| Phenol | 1750 mg L−1 | Rhodococcus aetherivorans UCMAc-603 | 18.2 mg L−1 h−l | NA | [92] |
| Phenol | NA | Rhodococcus ruber SD3 | 98 | 72 h | [93] |
| α-Naphthol | NA | Oscillatoria rubescens | 59.49 | 5 days | [95] |
| Phenol and p-cresol | NA | Pseudomonas putida ATCC 17484 | 100 | 48 h | [96] |
| 2,4-Dichlorophenol | NA | Chlorella pyrenoidosa | 100 | 120 h | [97] |
| Bisphenol A | NA | Desmodesmus sp.WR1 | 18–57 | 10 days | [98] |
| Membrane Bioreactor | Membrane Type | Contaminants | MBR Operating Parameters | Phenol Removal Efficiency | References |
|---|---|---|---|---|---|
| EMBR | PDMS/PMMA/MWCNTs | Phenol (1000–4000 mg L−1) | Saline wastewater; effective membrane surface area: 20 cm2; HRT: 24 h; and temperature: 24 ± 2 °C. | 100% | [161] |
| EMBR | Hytrel™ 3548 tubing | Methyl ethyl ketone, benzene, phenol, and acetic acid (1000 mg L−1) | Synthetic hydraulic fracturing wastewater; T: 30 ± 0.5 °C; effective membrane surface area: 0.132 m2; V: 3 L; and HRT: ~8 h and microorganisms: microbial consortium (Pseudomonas sp., Comamonas sp., Achromobacter sp., Lysinibacillus sp., and Oxalobacter sp.). | Benzene and phenol: 99% Methyl ethyl ketone: 96% Acetic acid: 53% | [162] |
| EMBR | Electro-spun fiber of polydimethylsiloxane/polymethyl methacrylate | Synthetic phenol-laden saline wastewater (phenol: 14.1–290.7 mg L−1) | Effective membrane surface area: 0.0048 m2; HRT: 24 h; T: 24–26 °C; and microorganism: ProtTeobacteria and Saccharibacteria. | 100% | [156] |
| EMBR | Silicone membrane (capillary membrane) | Benzene, toluene, ethylbenzene, and xylene (BTEX) | T: 25 °C; pH: 6.8–7.0; agitation rate: 300 rpm; and microorganism: Pseudomonas putida TX1 and BTE1. | 75–99% | [163] |
| MBBR | Polypropylene and polyurethane | Phenol (0.1 g L−1) and ammonia (0.1 g L−1) | pH: 6.5; HRT: 2–12 h; and air flow rate: 2.15 L min−1; and microorganism: acclimatized bacterial consortium. | 91.2% | [164] |
| MBBR | Polyethylene | Phenol (800 mg L−1) and saline (40 g L−1) wastewater | HRT: 18 h; T: 23 ± 2 °C; pH: neutral; dissolved oxygen (DO): 4–5 mg O2 L−1; and microorganism: activated biomass mixed consortia. | 99% | [165] |
| MBBR | Polyethylene | Synthetic wastewater; phenol (200 mg L−1) | HRT: 24 h; DO: 5.0 ± 1.0 mg L−1; pH: 7.0; T: 32 ± 2.0 °C; and microorganism: Bacillus cereus. | 87.64% | [166] |
| MBR | Flat sheet membrane from methacrylate | Hypersaline wastewater, phenol (8–15 mg L−1) and salt (150–160 mS cm−1) | HRT: 0.5–0.7 days; and pH: 7.5–8.3; and microorganism: Halomonas and Marinobacter. | >98% | [167] |
| MBR | Flat sheet PVDF membrane | Coir retting wastewater; phenol (90 mg L−1) | HRT: 8 h; pH: 6.5-7.2; effective membrane surface area: 0.8 m2; and microorganism: activated sludge. | 99% | [168] |
| MBR | Flat sheet ceramic membrane | Phenol-rich pharmaceutical wastewater | Phenol: 539 ± 67 mg L−1; HRT: 18 h; T: 27 ± 1 °C; pH: pH: 8.0 ± 0.5; effective membrane surface area: 0.008 m2; and microorganism: Rhodococcus sp. | >99% | [169] |
| AnMBR | PVDF membrane | Synthetic wastewater p-cresol (1200 mg p-cresol L−1) and phenol (2000 mg phenol L−1), resorcinol (800 mg resorcinol L−1) and phenol (2000 mg phenol L−1) | HRT: 6 d; T: 35 °C; operation time 77–112 d; and microorganism: Syntrophorhabdus sp. and Methanosaeta sp.; | 100% 100% | [170] |
