Charge Carrier Mobility in Poly(N,N′-bis-4-butylphenyl-N,N′-bisphenyl)benzidine Composites with Electron Acceptor Molecules
Abstract
1. Introduction
2. Materials and Methods
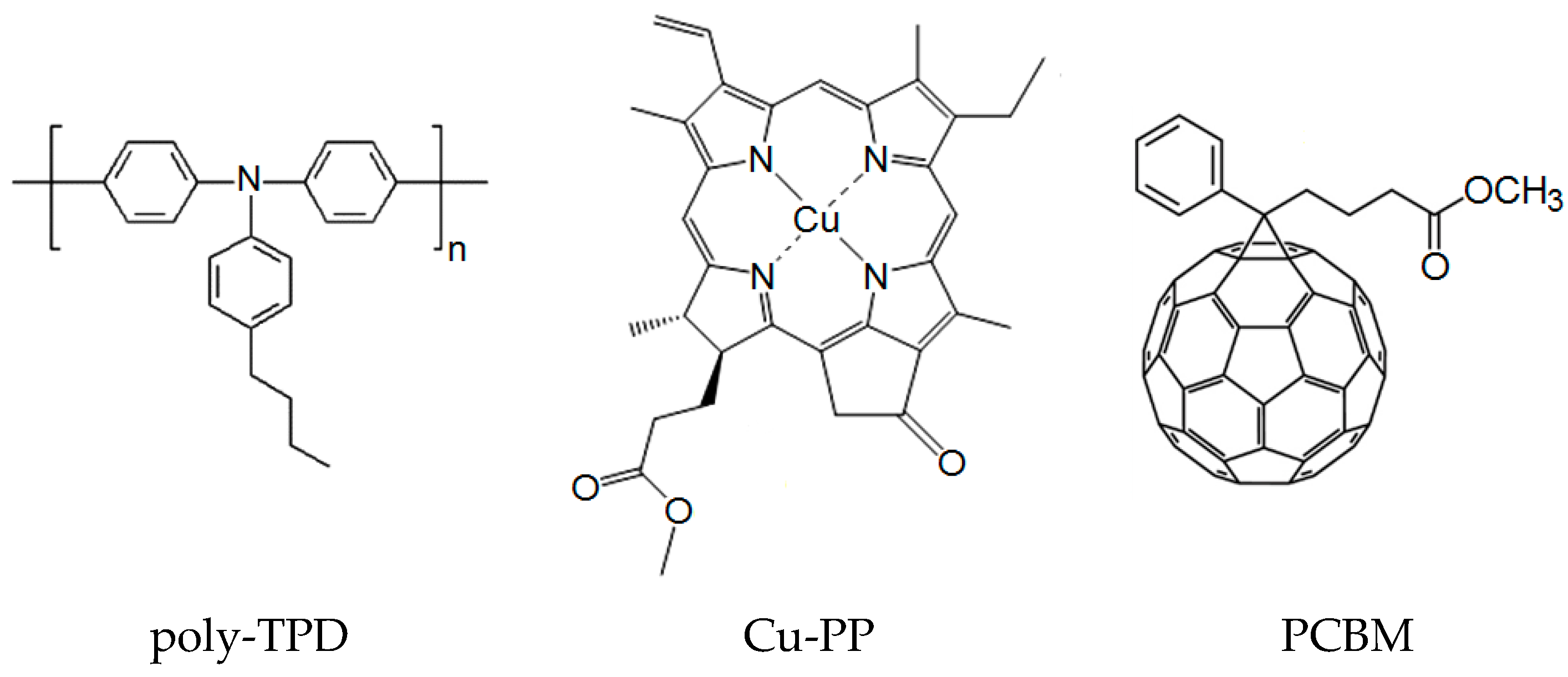
2.1. Materials and Synthesis
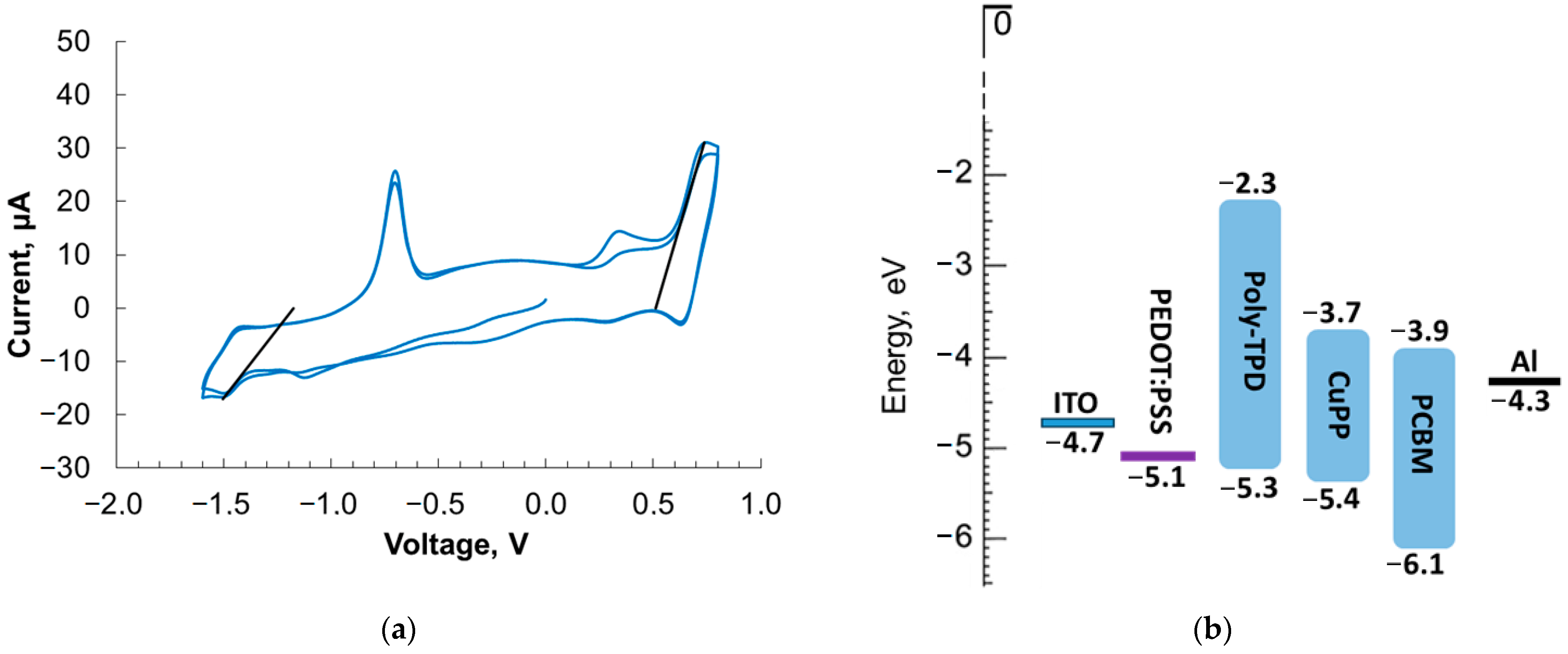
2.2. Cyclic Voltammetry and Energy Levels
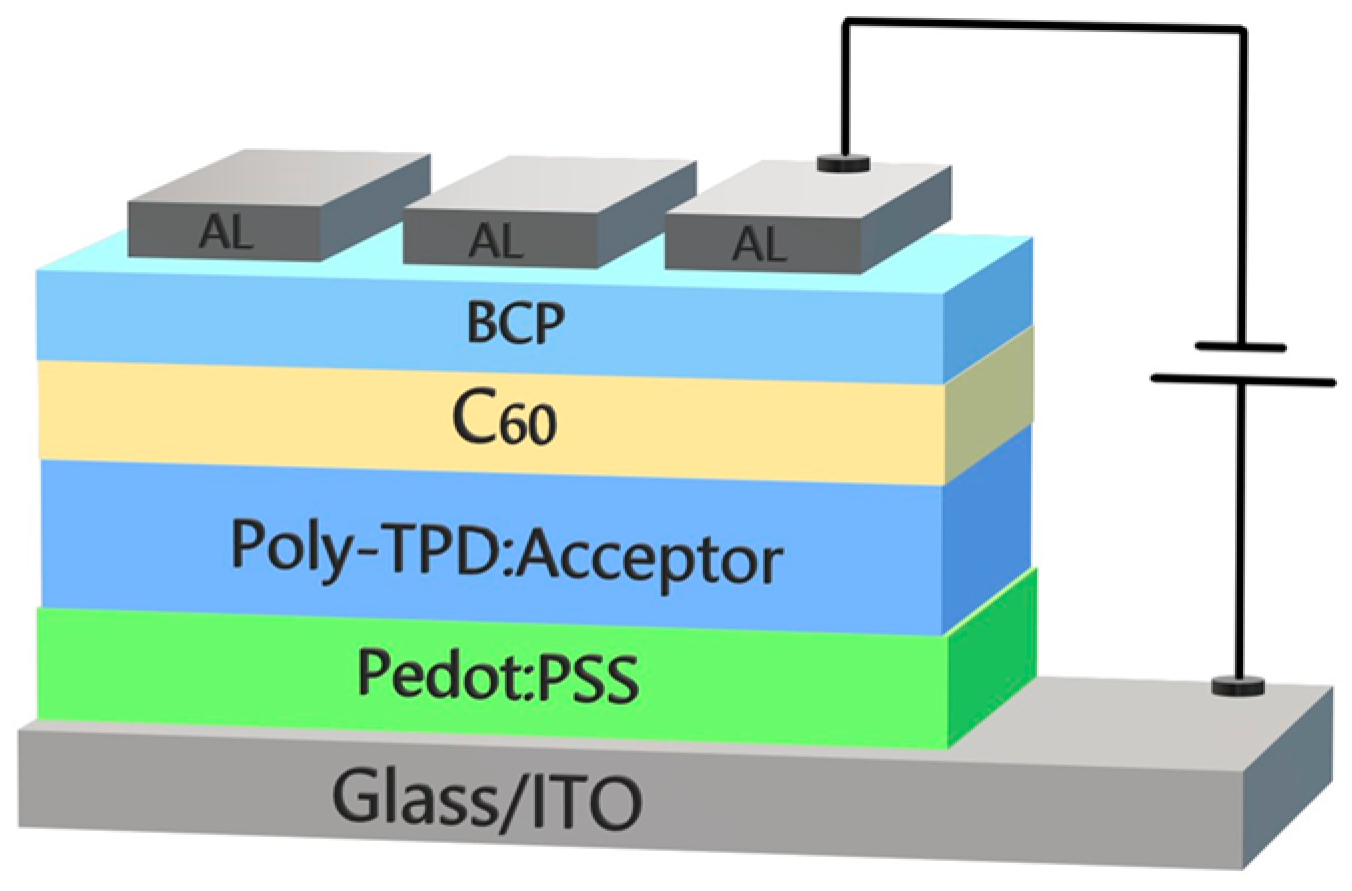
2.3. Thin Films and Device Preparation
2.4. Electrical Characterization
3. Results and Discussion
3.1. UV–Vis Spectroscopy
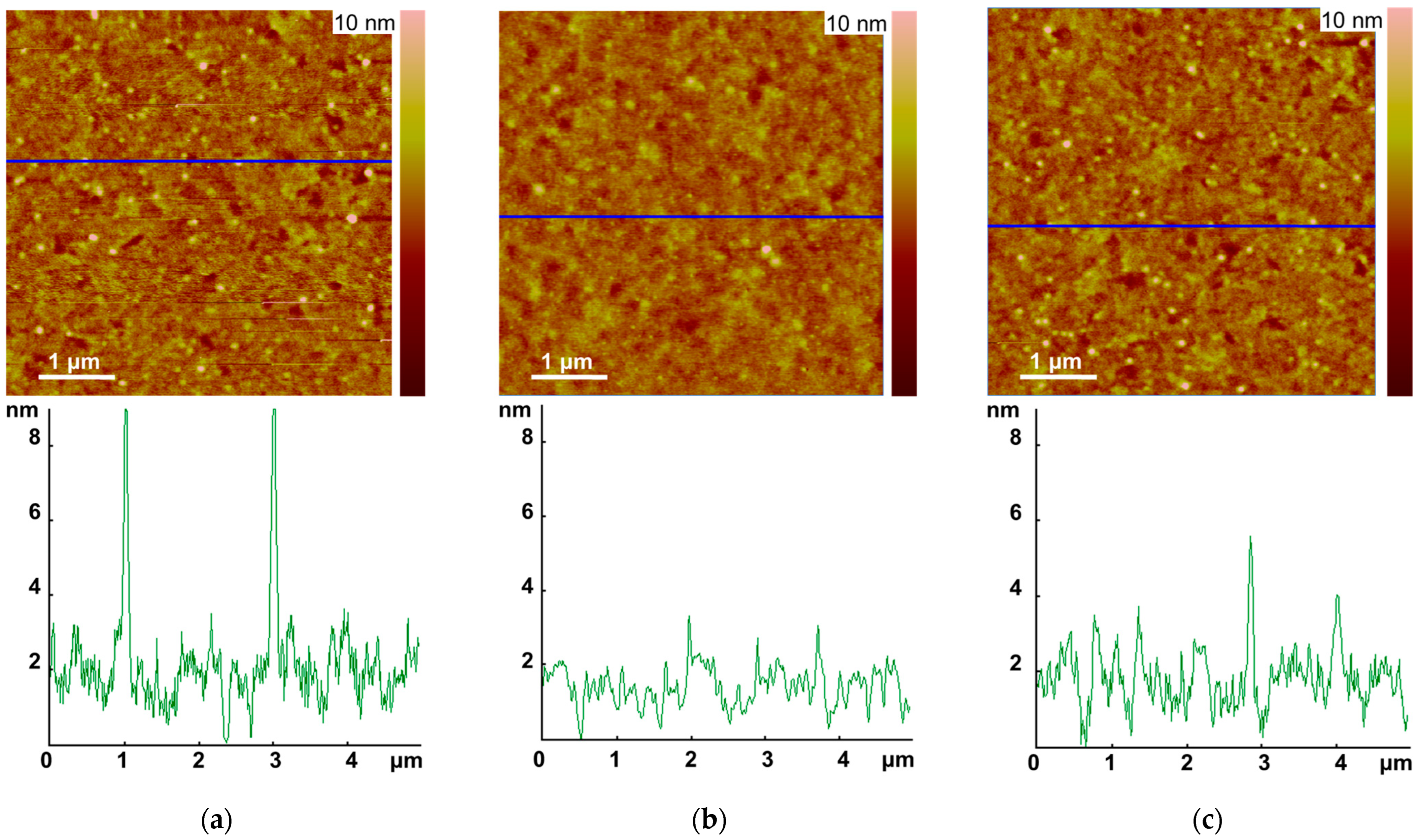
3.2. AFM of Solid Layers
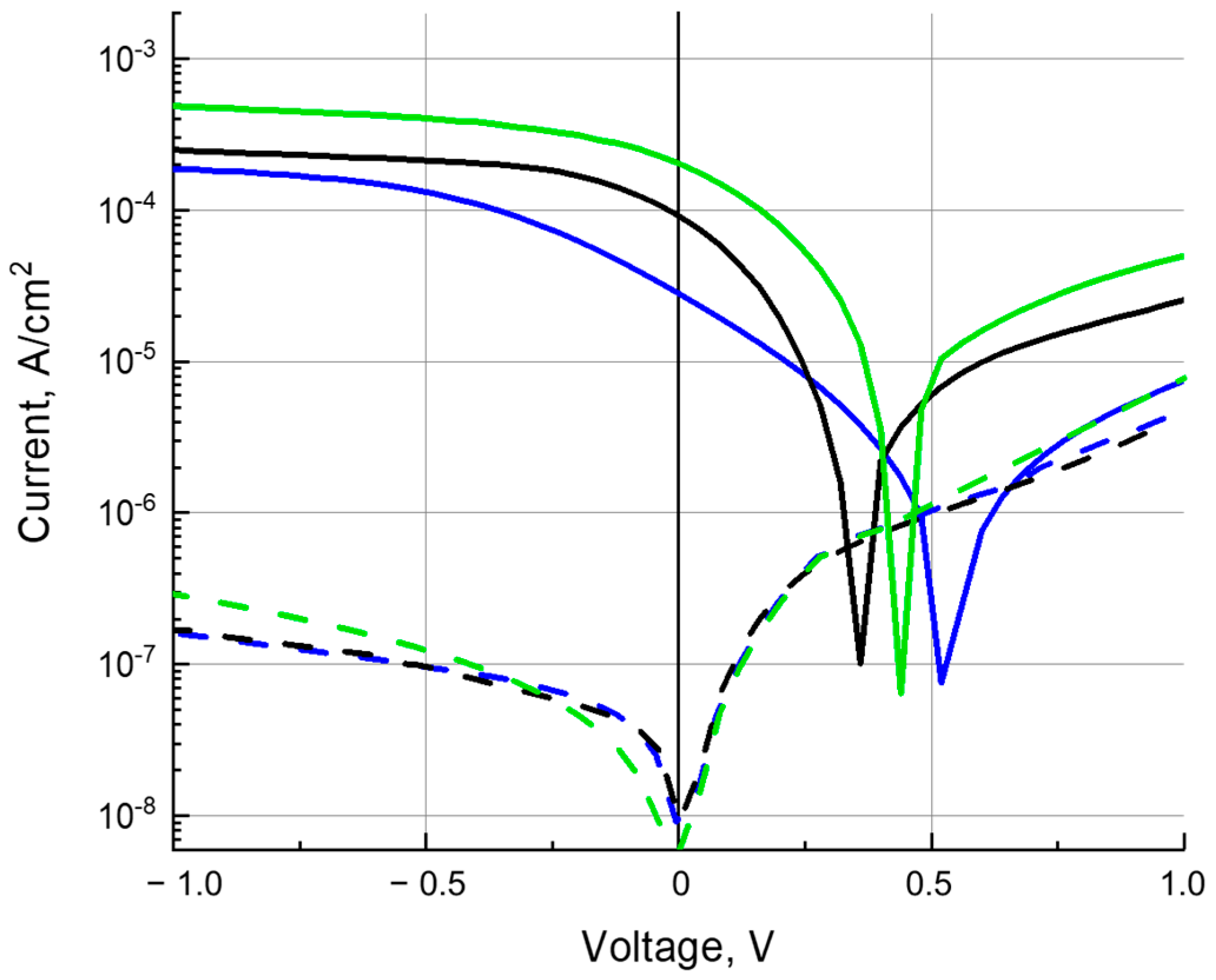
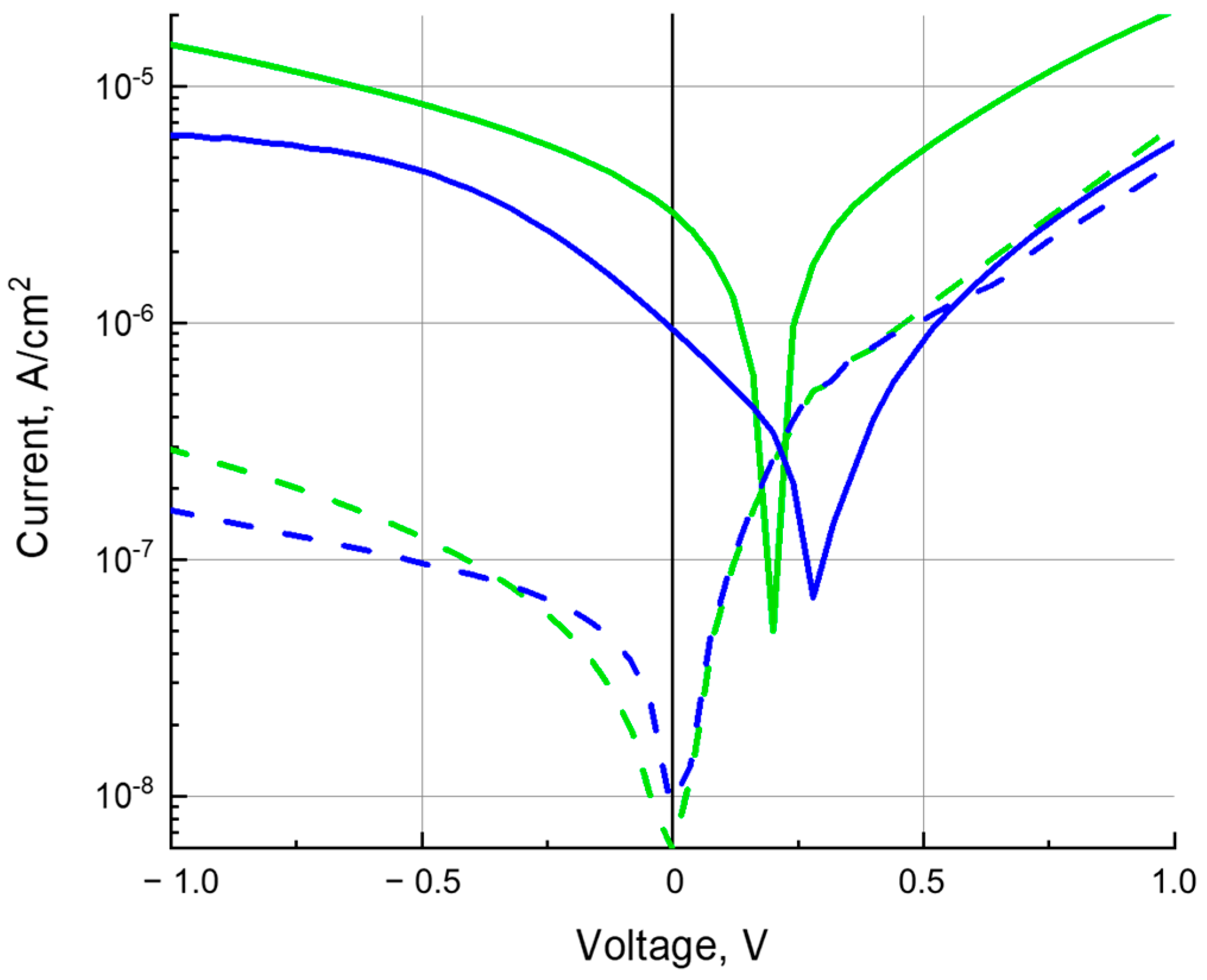
3.3. Charge Carrier Mobility
3.4. Photoconductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
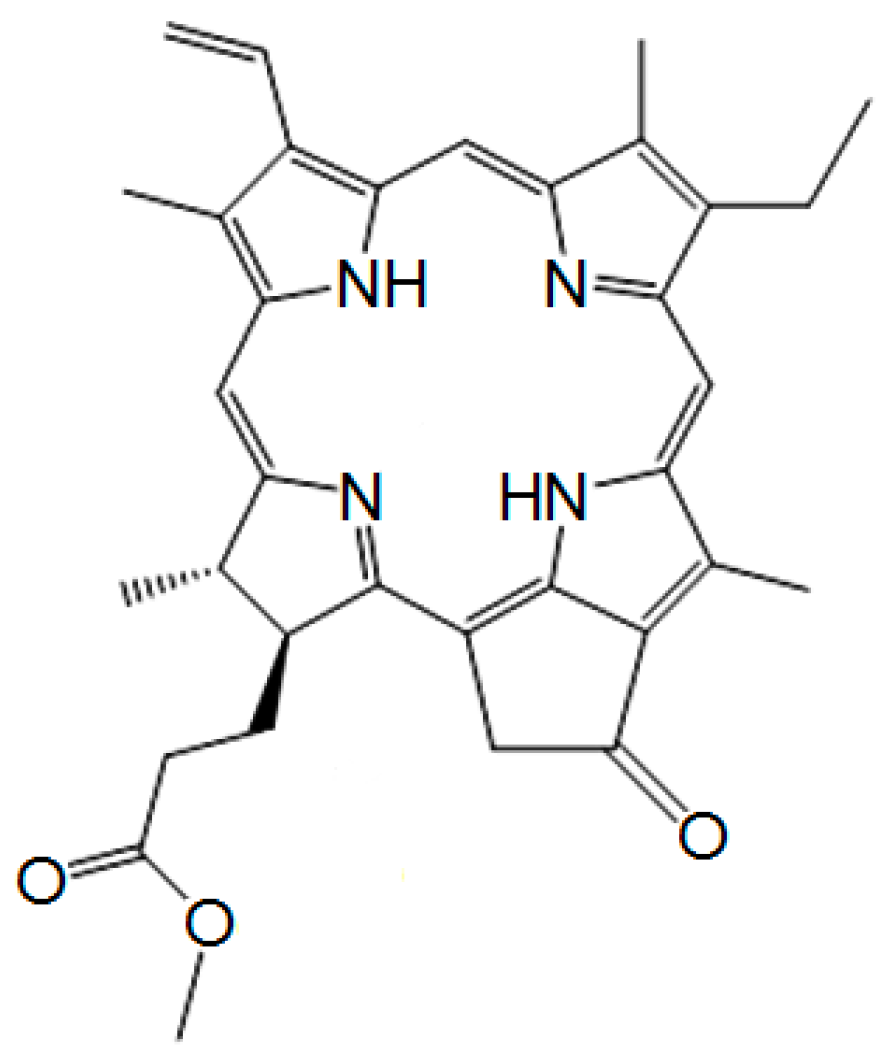
Appendix B
References
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Bässler, H.; Köhler, A. Electronic Processes in Organic Semiconductors: An Introduction; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Yang, K.; Wang, J.; Zhao, Z.; Zhou, Z.; Liu, M.; Zhang, J.; He, Z.; Zhang, F. Smart Strategy: Transparent Hole-Transporting Polymer as a Regulator to Optimize Photomultiplication-type Polymer Photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 21565–21572. [Google Scholar] [CrossRef]
