Synthesis, Characterization, and Evaluation of the Adsorption Behavior of Cellulose-Graft-Poly(Acrylonitrile-co-Acrylic Acid) and Cellulose-Graft-Poly(Acrylonitrile-co-Styrene) towards Ni(II) and Cu(II) Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graft Copolymerization of Binary Vinyl Monomers Mixture onto Cellulose Fibers
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Solid State NMR Measurement (13C CP MAC NMR)
2.3.3. Fourier Transform Infrared Spectroscopy (FT-IR Spectra)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. X-ray Measurements
2.4. Adsorption of Metal Ions from Aqueous Medium
3. Results and Discussion
3.1. Graft Copolymerization
3.2. Characterization of the Grafted Cellulose
3.2.1. SEM Analysis
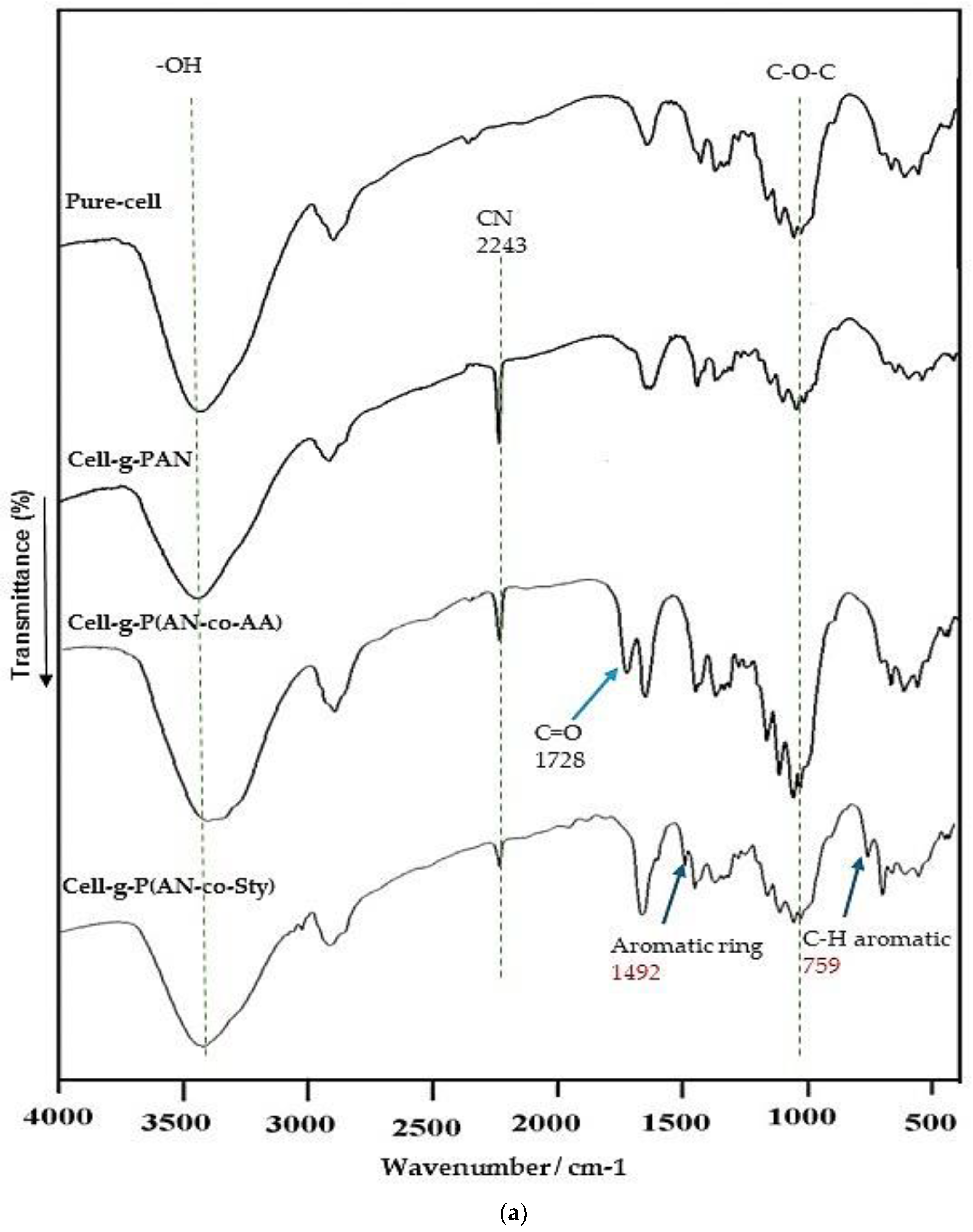
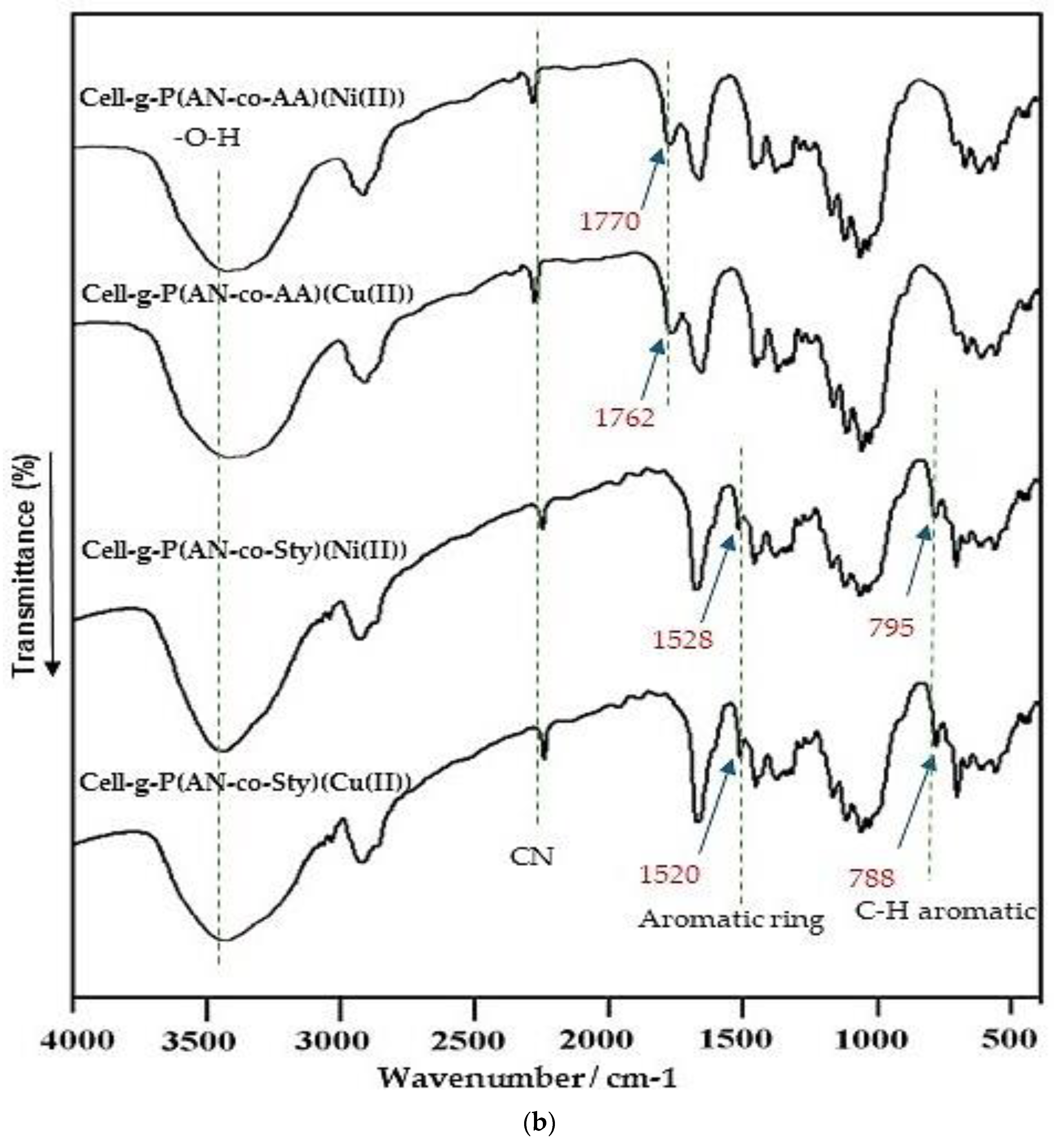
3.2.2. FT-IR Analysis
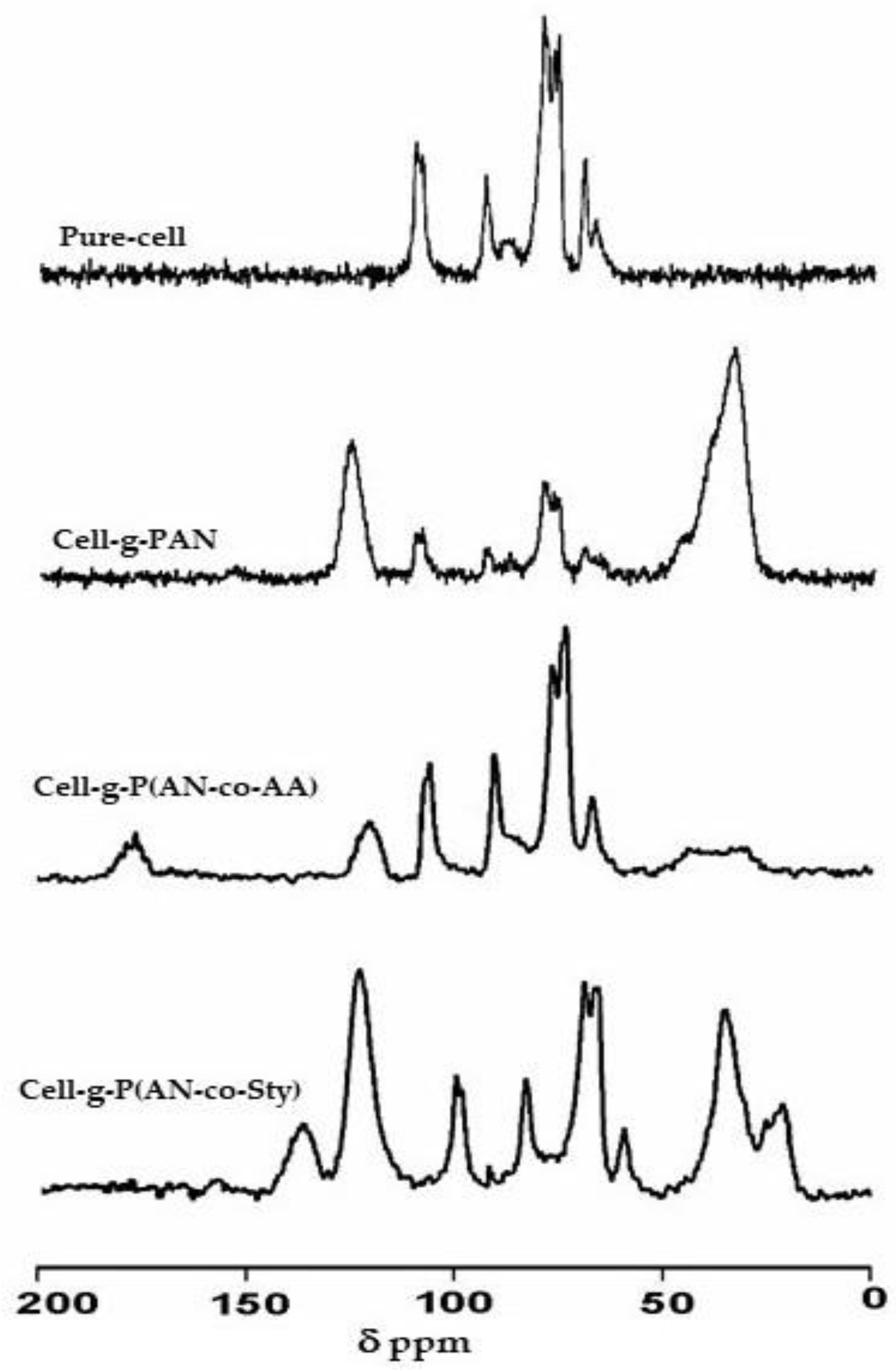
3.2.3. Solid State NMR Analysis
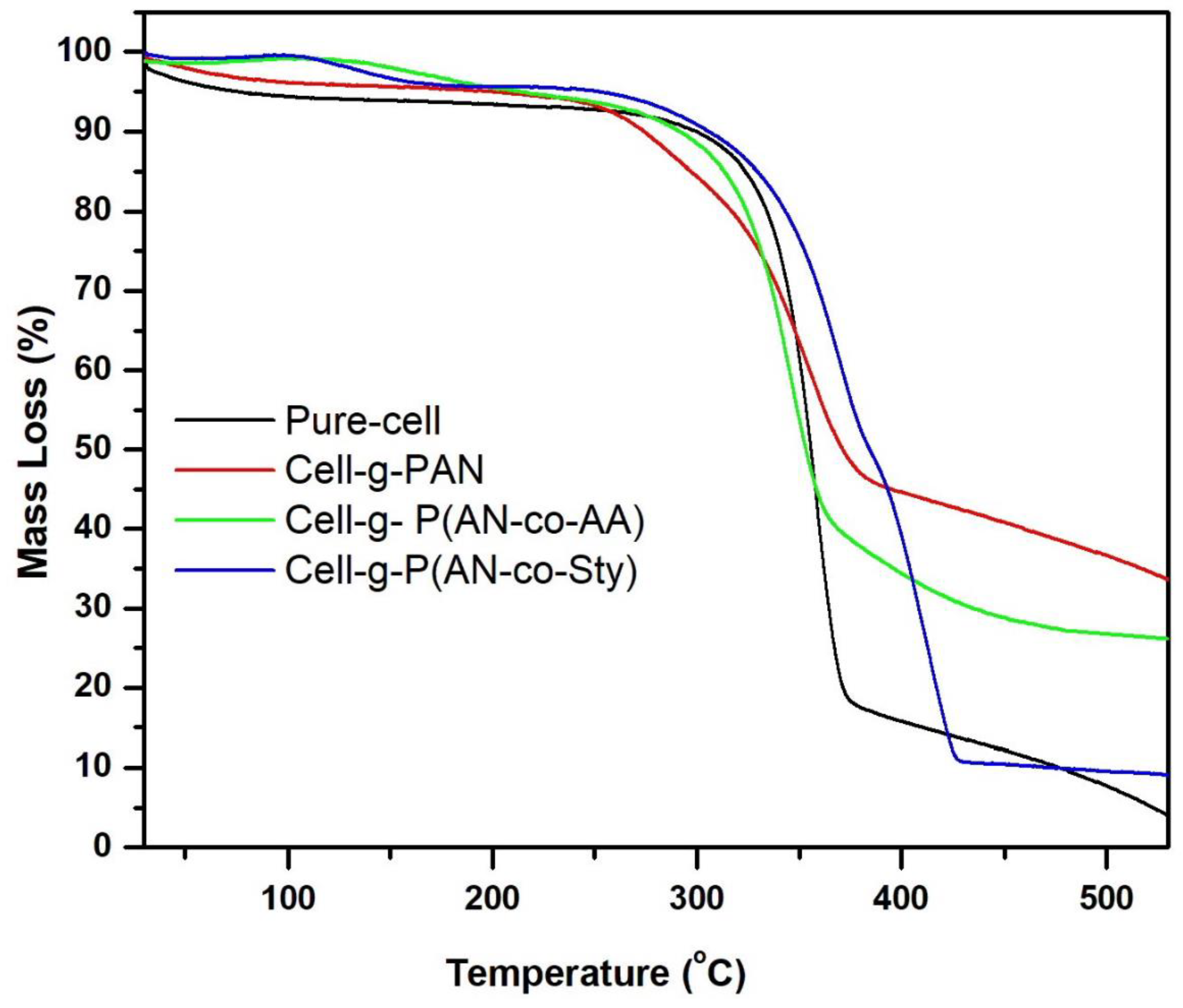
3.2.4. Thermal Analysis
3.2.5. X-ray Diffraction Analysis
3.3. Sorption Properties of Cellulose Graft Copolymer
3.3.1. Effect of the pH Medium and Contact Time
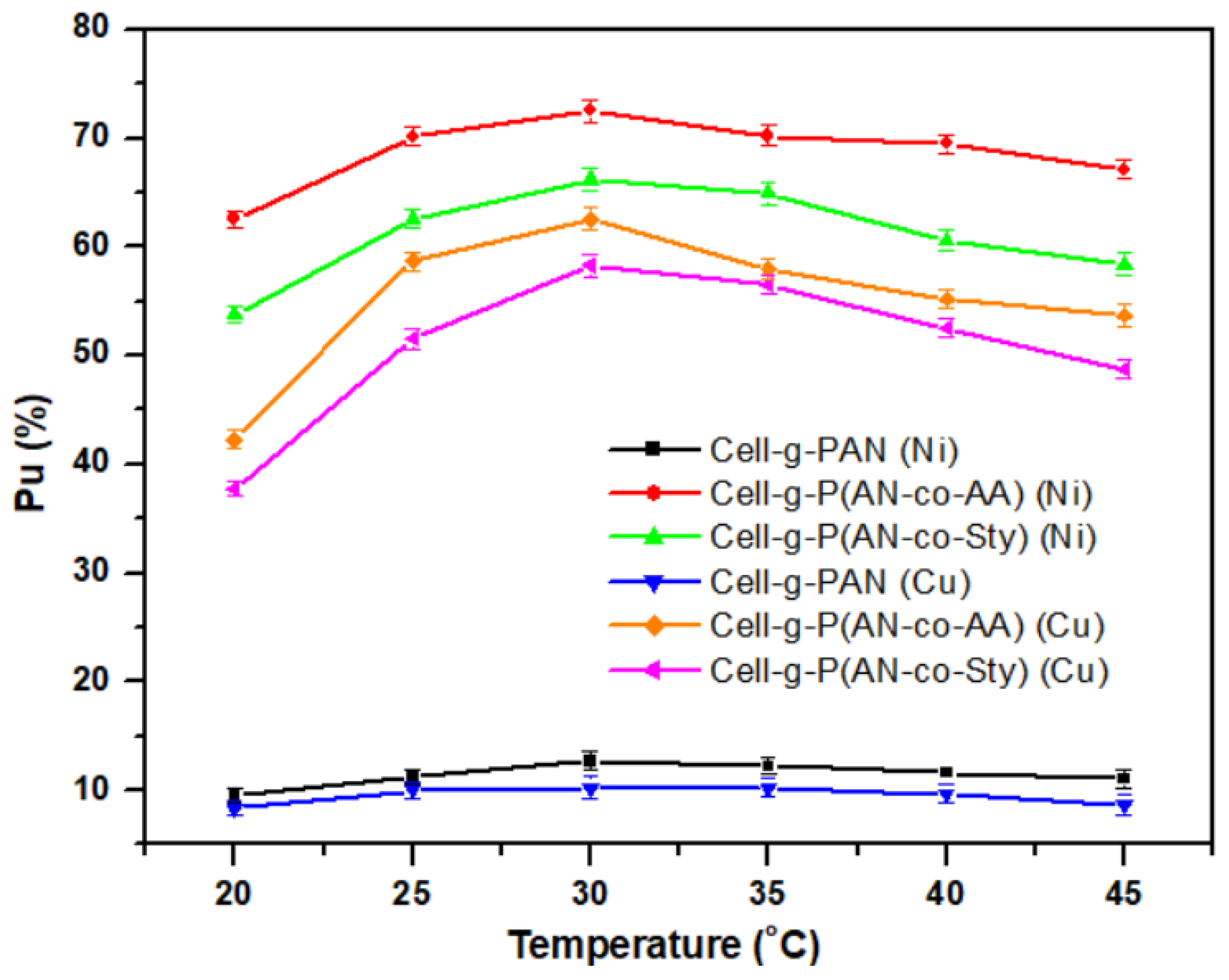
3.3.2. Effect of Temperature
3.3.3. Effect of Metal Ion Concentration
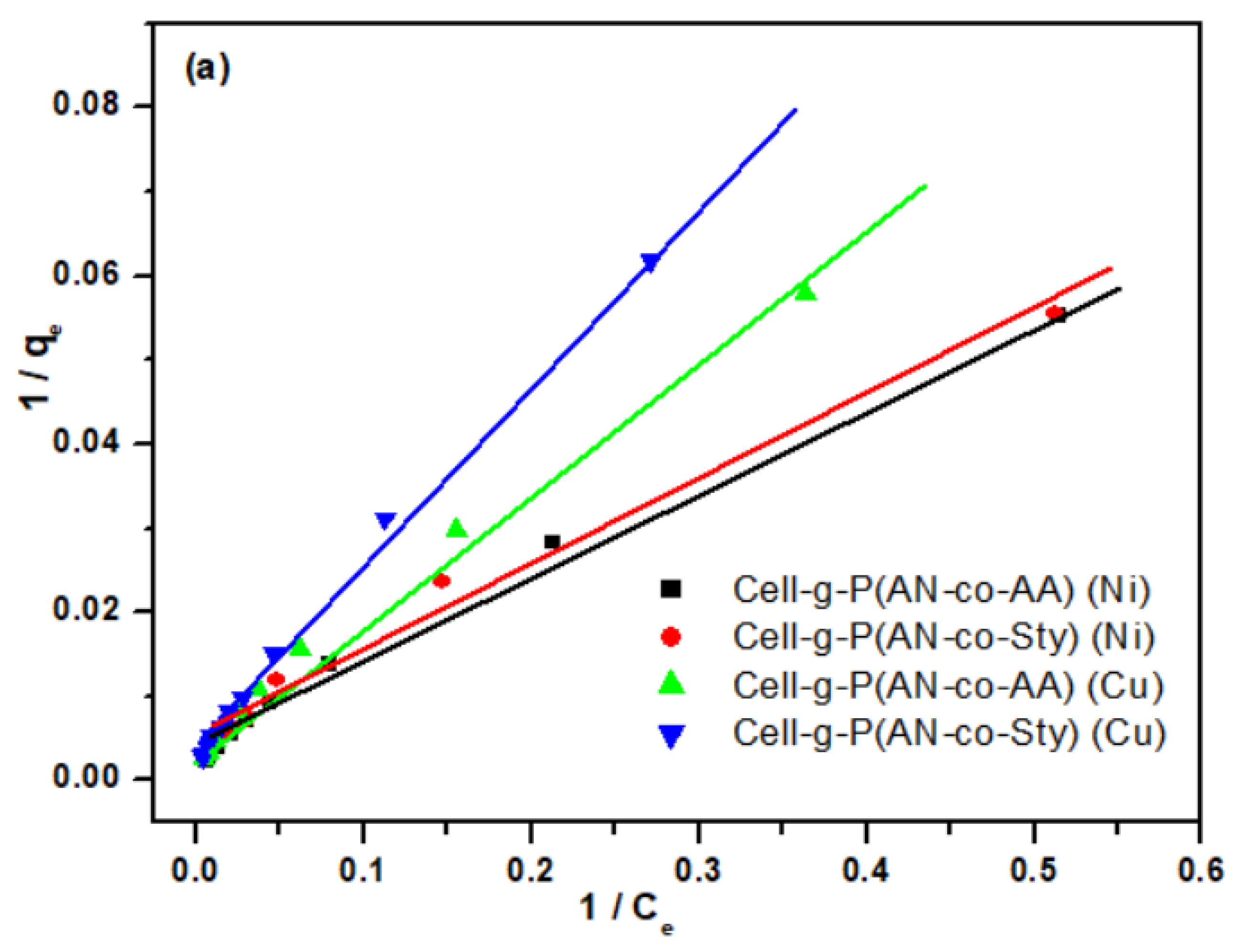
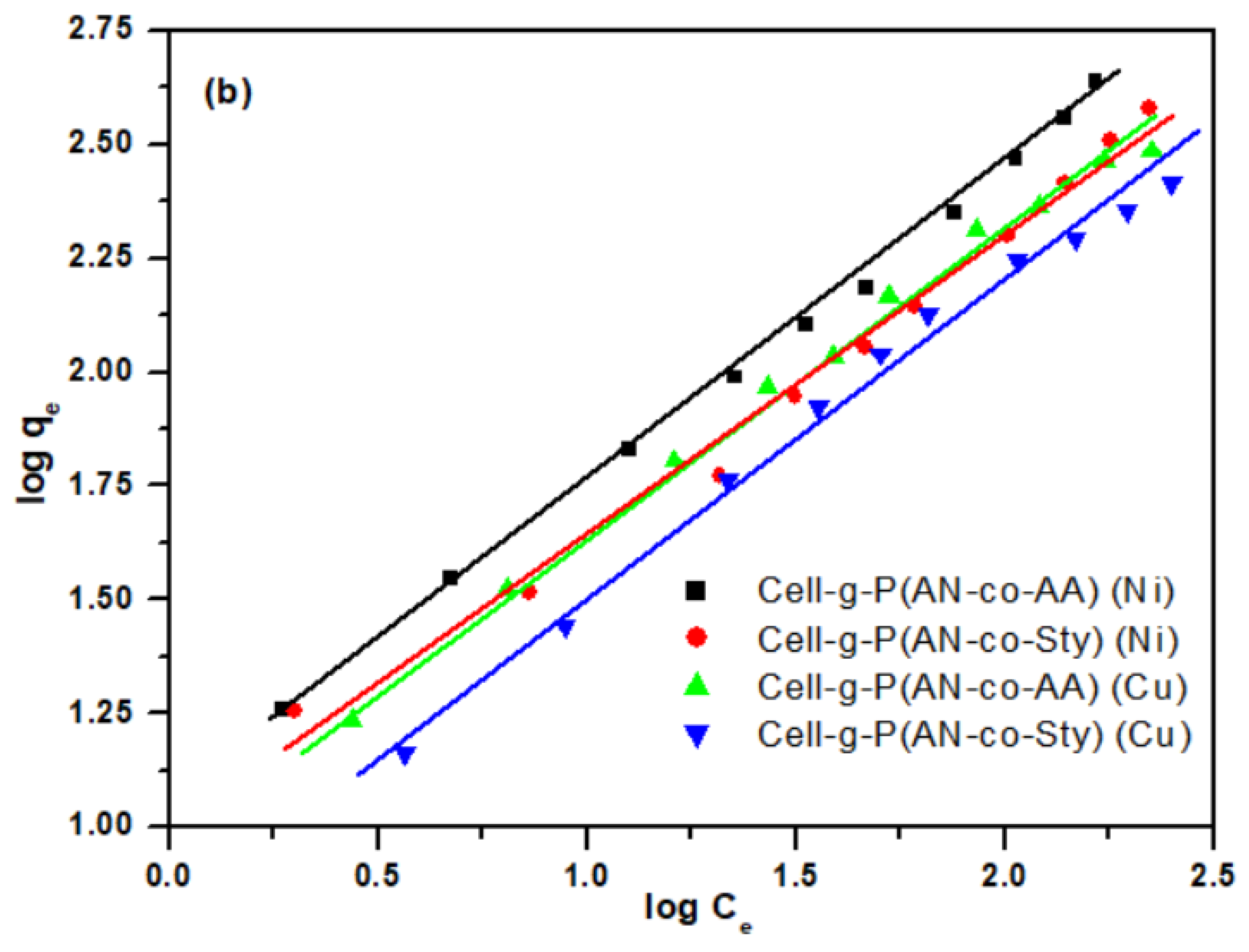
3.3.4. Adsorption Isotherm Studies
3.3.5. Comparative Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, B.; Zhao, C.; Yan, K.; Sun, G. Effects of Acid Diffusibility and Affinity to Cellulose on Strength Loss of Polycarboxylic Acid Crosslinked Fabrics. Carbohydr. Polym. 2016, 144, 282–288. [Google Scholar] [CrossRef]
- Qi, H.; Huang, Y.; Ji, B.; Sun, G.; Qing, F.L.; Hu, C.; Yan, K. Anti-Crease Finishing of Cotton Fabrics Based on Crosslinking of Cellulose with Acryloyl Malic Acid. Carbohydr. Polym. 2016, 135, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; You, X.; Rissanen, M.; Schlapp-Hackl, I.; Sixta, H. Sustainable Cross-Linking of Man-Made Cellulosic Fibers with Poly(carboxylic acids) for Fibrillation Control. ACS Sustain. Chem. Eng. 2021, 9, 16749–16756. [Google Scholar] [CrossRef]
- Quellmalz, A.; Mihranyan, A. Citric Acid Cross-Linked Nanocellulose-Based Paper for Size-Exclusion Nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Hufendiek, A.; Trouillet, V.; Meier, M.A.R.; Barner-Kowollik, C. Temperature Responsive Cellulose-graft-Copolymers via Cellulose Functionalization in an Ionic Liquid and RAFT Polymerization. Biomacromolecules 2014, 15, 2563–2572. [Google Scholar] [CrossRef] [PubMed]
