Photopolymerization Pattern of New Methacrylate Cellulose Acetate Derivatives
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Cellulose Acetate Derivatives (CA-M5–CA-M100)
2.3. Preparation of CA-Mx Photopolymerized Films
2.4. Characterization
3. Results and Discussion
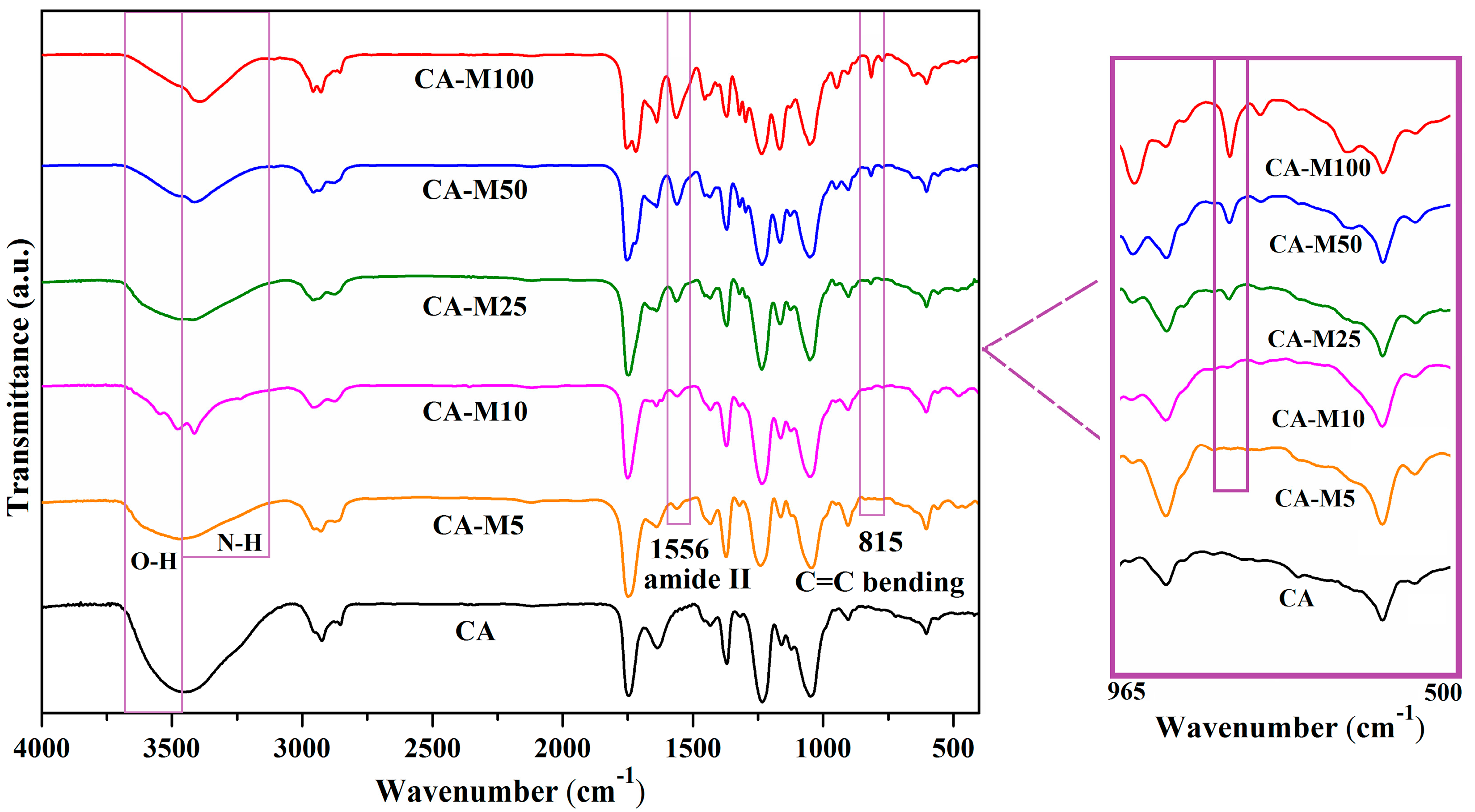
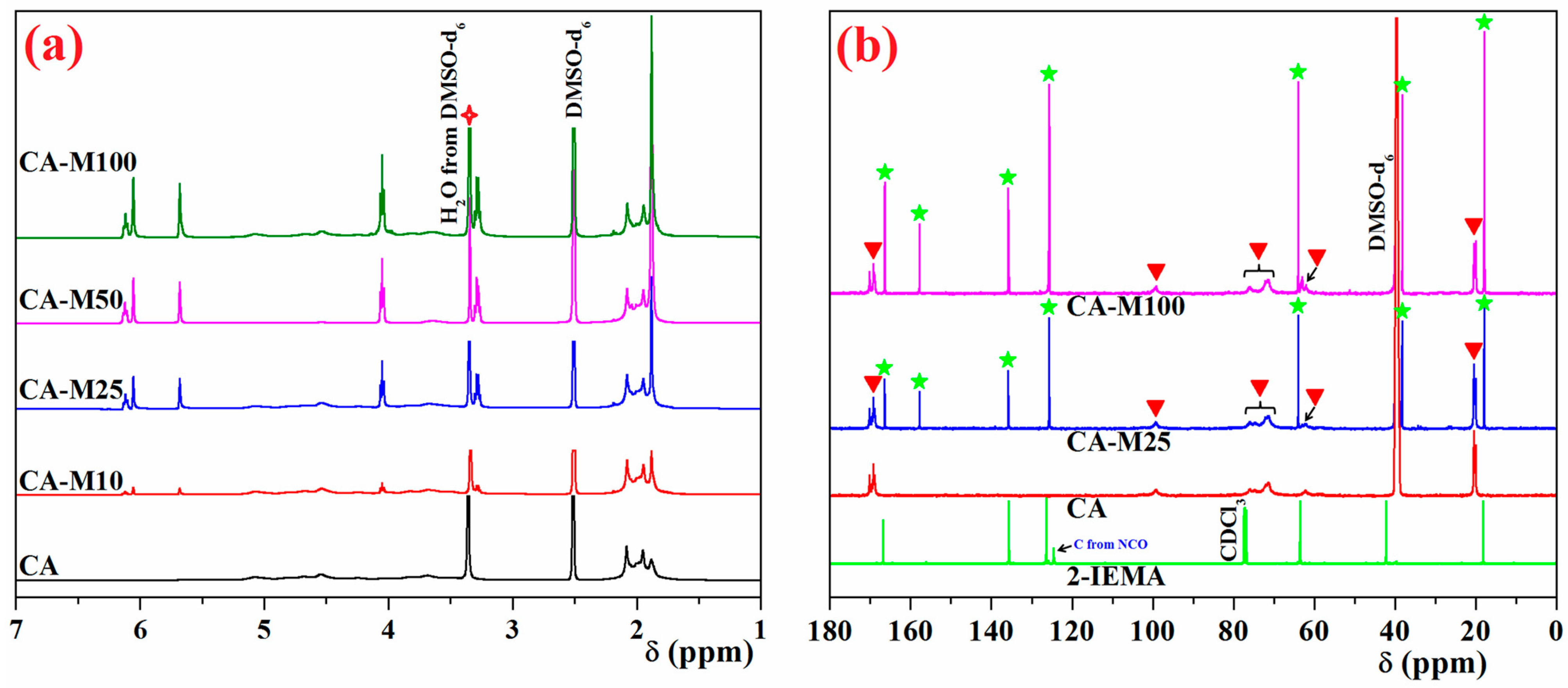
3.1. Investigation of the Chemical Structure
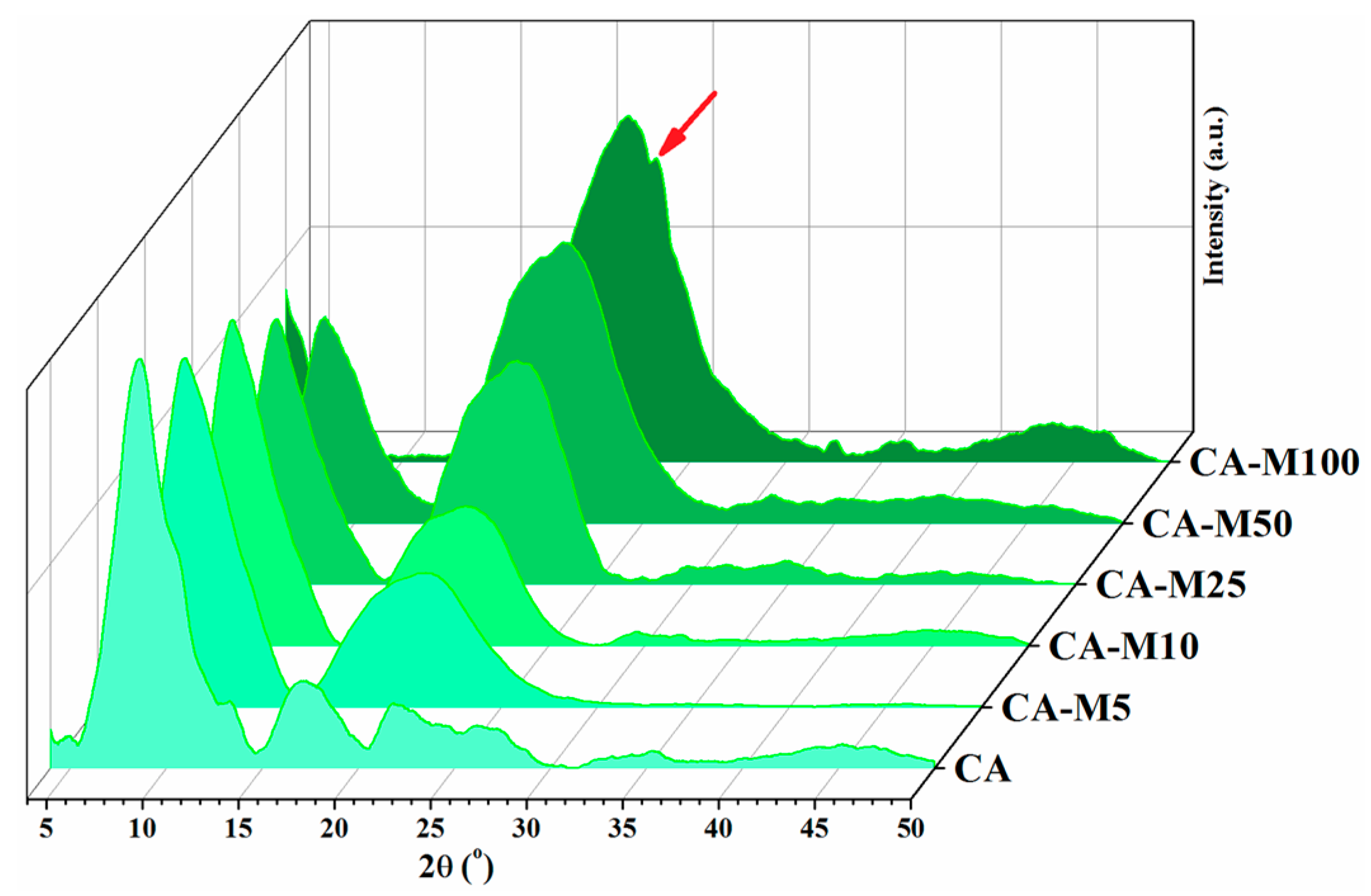
3.2. X-ray Diffraction (XRD) Analysis
3.3. Investigation of the Wettability of Cellulose Acetate and Its Derivatives
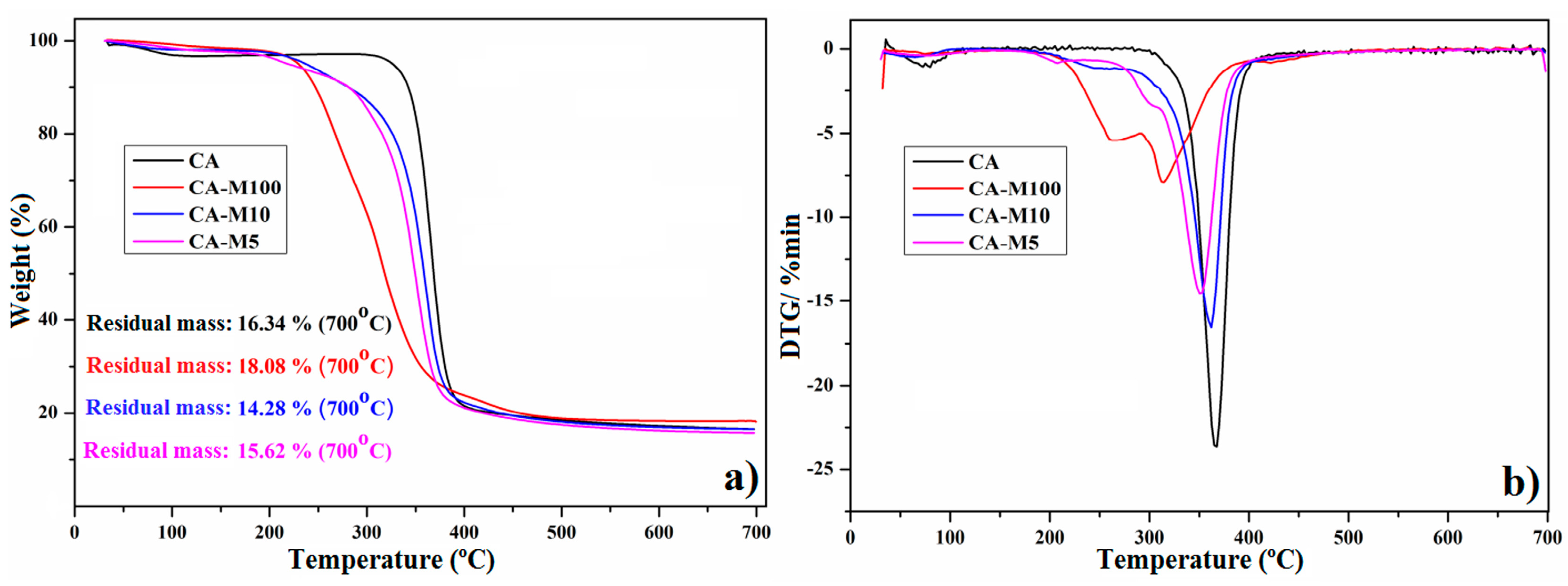
3.4. Thermal Stability Investigation
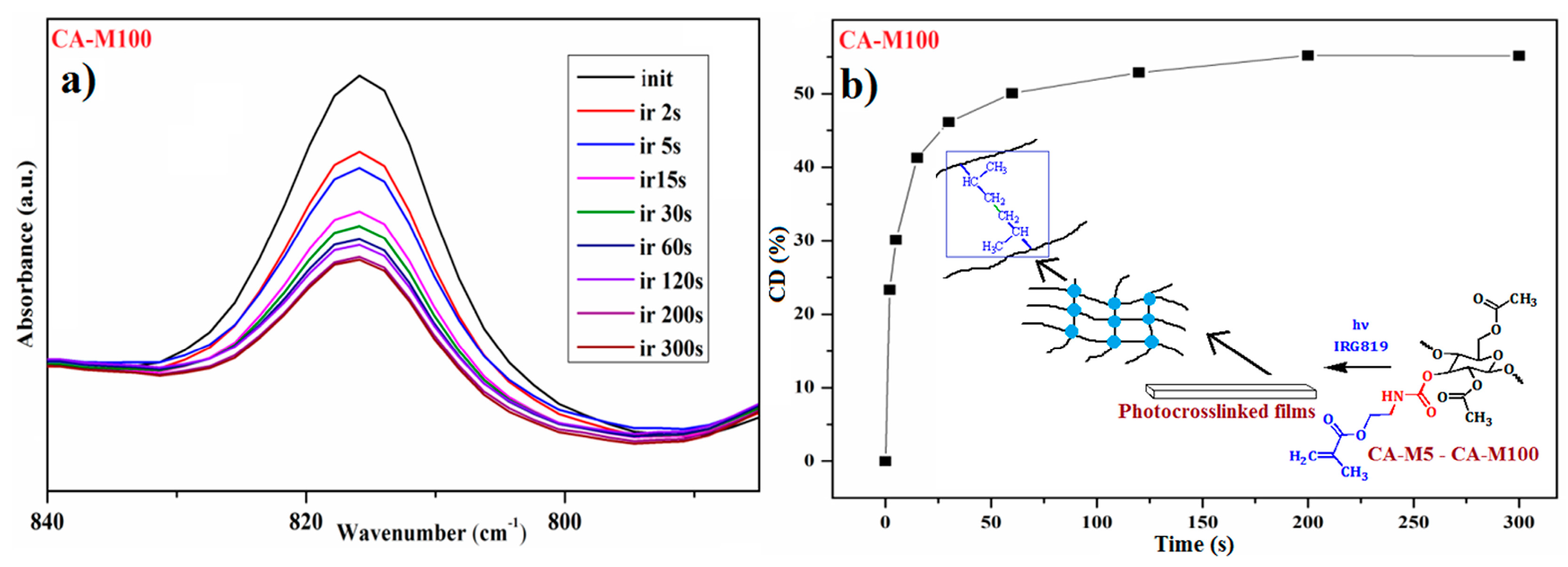
3.5. Photopolymerization Kinetics of Cellulose Acetate Derivatives (CA-M5–CA-M100)
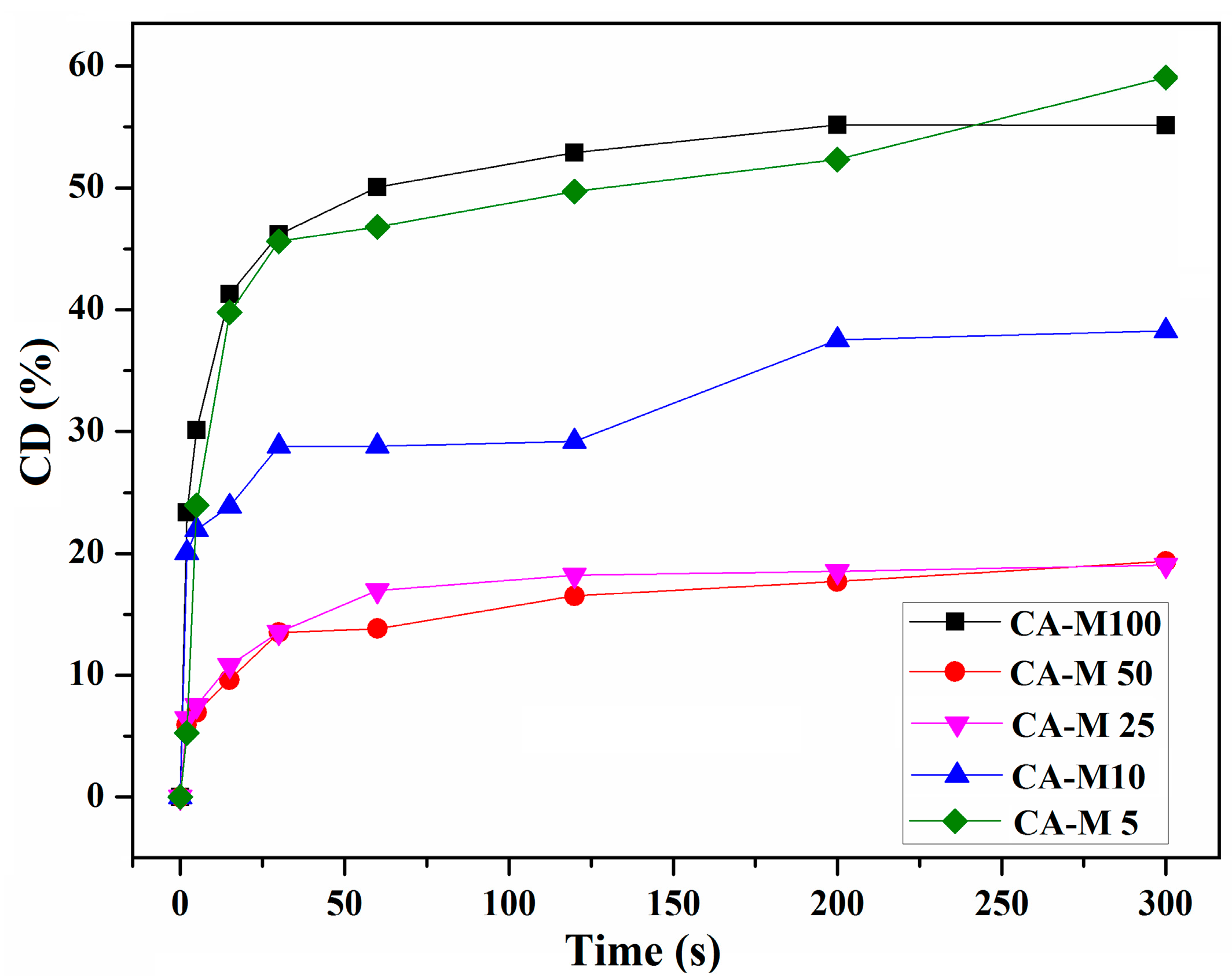
3.5.1. Investigation of the Photopolymerization Kinetics of CA-Mx Samples
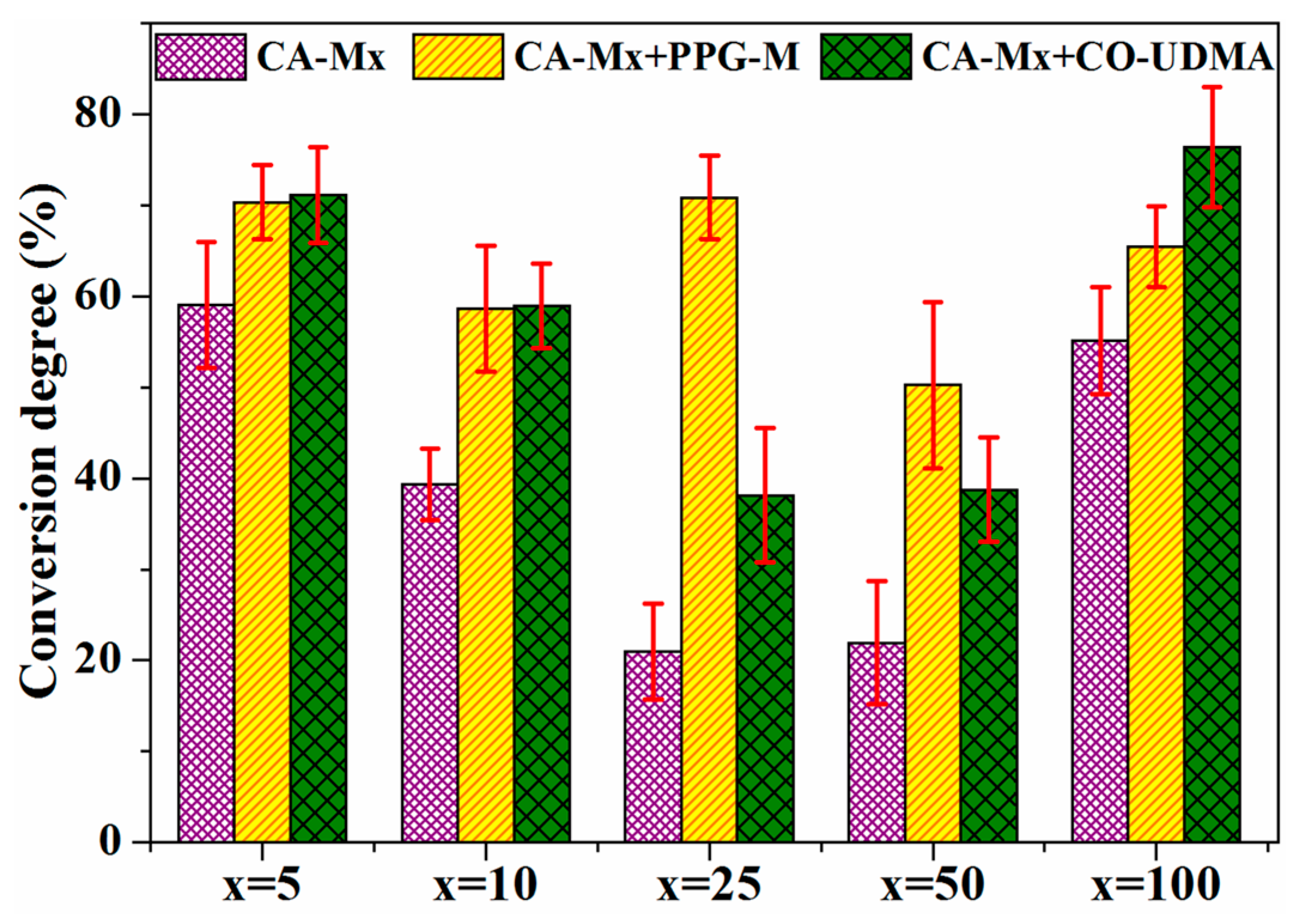
3.5.2. Photopolymerization Kinetics of CA-Mx Samples Used in Combination with Low Molecular Weight Photopolymerizable Monomers
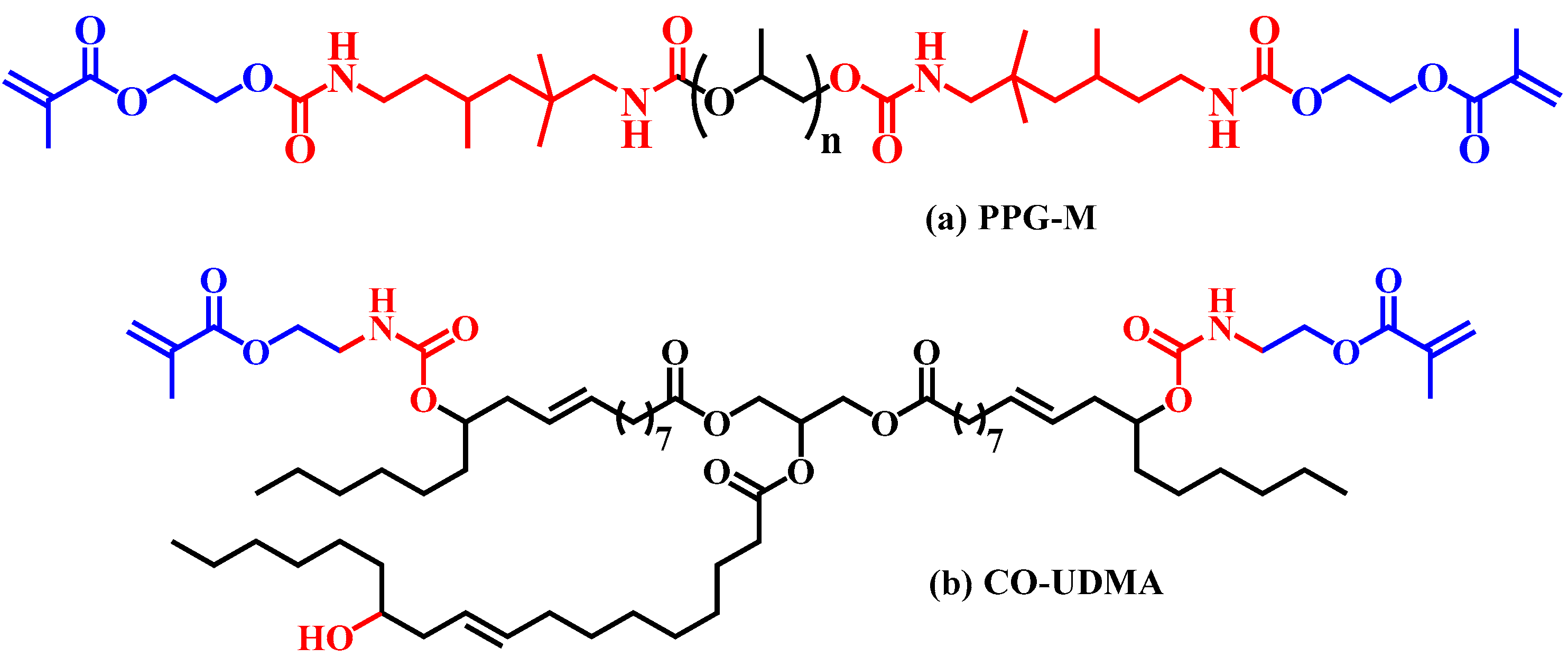
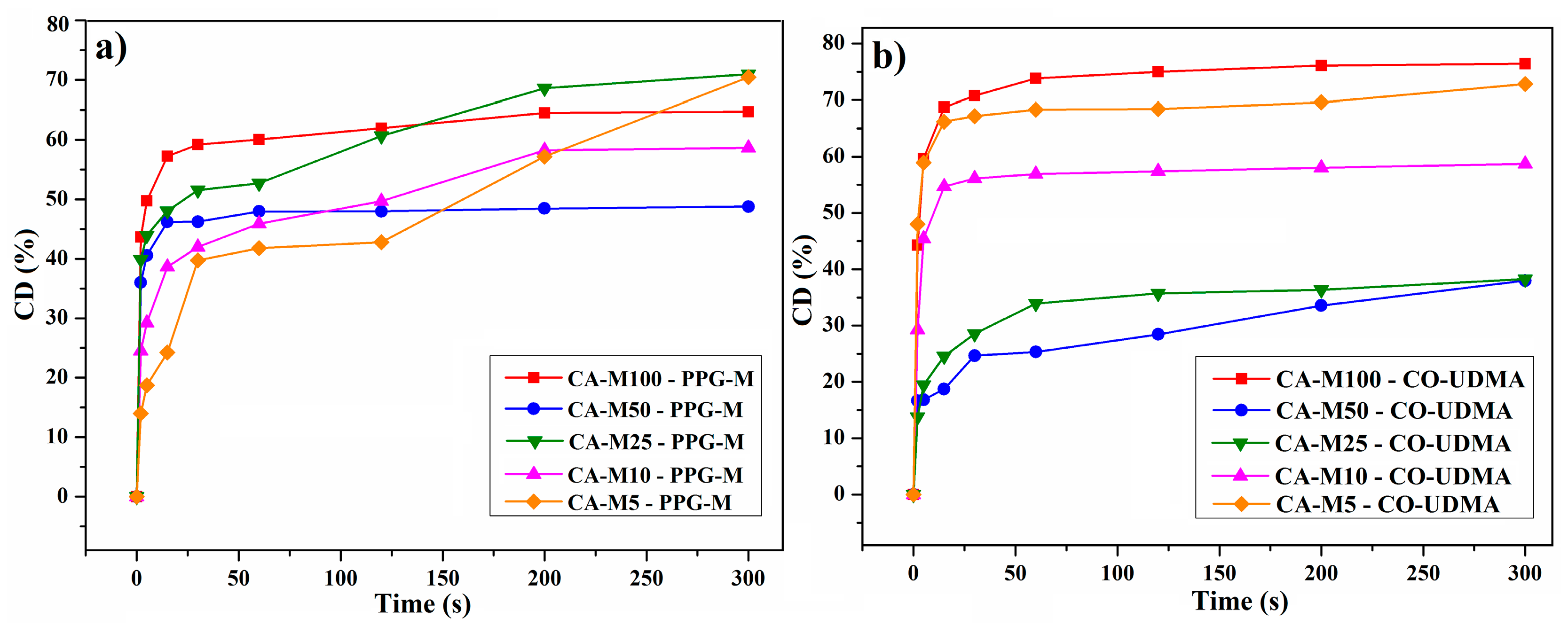
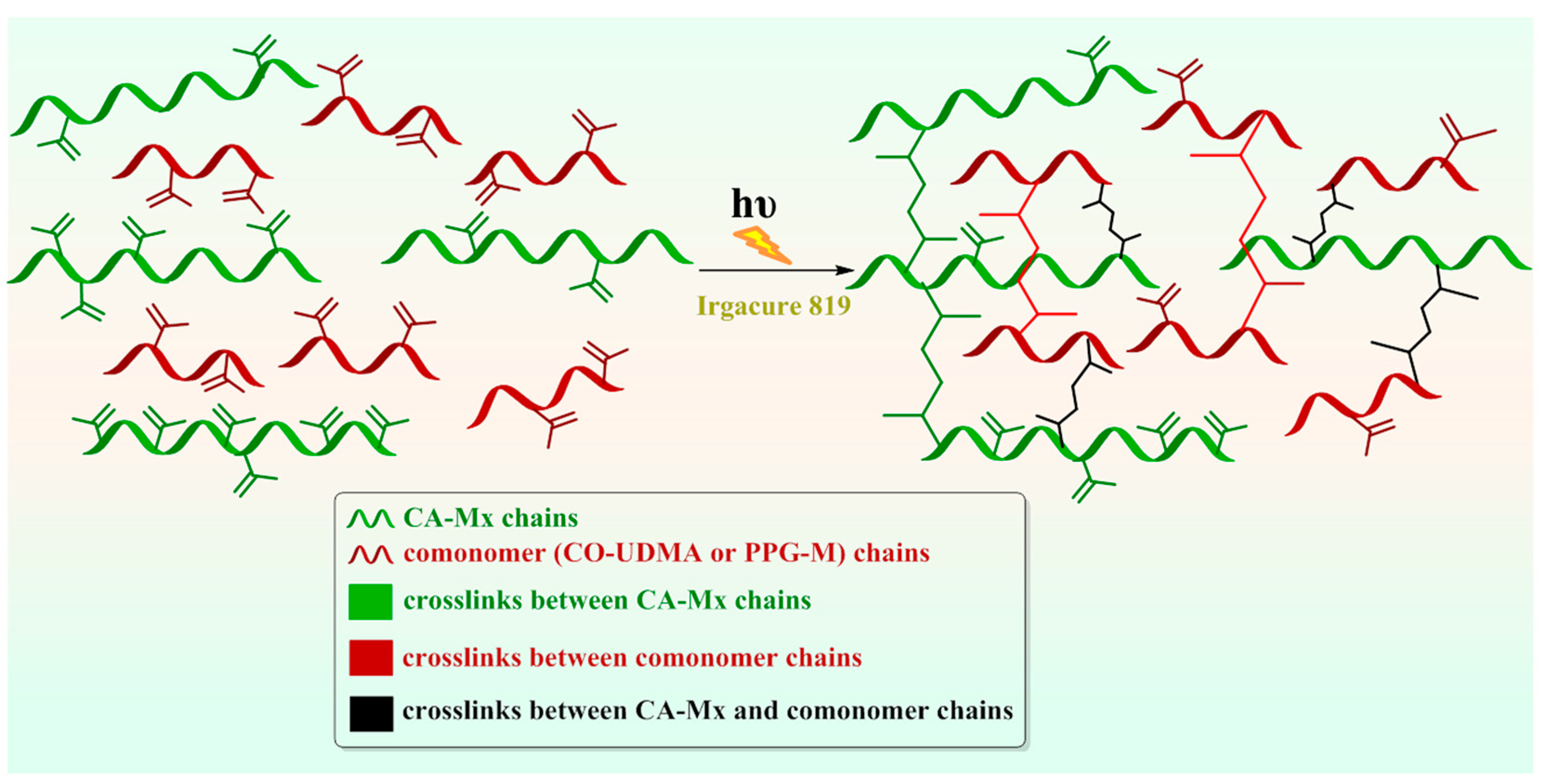
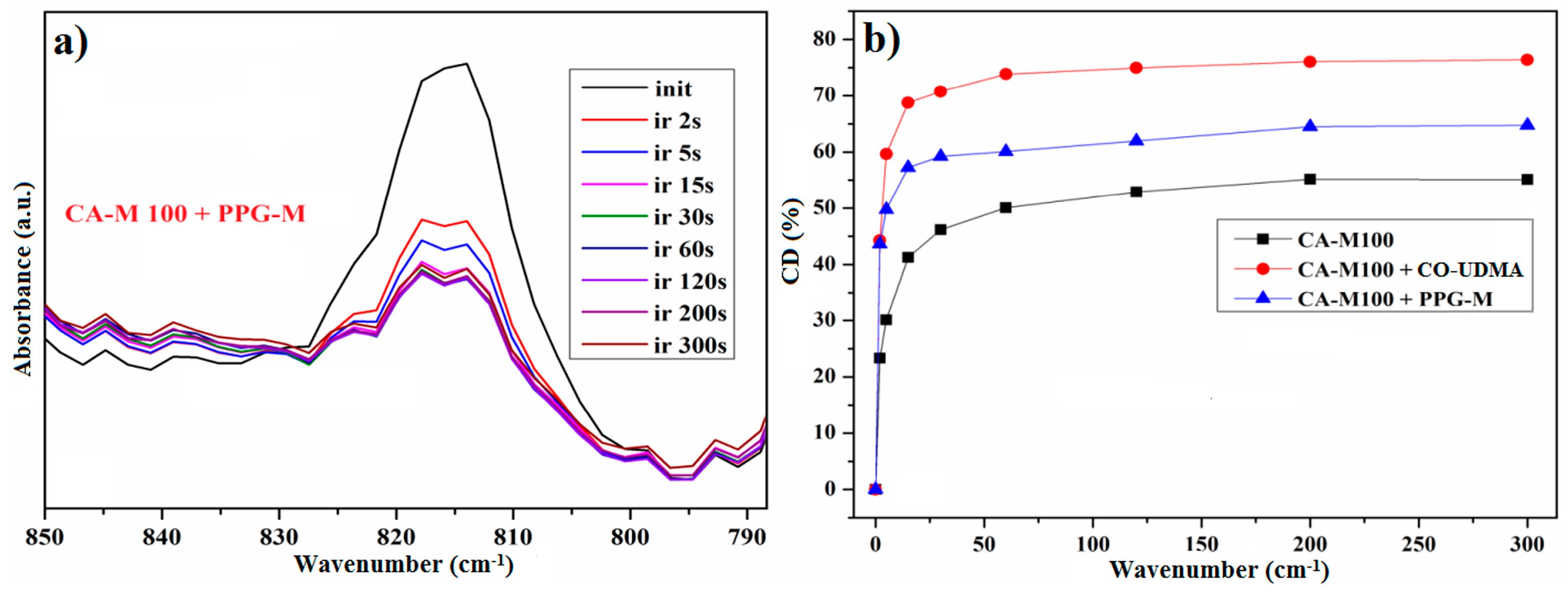
3.5.3. Investigation of the Photopolymerization Kinetics of CA-M Samples in a Mixture with PPG-M or CO-UDMA
3.5.4. Morphological Characterization of Photopolymerized CA Derivatives-Based Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S.; Biliuta, G. Bromide-Free Oxidizing System for Carboxylic Moiety Formation in Cellulose Chain. Carbohydr. Polym. 2012, 90, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Biliuta, G.; Coseri, S. Magnetic Cellulosic Materials Based on TEMPO-Oxidized Viscose Fibers. Cellulose 2016, 23, 3407–3415. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Naserifar, S.; Kuijpers, P.F.; Wojno, S.; Kádár, R.; Bernin, D.; Hasani, M. In Situ Monitoring of Cellulose Etherification in Solution: Probing the Impact of Solvent Composition on the Synthesis of 3-Allyloxy-2-Hydroxypropyl-Cellulose in Aqueous Hydroxide Systems. Polym. Chem. 2022, 13, 4111–4123. [Google Scholar] [CrossRef]