- Ha, H.; Shim, Y.J.; Lee, D.H.; Park, E.Y.; Lee, I.-H.; Yoon, S.-K.; Suh, M.C. Highly Efficient Solution-Processed Organic Light-Emitting Diodes Containing a New Cross-linkable Hole Transport Material Blended with Commercial Hole Transport Materials. ACS Appl. Mater. Interfaces 2021, 13, 21954–21963. [Google Scholar] [CrossRef]
- Chen, H.; Ding, K.; Fan, L.; Liu, W.; Zhang, R.; Xiang, S.; Zhang, Q.; Wang, L. All-Solution-Processed Quantum Dot Light Emitting Diodes Based on Double Hole Transport Layers by Hot Spin-Coating with Highly Efficient and Low Turn-On Voltage. ACS Appl. Mater. Interfaces 2018, 10, 29076–29082. [Google Scholar] [CrossRef]
- Hu, X.; Tao, C.; Liang, J.; Chen, C.; Zheng, X.; Li, J.; Li, J.; Liu, Y.; Fang, G. Molecular weight effect of poly-TPD hole-transporting layer on the performance of inverted perovskite solar cells. Sol. Energy 2021, 218, 368–374. [Google Scholar] [CrossRef]
- Thesen, M.W.; Höfer, B.; Debeaux, M.; Janietz, S.; Wedel, A.; Köhler, A.; Johannes, H.-H.; Krueger, H. Hole-Transporting Host-Polymer Series Consisting of Triphenylamine Basic Structures for Phosphorescent Polymer Light-Emitting Diodes. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3417–3430. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Hu, W. Organic photodiodes and phototransistors toward infrared detection: Materials, devices, and applications. Chem. Soc. Rev. 2020, 49, 653–670. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Liu, B.; Tao, H.; Zou, J.; Jiang, C.; Xu, M.; Wang, L.; Penga, J.; Cao, Y. Preparation of efficient quantum dot light-emitting diodes by balancing charge injection and sensitizing emitting layer with phosphorescent dye. J. Mater. Chem. C 2019, 7, 5755–5763. [Google Scholar] [CrossRef]
- Zhao, D.; Sexton, M.; Park, H.-Y.; Baure, G.; Nino, J.C.; So, F. High-Efficiency Solution-Processed Planar Perovskite Solar Cells with a Polymer Hole Transport Layer. Adv. Energy Mater. 2015, 5, 1401855. [Google Scholar] [CrossRef]
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Huang, F.; Li, J.Z.; Xu, Z.H.; Liu, Y.; Luo, R.P.; Zhang, S.W.; Nie, P.B.; Lv, Y.F.; Zhao, S.X.; Su, W.T.; et al. A Bilayer 2D-WS2/Organic-Based Heterojunction for High-Performance Photodetectors. Nanomaterials 2019, 9, 1312. [Google Scholar] [CrossRef]
- Blankenburg, L.; Sensfuss, S.; Schache, H.; Marten, J.; Milker, R.; Schrödner, M. TPD wide-bandgap polymers for solar cell application and their sensitization with small molecule dyes. Synth. Met. 2015, 199, 93–104. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. The Porphyrin Handbook; Academic Press: New York, NY, USA, 2000. [Google Scholar]
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met. Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef]