- Schenzel, A.; Hufendiek, A.; Barner-Kowollik, C.; Meier, M.A.R. Catalytic transesterification of cellulose in ionic liquids: Sustainable access to cellulose esters. Green Chem. 2014, 16, 3266–3271. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Huang, F.; Li, K. Synthesis and characterization of cellulose fibers grafted with hyperbranched poly(3-methyl-3- oxetanemethanol). Cellulose 2011, 18, 1611–1621. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Liu, F.; Chen, M.; Na, H.; Zhu, J. Impact of DBU on the synthesis of cellulose-graft-poly(l-lactide) copolymer in CO2 switchable solvent with different grafting strategies. Polymer 2021, 229, 124020. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Cui, B.; Wang, W. Biocomposites of Polylactic Acid Reinforced by DL-Lactic Acid-Grafted Microfibrillated Cellulose. J. Renew. Mater. 2022, 10, 2961–2972. [Google Scholar] [CrossRef]
- Mohammadbagheri, Z.; Rahmati, A.; Hoshyarmanesh, P. Synthesis of a novel superabsorbent with slow-release urea fertilizer using modified cellulose as a grafting agent and flexible copolymer. Int. J. Biol. Macromol. 2021, 182, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Liu, W.; Liu, R.; Huang, Y. A novel, amphiphilic ethyl cellulose grafting copolymer with poly(2-hydroxyethyl methacrylate) side chains and its micellization. Macromol. Chem. Phys. 2008, 209, 424–430. [Google Scholar] [CrossRef]
- Chun-Xiang, L.; Huai-Yu, Z.; Ming-Hua, L.; Shi-Yu, F.; Jia-Jun, Z. Preparation of cellulose graft poly(methyl methacrylate) copolymers by atom transfer radical polymerization in an ionic liquid. Carbohydr. Polym. 2009, 78, 432–438. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Inamuddin; Asiri, A.M. Carbon nanotube- and graphene-based advanced membrane materials for desalination. Environ. Chem. Lett. 2017, 15, 643–671. [Google Scholar] [CrossRef]
- Ibrahim, G.P.S.; Isloor, A.M.; Inamuddin; Asiri, A.M.; Ismail, N.; Ismail, A.F.; Ashraf, G.M. Novel, one-step synthesis of zwitterionic polymer nanoparticles via distillation-precipitation polymerization and its application for dye removal membrane. Sci. Rep. 2017, 7, 15889. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Dashti, A.; Harami, H.R.; Hajilari, N.; Inamuddin. Fouling-resistant membranes for water reuse. Environ. Chem. Lett. 2018, 16, 715–763. [Google Scholar] [CrossRef]
- Madhura, L.; Kanchi, S.; Sabela, M.I.; Singh, S.; Bisetty, K.; Inamuddin. Membrane technology for water purification. Environ. Chem. Lett. 2018, 16, 343–365. [Google Scholar] [CrossRef]
- Inamuddin, T.A.; Rangreez, M.N.; Al-Ahmad, A. Synthesis and characterisation of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) Zr(IV) monothiophosphate composite cation exchanger: Analytical application as lead ion selective membrane electrode. Int. J. Environ. Anal. Chem. 2015, 95, 312–323. [Google Scholar] [CrossRef]