- You, J.; Zhang, X.; Mi, Q.; Zhang, J.; Wu, J.; Zhang, J. Mild, Rapid and Efficient Etherification of Cellulose. Cellulose 2022, 29, 9583–9596. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional Nanomaterials through Esterification of Cellulose: A Review of Chemistry and Application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Gou, S.; Zhang, L.; Wang, Z. Esterification of Cellulose Using Carboxylic Acid-Based Deep Eutectic Solvents to Produce High-Yield Cellulose Nanofibers. Carbohydr. Polym. 2021, 251, 117018. [Google Scholar] [CrossRef]
- Culica, M.E.; Chibac-Scutaru, A.L.; Asandulesa, M.; Melinte, V.; Cojocaru, C.; Coseri, S. Convertible Cellulosic Platforms with Manageable Loads of 1-Hydroxybenzotriazole: Their Preparation and Conductive Behavior. Cellulose 2022, 29, 9847–9863. [Google Scholar] [CrossRef]
- Melinte, V.; Trifan, S.I.; Chibac-Scutaru, A.L.; Podasca, V.; Coseri, S. Reusable Catalysts Based on CeO2/Cellulose Derivative with Visible Light Photocatalytic Activity Tuned by Noble Metal Nanoparticles Inclusion. Int. J. Biol. Macromol. 2022, 222, 736–749. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Paul, U.C.; Barthel, M.J.; Goldoni, L.; Caputo, G.; Ceseracciu, L.; Athanassiou, A.; Heredia-Guerrero, J.A. Thermoplastic Cellulose Acetate Oleate Films with High Barrier Properties and Ductile Behaviour. Chem. Eng. J. 2018, 348, 840–849. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Kaschuk, J.J.; Borghei, M.; Solin, K.; Tripathi, A.; Khakalo, A.; Leite, F.A.S.; Branco, A.; Amores De Sousa, M.C.; Frollini, E.; Rojas, O.J. Cross-Linked and Surface-Modified Cellulose Acetate as a Cover Layer for Paper-Based Electrochromic Devices. ACS Appl. Polym. Mater. 2021, 3, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Yezer, I.; Demirkol, D.O. Cellulose Acetate–Chitosan Based Electrospun Nanofibers for Bio-Functionalized Surface Design in Biosensing. Cellulose 2020, 27, 10183–10197. [Google Scholar] [CrossRef]