- Beyene, B.B.; Hung, C.-H. Photocatalytic hydrogen evolution from neutral aqueous solution by a water-soluble cobalt(II) porphyrin. Sustain. Energy Fuels 2018, 2, 2036–2043. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Chernyadyev, A.Y.; Aleksandrov, A.E.; Lypenko, D.A.; Tyurin, V.S.; Tameev, A.R.; Tsivadze, A.Y. Copper (II) Me-so-Tetraphenyl and Meso-Tetafluorenyl Porphyrinates as Charge Carrier Transporting and Electroluminescent Compounds. ACS Omega 2022, 7, 8613–8622. [Google Scholar] [CrossRef]
- Lypenko, D.A.; Aleksandrov, A.E.; Chernyadyev, A.Y.; Pozin, S.I.; Tsivadze, A.Y.; Tameev, A.R. Photoconduction and Electroluminescence of Copper (II) Protoporphyrin and Chlorin Cu-C-e6. Int. J. Mol. Sci. 2023, 24, 3178. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.A. Photoluminescence of Solutions; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1968. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Bobrovsky, A.; Piryazev, A.; Ivanov, D.; Kozlov, M.; Utochnikova, V. Temperature-Dependent Circularly Polarized Luminescence of a Cholesteric Copolymer Doped with a Europium Complex. Polymers 2023, 15, 1344. [Google Scholar] [CrossRef]
- Scheer, H.; Gross, E.; Nitsche, B.; Cmiel, E.; Schneider, S.; Schäfer, W.; Schiebel, H.-M.; Schulten, H.-R. Structure of methylpheophorbide-RCI. Photochem. Photobiol. 1986, 43, 559–571. [Google Scholar] [CrossRef]