- Bushra, R.; Shahadat, M.; Khan, M.A.; Inamuddin; Adnan, R.; Rafatullah, M. Optimization of polyaniline supported Ti(IV) arsenophosphate composite cation exchanger based ion-selective membrane electrode for the determination of lead. Ind. Eng. Chem. Res. 2014, 53, 19387–19391. [Google Scholar] [CrossRef]
- Gokmen, F.O.; Yaman, E.; Temel, S. Eco-friendly polyacrylic acid based porous hydrogel for heavy metal ions adsorption: Characterization, adsorption behavior, thermodynamic and reusability studies. Microchem. J. 2021, 168, 106357. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Inamuddin; Abdullah, M.S.; Ismail, A.F.; Asiri, A.M. Fabrication of polyetherimide nanocomposite membrane with amine functionalised halloysite nanotubes for effective removal of cationic dye effluents. J. Taiwan Inst. Chem. Eng. 2018, 93, 42–53. [Google Scholar] [CrossRef]
- Kumar, M.; RaoT, S.; Isloor, A.M.; Ibrahim, G.S.; Ismail, N.; Ismail, A.F.; Asiri, A.M. Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. Int. J. Biol. Macromol. 2019, 129, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Zhou, W.; Gao, B. Removal of copper ions from aqueous solutions by adsorption onto wheat straw cellulose-based polymeric composites. J. Appl. Polym. Sci. 2018, 135, 46680. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, L.; Yu, H.; Ullah, R.S.; Zain-ul-Abdin; Khan, R.U.; Chen, Q.; Liu, J. Synthesis of carboxymethyl starch-g-polyvinylpyrolidones and their properties for the adsorption of rhodamine 6G and ammonia. Carbohydr. Polym. 2018, 186, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.M.; Sokker, H.H.; Zayed, E.M.; Eldien, F.A.N.; Alrahman, N.M.A. Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int. J. Biol. Macromol. 2018, 120, 2188–2199. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Mao, D.; Bai, N.; Dong, H. A versatile bio-based material for efficiently removing toxic dyes, heavy metal ions and emulsified oil droplets from water simultaneously. Bioresour. Technol. 2017, 245, 649–655. [Google Scholar] [CrossRef]
- Lalita, A.; Singh, A.P.; Sharma, R.K. Selective sorption of Fe(II) ions over Cu(II) and Cr(VI) ions by cross-linked graft copolymers of chitosan with acrylic acid and binary vinyl monomer mixtures. Int. J. Biol. Macromol. 2017, 105, 1202–1212. [Google Scholar] [CrossRef]
- Lalita, A.; Singh, A.P.; Sharma, R.K. Synthesis and characterization of graft copolymers of chitosan with NIPAM and binary monomers for removal of Cr(VI), Cu(II) and Fe (II) metal ions from aqueous solutions. Int. J. Biol. Macromol. 2017, 99, 409–426. [Google Scholar] [CrossRef]
- Sharma, R.K.; Lalita, A.; Singh, A.P. Sorption of Pb(II), Cu(II), Fe(II) and Cr(VI) metal ions onto cross-linked graft copolymers of chitosan with binary vinyl monomer mixtures. React. Funct. Polym. 2017, 121, 32–44. [Google Scholar] [CrossRef]