- Pandele, A.M.; Iovu, H.; Orbeci, C.; Tuncel, C.; Miculescu, F.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Surface Modified Cellulose Acetate Membranes for the Reactive Retention of Tetracycline. Sep. Purif. Technol. 2020, 249, 117145. [Google Scholar] [CrossRef]
- Matamá, T.; Araújo, R.; Gübitz, G.M.; Casal, M.; Cavaco-Paulo, A. Functionalization of Cellulose Acetate Fibers with Engineered Cutinases. Biotechnol. Prog. 2010, 26, 636–643. [Google Scholar] [CrossRef]
- Qu, Z.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Acrylic Functionalization of Cellulose Nanocrystals with 2-Isocyanatoethyl Methacrylate and Formation of Composites with Poly(Methyl Methacrylate). ACS Omega 2020, 5, 31092–31099. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Shi, M.; Xu, X.; He, S.; Yang, C.; Wang, L.; Wu, R.; Wang, W.; Wang, J. DMAEMA-Grafted Cellulose as an Imprinted Adsorbent for the Selective Adsorption of 4-Nitrophenol. Cellulose 2021, 28, 6481–6498. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, S.; Zhang, M.; He, J.; Ni, P. UV-Photopolymerized Cellulose Acetate-Acrylate Membranes for Lithium-Ion Battery Separator. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131359. [Google Scholar] [CrossRef]
- Dogan-Guner, E.M.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Acryloyl-Modified Cellulose Nanocrystals: Effects of Substitution on Crystallinity and Copolymerization with Acrylic Monomers. Cellulose 2021, 28, 10875–10889. [Google Scholar] [CrossRef]
- Chatani, S.; Kloxin, C.J.; Bowman, C.N. The Power of Light in Polymer Science: Photochemical Processes to Manipulate Polymer Formation, Structure, and Properties. Polym. Chem. 2014, 5, 2187–2201. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Fan, Q. Visible Light Initiating Systems for Photopolymerization: Status, Development and Challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Sangermano, M.; Marchi, S.; Valentini, L.; Bon, S.B.; Fabbri, P. Transparent and Conductive Graphene Oxide/Poly(Ethylene Glycol) Diacrylate Coatings Obtained by Photopolymerization. Macromol. Mater. Eng. 2011, 296, 401–407. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat Photopolymerization of Polymers and Polymer Composites: Processes and Applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Lai, H.; Peng, X.; Li, L.; Zhu, D.; Xiao, P. Novel Monomers for Photopolymer Networks. Prog. Polym. Sci. 2022, 128, 101529. [Google Scholar] [CrossRef]
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A Review on Modeling Cure Kinetics and Mechanisms of Photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W. Dimethacrylate Network Formation and Polymer Property Evolution as Determined by the Selection of Monomers and Curing Conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Hamel, C.M.; Mu, X.; Kuang, X.; Guo, Z.; Qi, H.J. Evolution of Material Properties during Free Radical Photopolymerization. J. Mech. Phys. Solids 2018, 112, 25–49. [Google Scholar] [CrossRef]
- Anastasio, R.; Peerbooms, W.; Cardinaels, R.; Van Breemen, L.C.A. Characterization of Ultraviolet-Cured Methacrylate Networks: From Photopolymerization to Ultimate Mechanical Properties. Macromolecules 2019, 52, 9220–9231. [Google Scholar] [CrossRef]
- Scherzer, T.; Decker, U. Real-Time FTIR–ATR Spectroscopy to Study the Kinetics of Ultrafast Photopolymerization Reactions Induced by Monochromatic UV Light. Vib. Spectrosc. 1999, 19, 385–398. [Google Scholar] [CrossRef]
- Kwaśny, M.; Polkowski, J.; Bombalska, A. A Study on the Photopolymerization Kinetics of Selected Dental Resins Using Fourier Infrared Spectroscopy (FTIR). Materials 2022, 15, 5850. [Google Scholar] [CrossRef]
- Chibac-Scutaru, A.L.; Podasca, V.; Timpu, D.; Melinte, V. Comparative Study on the Influence of Noble Metal Nanoparticles (Ag, Au, Pd) on the Photocatalytic Activity of ZnO NPs Embedded in Renewable Castor Oil Polymer Matrices. Materials 2020, 13, 3468. [Google Scholar] [CrossRef]