- Malov, V.V.; Tanwistha, G.; Nair, V.C.; Maslov, M.M.; Katin, K.P.; Unni, K.N.N.; Tameev, A.R. Hole mobility in thieno [3,2-b]thiophene oligomers]. Mendeleev Commun. 2019, 29, 218–219. [Google Scholar] [CrossRef]
- Lampert, M.A.; Mark, P. Current Injection in Solids; Academic: New York, NY, USA, 1970. [Google Scholar]
- Tameev, A.R.; Licea, J.L.; Pereshivko, L.Y.; Rychwalski, R.W.; Vannikov, A.V. Charge carrier mobility in films of carbon-nanotube-polymer composites. J. Phys. Conf. Ser. 2007, 61, 1152–1156. [Google Scholar] [CrossRef]
- Low, P.J.; Paterson, M.A.J.; Puschmann, H.; Goeta, A.E.; Howard, J.A.K.; Lambert, C.; Cherryman, J.C.; Tackley, D.R.; Leeming, S.; Brown, B. Crystal, molecular and electronic structure of N,N′-diphenyl-N,N′-bis(2,4-dimethylphenyl)-(1,1′-biphenyl)-4,4′-diamine and the corresponding radical cation. Chem. Eur. J. 2004, 10, 83–91. [Google Scholar] [CrossRef]
- Liu, F.; Li, C.; Li, J.; Wang, C.; Xiao, C.; Wu, Y.; Li, W. Ternary organic solar cells based on polymer donor, polymer acceptor and PCBM components. Chinese Chem. Lett. 2020, 31, 865–868. [Google Scholar] [CrossRef]
- Bässler, H. Charge Transport in Disordered Organic Photoconductors: A Monte Carlo Simulation Study. Phys. Status Solidi B 1993, 175, 15–56. [Google Scholar] [CrossRef]
- Novikov, S.V.; Dunlap, D.H.; Kenkre, V.M.; Parris, P.E.; Vannikov, A.V. Essential Role of Correlations in Governing Charge Transport in Disordered Organic Materials. Phys. Rev. Lett. 1998, 81, 4472–4475. [Google Scholar] [CrossRef]