- Sharma, R.K.; Kumar, R.; Singh, A.P. Metal ions and organic dyes sorption applications of cellulose grafted with binary vinyl monomers. Sep. Purif. Technol. 2019, 209, 684–697. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Removal of organic dyes and metal ions by crosslinked graft copolymers of cellulose obtained from the agricultural residue. J. Environ. Chem. Eng. 2018, 6, 6037–6048. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Adala, O.B.; Okabe, H.; Hidaka, Y.; Hara, K. Selective adsorption of trivalent metal ions from multielement solution by using gamma radiation-induced pectin-acrylamide-(2-acrylamido-2-methyl-1-propanesulfonic acid) hydrogel. J. Environ. Chem. Eng. 2019, 7, 102844. [Google Scholar] [CrossRef]
- Karmakar, M.; Mondal, H.; Mahapatra, M.; Chattopadhyay, P.K.; Chatterjee, S.; Singha, N.R. Pectin-grafted terpolymer superadsorbent via N–H activated strategic protrusion of monomer for removals of Cd(II), Hg(II), and Pb(II). Carbohydr. Polym. 2019, 206, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, Z.; Zhang, S.; Wang, W.; Dong, W. Interpenetrating polymer networks in polyvinyl alcohol/cellulose nanocrystals hydrogels to develop absorbent materials. Carbohydr. Polym. 2018, 200, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, Z.; Wen, J.; Tang, T.; Jian, L.; Hu, G.; Li, M. Synthesis of cellulose-poly(acrylic acid) using sugarcane bagasse extracted cellulose fibers for the removal of heavy metal ions. Mol. Sci. 2023, 24, 8922. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.S.; Mahajan, S. Use of novel hydrogels based on modified cellulosics and methacrylamide for separation of metal ions from water systems. J. Appl. Polym. Sci. 2002, 86, 667–671. [Google Scholar] [CrossRef]
- Ozbas, Z.; Sahin, C.P.; Esen, E.; Gurdag, G.; Kasgoz, H. The effect of extractant on the removal of heavy metal ions by thermoresponsive cellulose graft copolymer. J. Environ. Chem. Eng. 2016, 4, 1948–1954. [Google Scholar] [CrossRef]
- Barsbay, M.; Kavaklı, P.A.; Tilki, S.; Kavaklı, C.; Güven, O. Porous cellulosic adsorbent for the removal of Cd (II), Pb(II) and Cu(II) ions from aqueous media. Radiat. Phys. Chem. 2018, 142, 70–76. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Barathi, M.; Puvvada, S.; Rajesh, N. Microwave assisted preparation of glycidyl methacrylate grafted cellulose adsorbent for the effective adsorption of mercury from a coal fly ash sample. J. Environ. Chem. Eng. 2013, 1, 1359–1367. [Google Scholar] [CrossRef]