- Halake, K.S.; Choi, S.Y.; Hong, S.M.; Seo, S.Y.; Lee, J. Regioselective Substitution of 2-Isocyanatoethylmethacrylate onto Cellulose. J. Appl. Polym. Sci. 2013, 128, 2056–2062. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of Acrylamide/Acrylic Acid Cellulose Hydrogels for the Adsorption of Heavy Metal Ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Chibac, A.L.; Buruiana, T.; Melinte, V.; Mangalagiu, I.; Epurescu, G.; Buruiana, E.C. Synthesis of New Photoactive Urethane Carbohydrates and Their Behavior in UV or Femtosecond Laser-Induced Two-Photon Polymerization. Des. Monomers Polym. 2016, 19, 12–23. [Google Scholar] [CrossRef][Green Version]
- Sato, H.; Suttiwijitpukdee, N.; Hashimoto, T.; Ozaki, Y. Simultaneous Synchrotron SAXS/WAXD Study of Composition Fluctuations, Cold-Crystallization, and Melting in Biodegradable Polymer Blends of Cellulose Acetate Butyrate and Poly(3-Hydroxybutyrate). Macromolecules 2012, 45, 2783–2795. [Google Scholar] [CrossRef]
- Tsioptsias, C. Glass Chemical Transition: An Unknown Thermal Transition Observed in Cellulose Acetate Butyrate. Carbohydr. Polym. 2021, 259, 117754. [Google Scholar] [CrossRef]
- Nejström, M.; Andreasson, B.; Sjölund, J.; Eivazi, A.; Svanedal, I.; Edlund, H.; Norgren, M. On Structural and Molecular Order in Cellulose Acetate Butyrate Films. Polymers 2023, 15, 2205. [Google Scholar] [CrossRef]
- Soroush, M.; Grady, M.C.; Kalfas, G.A. Free-Radical Polymerization at Higher Temperatures: Systems Impacts of Secondary Reactions. Comput. Chem. Eng. 2008, 32, 2155–2167. [Google Scholar] [CrossRef]
- Asandulesa, M.; Chibac-scutaru, A.L.; Culica, M.E.; Melinte, V.; Coseri, S. Cellulose-Based Films with Enhanced Load of Nitrogen Containing Heterocycles: The Impact on the Surface Morphology and Proton Conductivity. Appl. Surf. Sci. 2023, 607, 155077. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Wang, K.; Cao, X.; Sun, R. Cellulose Acetate Fibers Prepared from Different Raw Materials with Rapid Synthesis Method. Carbohydr. Polym. 2016, 137, 685–692. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; dos Santos, D.C.; Motta, J.F.G.; dos Santos, R.R.; Chávez, D.W.H.; Melo, N.R. de Structure and Functional Properties of Cellulose Acetate Films Incorporated with Glycerol. Carbohydr. Polym. 2019, 209, 190–197. [Google Scholar] [CrossRef]