- Baranovskii, S.D. Theoretical description of charge transport in disordered organic semiconductors. Phys. Status Solidi B 2014, 251, 487–525. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Halls, J.J.M.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient photodiodes from interpenetrating polymer networks. Nature 1995, 376, 498–500. [Google Scholar] [CrossRef]
- Waldauf, C.; Schilinsky, P.; Perisutti, M.; Hauch, J.; Brabec, C.J. Solution-Processed Organic n-Type Thin-Film Transistors. Adv. Mater. 2003, 15, 2084–2088. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Tanase, C.; Setayesh, S.; Meijer, E.J.; Hummelen, J.C.; Blom, P.W.M.; de Leeuw, D.M. Ambipolar Organic Field-Effect Transistors Based on a Solution-Processed Methanofullerene. Adv. Mater. 2004, 16, 2174–2179. [Google Scholar] [CrossRef]
- Zhu, W.W.; Xiao, S.; Shih, I. Field-effect mobilities of polyhedral oligomeric silsesquioxanes anchored semiconducting polymers. Appl. Surf. Sci. 2004, 221, 358–363. [Google Scholar] [CrossRef]
- Andersson, L.M.; Zhang, F.; Inganäs, O. Bipolar transport observed through extraction currents on organic photovoltaic blend materials. Appl. Phys. Lett. 2006, 89, 142111. [Google Scholar] [CrossRef]