- Sokker, H.H.; Gad, Y.H.; Ismail, S.A. Synthesis of bifunctional cellulosic adsorbent by radiation induced graft polymerization of glycidyl methacrylate-co-methacrylic acids. J. Appl. Polym. Sci. 2012, 126, 54–62. [Google Scholar] [CrossRef]
- Monier, M.; Akl, M.A.; Ali, W.M. Modification and characterization of cellulose cotton fibers for fast extraction of some precious metal ions. Int. J. Biol. Macromol. 2014, 66, 125–134. [Google Scholar] [CrossRef]
- Hajeeth, T.; Sudha, P.N.; Vijayalakshmi, K.; Gomathi, T. Sorption studies on Cr (VI) removal from aqueous solution using cellulose grafted with acrylonitrile monomer. Int. J. Biol. Macromol. 2014, 66, 295–301. [Google Scholar] [CrossRef]
- Hajeeth, T.; Vijayalakshmi, K.; Gomathi, T.; Sudha, P.N. Removal of Cu(II) and Ni(II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int. J. Biol. Macromol. 2013, 62, 59–65. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Sogbaike, C.E.; Ebhoaye, J.E. Removal of cadmium and copper ions from aqueous solution with cellulose graft copolymers. Sep. Purif. Technol. 2005, 44, 85–89. [Google Scholar] [CrossRef]
- Rani, K.; Gomathi, T.; Vijayalakshmi, K.; Saranya, M.; Sudha, P.N. Banana fiber Cellulose Nano Crystals grafted with butyl acrylate for heavy metal lead (II) removal. Inter. J. Bio. Macro. 2019, 131, 461–472. [Google Scholar]
- Zhang, X.; Zhao, J.; Cheng, L.; Lu, C.; Wang, Y.; He, X.; Zhang, W. Acrylic acid grafted and acrylic acid/sodium humate grafted bamboo cellulose nanofibers for Cu2+ adsorption. RSC Adv. 2014, 4, 55195–55201. [Google Scholar] [CrossRef]
- Shainy, F.; Anirudhan, T.S. Effective removal of mercury (II) ions from chlor-alkali industrial wastewater using 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose composite. J. Colloid Interface Sci. 2015, 456, 22–31. [Google Scholar]
- Gouda, M.; Aljaafari, A. Removal of Heavy Metal Ions from Wastewater Using Hydroxyethyl Methacrylate-Modified Cellulose Nanofibers: Kinetic, Equilibrium and Thermodynamic Analysis. Int. J. Environ. Res. Public Health. 2021, 18, 6581. [Google Scholar] [CrossRef]
- Singha, A.S.; Guleria, A. Application of vinyl monomers functionalized cellulosic biopolymer for removal of dissolved toxic metal ions from polluted water samples. J. Environ. Chem. Eng. 2014, 2, 1456–1466. [Google Scholar] [CrossRef]
- Sharma, R.K.; Chauhan, G.S. Synthesis and characterization of graft copolymers of 2-hydroxyethyl methacrylate and some comonomers onto extracted cellulose for use in separation technologies. Bioresources 2009, 4, 986–1005. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Guleria, L.; Sharma, R. Synthesis, characterization and metal ion sorption studies of graft copolymers of cellulose with glycidyl methacrylate and some comonomers. Cellulose 2005, 12, 97–110. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Synthesis and characterization of cellulose based graft copolymers with binary vinyl monomers for efficient removal of cationic dyes and Ph(II) ions. J. Poly. Res. 2019, 26, 135. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K. Synthesis and characterization of cellulose based adsorbents for removal of Ni(II), Cu(II) and Pb(II) ions from aqueous solutions. React. Funct. Polym. 2019, 140, 82–92. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafting of cellulose with N-isopropylacrylamide and glycidyl methacrylate for efficient removal of Ni(II), Cu(II) and Pd(II) ions from aqueous solution. Sep. Purif. Technol. 2019, 219, 249–259. [Google Scholar] [CrossRef]
- Sharma, R.K.; Kumar, R. Functionalized cellulose with hydroxyethyl methacrylate and glycidyl methacrylate for metal ions and dye adsorption applications. Int. J. Bio. Macro. 2019, 134, 704–721. [Google Scholar] [CrossRef]
- El-Khouly, A.S.; Takahashi, Y.; Takada, A.; Safaan, A.A.; Kenawy, E.; Hafiz, Y.A. Characterization and thermal stability of cellulose-graft-polyacryloniytrile prepared by using KMnO4/citric acid redox system. J. Appl. Polym. Sci. 2010, 16, 1788–1795. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Sorption of Ni(II), Pb(II) and Cu(II) ions from aqueous solutions by cellulose grafted with poly(HEMA-co-AAc): Kinetic, isotherm and thermodynamic study. J. Env. Chem. Eng. 2019, 7, 103088. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Kouta, E.Y. Retention of copper, cadmium and lead from water by Na-Y-Zeolite confined in methyl methacrylate shell. J. Environ. Chem. Eng. 2017, 5, 3698–3710. [Google Scholar] [CrossRef]
- Sharma, R.K.; Lalita. Synthesis and characterization of graft copolymers of N-Vinyl-2-Pyrrolidone onto guar gum for sorption of Fe2+ and Cr6+ ions. Carbohydr. Polym. 2011, 83, 1929–1936. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Maiti, M. Induction of Chemical and Moisture Resistance in Saccharum spontaneum L Fiber Through Graft Copolymerization with Methyl Methacrylate and Study of Morphological Change. J. Appl. Polym. Sci. 2009, 113, 1781–1791. [Google Scholar] [CrossRef]
- Kaith, B.S.; Kalia, S. Synthesis and Characterization of Graft Co-Polymers of Flax Fiber with Binary Vinyl Monomers. Int. J. Polym. Anal. Charact. 2007, 12, 401–412. [Google Scholar] [CrossRef]
- Maiti, M.; Jindal, R.; Kaith, B.S.; Jana, A.K. Synthesis of Graft Copolymers of Binary Vinyl monomer Mixtures onto Acetylated Saccharum spontaneum L. and Characterization. J. Appli. Polym. Sci. 2011, 121, 2060–2071. [Google Scholar] [CrossRef]
- Kong, W.; Chang, M.; Zhang, C.; Liu, X.; He, B.; Ren, J. Preparation of Xylan-g-/P(AA-co-AM)/GO Nanocomposite Hydrogel and its Adsorption for Heavy Metal Ions. Polymers 2019, 11, 621. [Google Scholar] [CrossRef]