- Huang, A.; Wei, L.; Zhao, Z.; Wei, G.; Zhang, Y.; Huang, Z.; Li, X.; Hu, H.; Qin, X.; Yang, M. A Comparative Analysis of the Preparation of Cellulose Acetate Butyrate and the Characteristics of Applying in Pearlescent Coating Film. Polym. Bull. 2020, 77, 2873–2887. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, L.M.; Barbalata-Mandru, M.; Vlad, S.; Petrescu, M. Surface Energy Evaluation of Casting and Nanofiber Polyurethane Films by Using Different Models. J. Appl. Polym. Sci. 2021, 138, 50834. [Google Scholar] [CrossRef]
- Kramar, A.; Rodríguez, I.; Gaitano, G.G.; Benito, J.G. Solution Casting of Cellulose Acetate Films: Influence of Surface Substrate and Humidity on Wettability, Morphology and Optical Properties. Cellulose 2023, 30, 2037–2052. [Google Scholar] [CrossRef]
- Refaat, H.M.; Ashraf, N.; El-Dissouky, A.; Tieama, H.A.; Kamoun, E.A.; Showman, M.S. Efficient Removal of Bovine Serum Albumin from Water by Cellulose Acetate Membranes Modified with Clay and Titania Nano Particles. Front. Chem. 2023, 11, 1111558. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Gardner, D.J.; Shen, W. Contact Angles and Wettability of Cellulosic Surfaces: A Review of Proposed Mechanisms and Test Strategies. BioResources 2015, 10, 8657–8749. [Google Scholar] [CrossRef]
- Kramar, A.; González-Benito, J. Preparation of Cellulose Acetate Film with Dual Hydrophobic-Hydrophilic Properties Using Solution Blow Spinning. Mater. Des. 2023, 227, 111788. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The Correlation between the Surface Energy, the Contact Angle and the Spreading Parameter, and Their Relevance for the Wetting Behaviour of DLC with Lubricating Oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Kuang, P.; Constant, K. Increased Wettability and Surface Free Energy of Polyurethane by Ultraviolet Ozone Treatment. In Wetting and Wettability; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2015; ISBN 978-953-51-2215-9. [Google Scholar]