- Andersson, L.M.; Zhang, F.; Inganäs, O. Stoichiometry, mobility, and performance in bulk heterojunction solar cells. Appl. Phys. Lett. 2007, 91, 071108. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design Rules for Donors in Bulk-Heterojunction Solar Cells—Towards 10% Energy-Conversion Efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Wang, J.; Yao, H.; Xu, Y.; Ma, L.; Hou, J. Recent Progress in Reducing Voltage Loss in Organic Photovoltaic Cells. Mater. Chem. Front. 2021, 5, 709–722. [Google Scholar] [CrossRef]
- Chen, X.K.; Qian, D.; Wang, Y.; Kirchartz, T.; Tress, W.; Yao, H.; Yuan, J.; Hülsbeck, M.; Zhang, M.; Zou, Y.; et al. A unified description of non-radiative voltage losses in organic solar cells. Nat. Energy 2021, 6, 799–806. [Google Scholar] [CrossRef]

| Composite | Mobility, cm2V−1s−1 | |
|---|---|---|
| Electrons | Holes | |
| poly-TPD:PCBM | (8.0 ± 0.8) × 10−5 | (1.4 ± 0.1) × 10−4 |
| poly-TPD:Cu-PP | (3.5 ± 0.5) × 10−5 | (2.2 ± 0.1) × 10−4 |
| poly-TPD:PCBM:Cu-PP | (1.0 ± 0.1) × 10−4 | (1.5 ± 0.1) × 10−4 |
| poly-TPD | - | (2.8 ± 0.2) × 10−4 |
| PCBM | (8.0 ± 0.8) × 10−4 | - |
| Cu-PP | (6.0 ± 0.6) × 10−5 | (4.5 ± 0.5) × 10−5 |
| Composite | Mobility, cm2V−1s−1 | |
|---|---|---|
| Electrons | Holes | |
| polyTPD:PCBM | (4.2 ± 0.5) × 10−6 | (6.0 ± 0.6) × 10−6 |
| polyTPD:Cu-PP | (1.9 ± 0.2) × 10−6 | (7.0 ± 0.6) × 10−6 |