| A/O-MBR | PVDF hollow-fiber membrane | Coal gasification wastewater with abundant phenols | HRT: 12 h and 47 h; T: 20–25 °C; total membrane surface area: 0.2 m2; and microorganisms: Flavobacterium sp., Holophaga sp., and Geobacter sp. | >97% | [171] |
| SMBR | Polyvinylidene fluoride | Synthetic phenolic wastewater (phenol: 1000 mg L−1) | HRT: 16.6 h; T: 23–24 °C; effective membrane surface area: 5 × 10−2 m2; DO: 2 mg L−1; and microorganisms: activated sludge. | >99% | [154] |
| HFMBR | Trioctylphosphine oxide (TOPO) impregnated in polypropylene | Phenolic wastewater (phenol: 100 mg L−1) | HRT: 12 h; pH: 6.5–7.0; and microorganisms: Pseudomonas putida ATCC 11172. | 100% | [172] |
| HFMBR | Polyether sulfone + granular activated carbon | Synthetic wastewater (phenol: 1000 mg L−1) | HRT: 18 h; and microorganisms: Pseudomonas putida. | 100% | [173] |
| Wastewater Treatment Technologies | Wastewater Type | Capital/Operational Cost (CAPEX/OPEX) | References |
|---|---|---|---|
| CAS | Municipal wastewater | 0.11 USD/m3 | [217,219] |
| Pervaporation | Organic-contaminated Wastewater | Capital cost: USD 180K Operating cost: USD 50K | [218] |
| MBR | Wastewater | Capital cost USD 2.9–6.9 million (1 megaliter per day flow capacity plant) | [220,221] |
| MBR | Municipal wastewater Industrial wastewater | Capital cost: 600 USD/(m3/d) Capital cost: 900 USD/(m3/d) | [222] |
| MBR | Municipal wastewater | 0.48–0.59 USD/m3 | [223] |
| HFMBR | Municipal wastewater | 0.55–0.68 USD/m3 | [213] |
| HFMBR | Domestic wastewater | 0.24–0.25 USD/m3 | [224] |
| SMBR | Greywater (operating capacity 3 m3/day) (operating capacity 30 m3/day) | SMBR: 7.40 USD/m3 NF: 7.80 USD/m3 SMBR: 4.40 USD/m3 NF: 4.82 USD/m3 | [225] |
| FSMBR | Municipal wastewater | 0.42 USD/m3 | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.J.; Wibowo, A.; Karim, Z.; Posoknistakul, P.; Matsagar, B.M.; Wu, K.C.-W.; Sakdaronnarong, C. Wastewater Treatment Using Membrane Bioreactor Technologies: Removal of Phenolic Contaminants from Oil and Coal Refineries and Pharmaceutical Industries. Polymers 2024, 16, 443. https://doi.org/10.3390/polym16030443
Khan MJ, Wibowo A, Karim Z, Posoknistakul P, Matsagar BM, Wu KC-W, Sakdaronnarong C. Wastewater Treatment Using Membrane Bioreactor Technologies: Removal of Phenolic Contaminants from Oil and Coal Refineries and Pharmaceutical Industries. Polymers. 2024; 16(3):443. https://doi.org/10.3390/polym16030443
Chicago/Turabian StyleKhan, Mohd Jahir, Agung Wibowo, Zoheb Karim, Pattaraporn Posoknistakul, Babasaheb M. Matsagar, Kevin C.-W. Wu, and Chularat Sakdaronnarong. 2024. "Wastewater Treatment Using Membrane Bioreactor Technologies: Removal of Phenolic Contaminants from Oil and Coal Refineries and Pharmaceutical Industries" Polymers 16, no. 3: 443. https://doi.org/10.3390/polym16030443
APA StyleKhan, M. J., Wibowo, A., Karim, Z., Posoknistakul, P., Matsagar, B. M., Wu, K. C.-W., & Sakdaronnarong, C. (2024). Wastewater Treatment Using Membrane Bioreactor Technologies: Removal of Phenolic Contaminants from Oil and Coal Refineries and Pharmaceutical Industries. Polymers, 16(3), 443. https://doi.org/10.3390/polym16030443