- Wang, Q.; Cui, S.W. Understanding the Physical Properties of Food Polysaccharides. In Food Carbohydrates: Chemistry, Physical Properties, and Applications; Cui, S.W., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 2005; ISBN 9780429095641. [Google Scholar]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Aldeligan, S.H.; Alsubaie, F.S. Synthesis and Characterization of Cellulose Triacetate Obtained from Date Palm (Phoenix dzactylifera L.) Trunk Mesh-Derived Cellulose. Molecules 2022, 27, 1434. [Google Scholar] [CrossRef]
- Sousa, M.; Rita Brás, A.; Isabel, M.; Veiga, H.; Castelo Ferreira, F.; Norberta de Pinho, M.; Correia, N.T.; Dionísio, M. Dynamical Characterization of a Cellulose Acetate Polysaccharide. J. Phys. Chem. B 2010, 114, 10939–10953. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Wu, L.; Hua, M.; Jiang, C.; Pan, Y.; Yao, L.; Xu, S.; Ge, J.; Pan, G. Synthesis and Characterization of Cellulose Diacetate-Graft-Polylactide via Solvent-Free Melt Ring-Opening Graft Copolymerization. Polymers 2023, 15, 143. [Google Scholar] [CrossRef]
- Shalaby, A.A.; Abd Elmageed, M.H.; Malash, G.F.; Tamer, T.M.; Omer, A.M.; Mohy-Eldin, M.S.; Khalifa, R.E. Development of Novel Cellulose Acetate-g-Poly(Sodium 4-Styrenesulfonate) Proton Conducting Polyelectrolyte Polymer. J. Saudi Chem. Soc. 2021, 25, 101327. [Google Scholar] [CrossRef]
- Zhao, X.; Anwar, I.; Zhang, X.; Pellicciotti, A.; Storts, S.; Nagib, D.A.; Vodovotz, Y. Thermal and Barrier Characterizations of Cellulose Esters with Variable Side-Chain Lengths and Their Effect on PHBV and PLA Bioplastic Film Properties. ACS Omega 2021, 6, 24700–24708. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, T.; Melinte, V.; Jitaru, F.; Aldea, H.; Buruiana, E.C. Photopolymerization Experiments and Properties of Some Urethane/Urea Methacrylates Tested in Dental Composites. J. Compos. Mater. 2012, 46, 371–382. [Google Scholar] [CrossRef]