| polyTPD:PCBM:Cu-PP | (8.3 ± 0.8) × 10−6 | (9.8 ± 0.8) × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tameev, A.R.; Aleksandrov, A.E.; Sayarov, I.R.; Pozin, S.I.; Lypenko, D.A.; Dmitriev, A.V.; Nekrasova, N.V.; Chernyadyev, A.Y.; Tsivadze, A.Y. Charge Carrier Mobility in Poly(N,N′-bis-4-butylphenyl-N,N′-bisphenyl)benzidine Composites with Electron Acceptor Molecules. Polymers 2024, 16, 570. https://doi.org/10.3390/polym16050570
Tameev AR, Aleksandrov AE, Sayarov IR, Pozin SI, Lypenko DA, Dmitriev AV, Nekrasova NV, Chernyadyev AY, Tsivadze AY. Charge Carrier Mobility in Poly(N,N′-bis-4-butylphenyl-N,N′-bisphenyl)benzidine Composites with Electron Acceptor Molecules. Polymers. 2024; 16(5):570. https://doi.org/10.3390/polym16050570
Chicago/Turabian StyleTameev, Alexey R., Alexey E. Aleksandrov, Ildar R. Sayarov, Sergey I. Pozin, Dmitry A. Lypenko, Artem V. Dmitriev, Natalia V. Nekrasova, Andrey Yu. Chernyadyev, and Aslan Yu. Tsivadze. 2024. "Charge Carrier Mobility in Poly(N,N′-bis-4-butylphenyl-N,N′-bisphenyl)benzidine Composites with Electron Acceptor Molecules" Polymers 16, no. 5: 570. https://doi.org/10.3390/polym16050570
APA StyleTameev, A. R., Aleksandrov, A. E., Sayarov, I. R., Pozin, S. I., Lypenko, D. A., Dmitriev, A. V., Nekrasova, N. V., Chernyadyev, A. Y., & Tsivadze, A. Y. (2024). Charge Carrier Mobility in Poly(N,N′-bis-4-butylphenyl-N,N′-bisphenyl)benzidine Composites with Electron Acceptor Molecules. Polymers, 16(5), 570. https://doi.org/10.3390/polym16050570