- Gharekhani, H.; Olad, A.; Mirmohseni, A.; Bybordi, A. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: Synthesis, characterization, and swelling kinetic studies. Carbohydr. Polym. 2017, 168, 1–13. [Google Scholar] [CrossRef]
- Ouajai, S.; Hodzic, A.; Shanks, R.A. Morphological and grafting modification of natural cellulose fibers. J. Appl. Polym. Sci. 2004, 94, 2456–2465. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Muntha, S.; Ambreen, J.; Siddiq, M. Synthesis and characterization of graphene oxide sheets integrated with gold nanoparticles and their applications to adsorptive removal and catalytic reduction of water contaminants. RSC Adv. 2018, 8, 3599–3610. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, Y.; Sun, D.; Wang, Q.; Zhang, X.; Weeks, B.L.; O’Connor, R.; Huang, X.; Wei, S.; Guo, Z. Cr(vi) Removal by Magnetic Carbon Nanocomposites Derived from Cellulose at Different Carbonization Temperatures. J. Mater. Chem. A 2015, 3, 9817–9825. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, M.; Wu, W.; Vasiliev, A.L.; Zhu, K.; Padture, N.P. Room-Temperature Crystallization of Hybrid-Perovskite Thin Films via Solvent−Solvent Extraction for High-Performance Solar Cells. J. Mater. Chem. A 2015, 3, 8178–8184. [Google Scholar] [CrossRef]
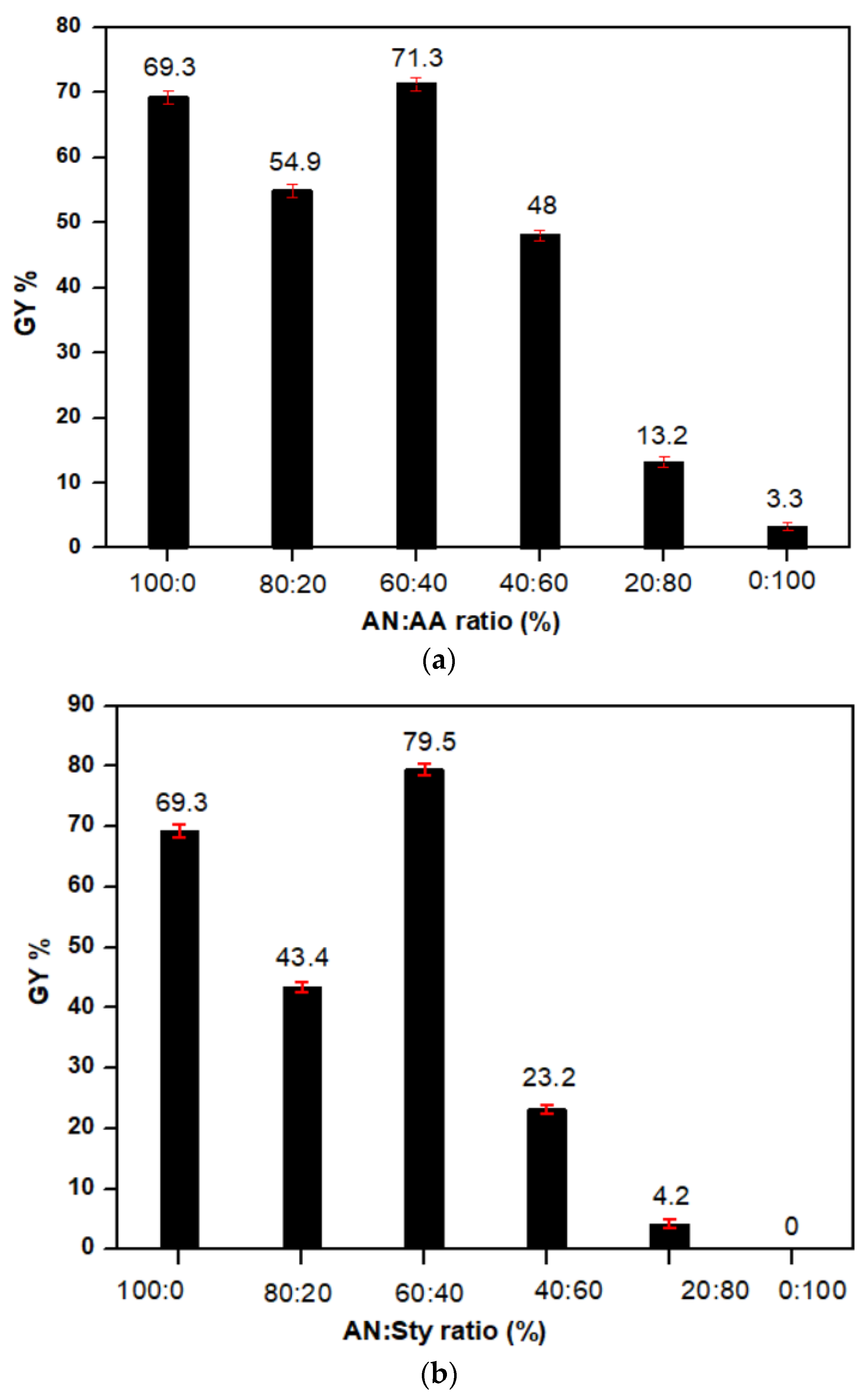




| Monomer (V%) | Percent Graft Yield (GY%) | Monomer Ratio (10%) (AN: Vinyl Monomer) | Percent Graft Yield (GY%) | |||
|---|---|---|---|---|---|---|
| AN | AA | Sty | AN:AA | AN:Sty | ||
| 2 | 7.8 | 00.0 | 00.0 | 100:00 | 69.3 | 69.3 |
| 4 | 19.8 | 00.0 | 00.0 | 80:20 | 54.9 | 43.4 |
| 6 | 49.1 | 00.0 | 00.0 | 60:40 | 71.3 | 79.5 |
| 8 | 68.4 | 00.0 | 00.0 | 40:60 | 48 | 23.2 |
| 10 | 69.3 | 3.3 | 00.0 | 20:80 | 13.2 | 4.2 |
| Sample | (IDT-FDT °C) Mass Loss of Stages (%) | Residue (%) at 530 °C | |||
|---|---|---|---|---|---|
| First | Second | Third | Fourth | ||
| UN-C | 28–115 °C 6% | 270–382 °C 75.5% | -- | -- | 2.00% |
| Cell-g-PAN | 28–103 °C 4% | 250–300 °C 10.3% | 300–384 °C 37.2% | -- | 33% |
| Cell-g-P(AN-co-AA) | 28–100 °C 4.5% | 235–300 °C 4.2% | 300–370 °C 49.9% | 370–465 °C 15.13% | 25% |
| Cell-g-P(AN-co-Sty) | 28–150 °C 3.4% | 260–320 °C 7.7% | 320–380 °C 33.3% | 390–434 °C 39.11% | 9% |
| Monomers Ratio (AN Monomer) Total Conc. = 10 v% | Degree of Crystallinity (Cr%) | Monomer Concentration (v%) | Degree of Crystallinity (Cr %) | |
|---|---|---|---|---|
| AN:AA | AN:Sty | AN | ||
| 100:00 | 46.2 | 46.2 | 10 | 46.2 |
| 80:20 | 52.6 | 42.9 | 8 | 47.3 |
| 60:40 | 53.3 | 41.7 | 6 | 50.0 |
| 40:60 | 55.2 | 44.7 | 4 | 53.0 |
| 20:80 | 57.7 | 55.6 | 2 | 57.1 |
| 00:100 | 69.5 | -- | ||