- Lovell, L.G.; Stansbury, J.W.; Syrpes, D.C.; Bowman, C.N. Effects of Composition and Reactivity on the Reaction Kinetics of Dimethacrylate/Dimethacrylate Copolymerizations. Macromolecules 1999, 32, 3913–3921. [Google Scholar] [CrossRef]
- Feng, L.; Suh, B.I. Exposure Reciprocity Law in Photopolymerization of Multi-Functional Acrylates and Methacrylates. Macromol. Chem. Phys. 2007, 208, 295–306. [Google Scholar] [CrossRef]


| Product Name | CA (g) | CA (mmol) | 2-IEMA (mL) | 2-IEMA (mmol) |
|---|---|---|---|---|
| CA-M5 | 5 | 0.1 | 0.14 | 1 |
| CA-M10 | 5 | 0.1 | 0.29 | 2 |
| CA-M25 | 5 | 0.1 | 0.72 | 5 |
| CA-M50 | 5 | 0.1 | 1.44 | 10 |
| CA-M100 | 5 | 0.1 | 2.88 | 20 |
| Sample | WCA |
|---|---|
| CA | 62 ± 1° |
| CA-M5 | 75 ± 1° |
| CA-M10 | 65 ± 1° |
| CA-M25 | 57 ± 1° |
| CA-M50 | 78 ± 1° |
| CA-M100 | 44 ± 1° |
| Films | CA-Mx (wt%) | PPG-M (wt%) | CO-UDMA (wt%) | Irg 819 (wt%) |
|---|---|---|---|---|
| CA-Mx–PPG-M | 50 | 50 | - | 1 |
| CA-Mx–CO-UDMA | 50 | - | 50 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifan, I.-S.; Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Photopolymerization Pattern of New Methacrylate Cellulose Acetate Derivatives. Polymers 2024, 16, 560. https://doi.org/10.3390/polym16040560
Trifan I-S, Chibac-Scutaru AL, Melinte V, Coseri S. Photopolymerization Pattern of New Methacrylate Cellulose Acetate Derivatives. Polymers. 2024; 16(4):560. https://doi.org/10.3390/polym16040560
Chicago/Turabian StyleTrifan, Ioana-Sabina, Andreea L. Chibac-Scutaru, Violeta Melinte, and Sergiu Coseri. 2024. "Photopolymerization Pattern of New Methacrylate Cellulose Acetate Derivatives" Polymers 16, no. 4: 560. https://doi.org/10.3390/polym16040560
APA StyleTrifan, I.-S., Chibac-Scutaru, A. L., Melinte, V., & Coseri, S. (2024). Photopolymerization Pattern of New Methacrylate Cellulose Acetate Derivatives. Polymers, 16(4), 560. https://doi.org/10.3390/polym16040560