| Isotherm | Constants | Cell-g-P(AN-co-AA) | Cell-g-P(AN-co-Sty) | ||
|---|---|---|---|---|---|
| Ni(II) | Cu(II) | Ni(II) | Cu(II) | ||
| Langmuir | KL (L/mg) | 0.0018 | 0.0016 | 0.0017 | 0.0013 |
| qm (mg/g) | 446.43 | 384.62 | 387.59 | 362.32 | |
| R2 | 0.9989 | 0.9997 | 0.9988 | 0.9991 | |
| RL | 0.7976 | 0.5142 | 0.4905 | 0.5709 | |
| Freundlich | KF (L/mg) | 11.5659 | 9.4146 | 9.7029 | 6.6039 |
| n | 1.4432 | 1.5051 | 1.5058 | 1.4535 | |
| R2 | 0.9981 | 0.9941 | 0.9962 | 0.9942 | |
| Adsorbent | qmax (mg/g) | Ci (mg/L) | pH | T (°C) | Time h | Ref. | |
|---|---|---|---|---|---|---|---|
| Ni (II) | Cu(II) | ||||||
| Cell-g-AASO3H-co-AAc | 112.74 | 109.77 | 200 | 6 | 30 | 2 | [32] |
| CC-g-(AA-co-AM) | -- | 157.51 | 600 | 5 | 27 | 0.5 | [35] |
| CE–PAANa | -- | 106.3 | 600 | 5 | 24 | [37] | |
| Cell-g-NIPAM-co-AAc | 79.78 | 84.67 | 200 | 5 | 30 | 6 | [55] |
| Cell-g-NIPAM-co-GMA | 74.68 | 82.92 | 200 | 6 (Ni) 5(Cu) | 30 | 6 | [56] |
| Cell-g-HEMA-co-GMA | 83.80 | 71.40 | 200 | 6 | 30 | 6 | [57] |
| Cell-g-HEMA-co-AAc | 85.32 | 84.82 | 200 | 5 (Ni) 6(Cu) | 30 | 6 | [59] |
| Cell-g-P(AN-co-AA) | 153.21 | 147.2 | 200 | 5.5 | 30 | 8 | Current work |
| Cell-g-P(AN-co-Sty) | 139 | 134.42 | 200 | 5.5 | 30 | 8 | Current work |
| Cell-g-P(AN-co-AA) | 435.07 | 375.48 | 600 | 5.5 | 30 | 8 | Current work |
| Cell-g-P(AN-co-Sty) | 379.2 | 349.68 | 600 | 5.5 | 30 | 8 | Current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Khouly, A.S.; Takahashi, Y. Synthesis, Characterization, and Evaluation of the Adsorption Behavior of Cellulose-Graft-Poly(Acrylonitrile-co-Acrylic Acid) and Cellulose-Graft-Poly(Acrylonitrile-co-Styrene) towards Ni(II) and Cu(II) Heavy Metals. Polymers 2024, 16, 445. https://doi.org/10.3390/polym16030445
El-Khouly AS, Takahashi Y. Synthesis, Characterization, and Evaluation of the Adsorption Behavior of Cellulose-Graft-Poly(Acrylonitrile-co-Acrylic Acid) and Cellulose-Graft-Poly(Acrylonitrile-co-Styrene) towards Ni(II) and Cu(II) Heavy Metals. Polymers. 2024; 16(3):445. https://doi.org/10.3390/polym16030445
Chicago/Turabian StyleEl-Khouly, Amany S., and Yoshiaki Takahashi. 2024. "Synthesis, Characterization, and Evaluation of the Adsorption Behavior of Cellulose-Graft-Poly(Acrylonitrile-co-Acrylic Acid) and Cellulose-Graft-Poly(Acrylonitrile-co-Styrene) towards Ni(II) and Cu(II) Heavy Metals" Polymers 16, no. 3: 445. https://doi.org/10.3390/polym16030445
APA StyleEl-Khouly, A. S., & Takahashi, Y. (2024). Synthesis, Characterization, and Evaluation of the Adsorption Behavior of Cellulose-Graft-Poly(Acrylonitrile-co-Acrylic Acid) and Cellulose-Graft-Poly(Acrylonitrile-co-Styrene) towards Ni(II) and Cu(II) Heavy Metals. Polymers, 16(3), 445. https://doi.org/10.3390/polym16030445





