New Inhibitor Based on Hydrolyzed Keratin Peptides for Stainless Steel Corrosion in Physiological Serum: An Electrochemical and Thermodynamic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Electrochemical Measurements
Electrochemical Impedance Spectroscopy (EIS)
Potentiodynamic Polarization (PDP)
2.2.2. Surface Characterization
Optical Microscopy
Atomic Force Microscopy (AFM)
3. Results and Discussion
3.1. Open Circuit Potential (OCP)
3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3. Potentiodynamic Polarization
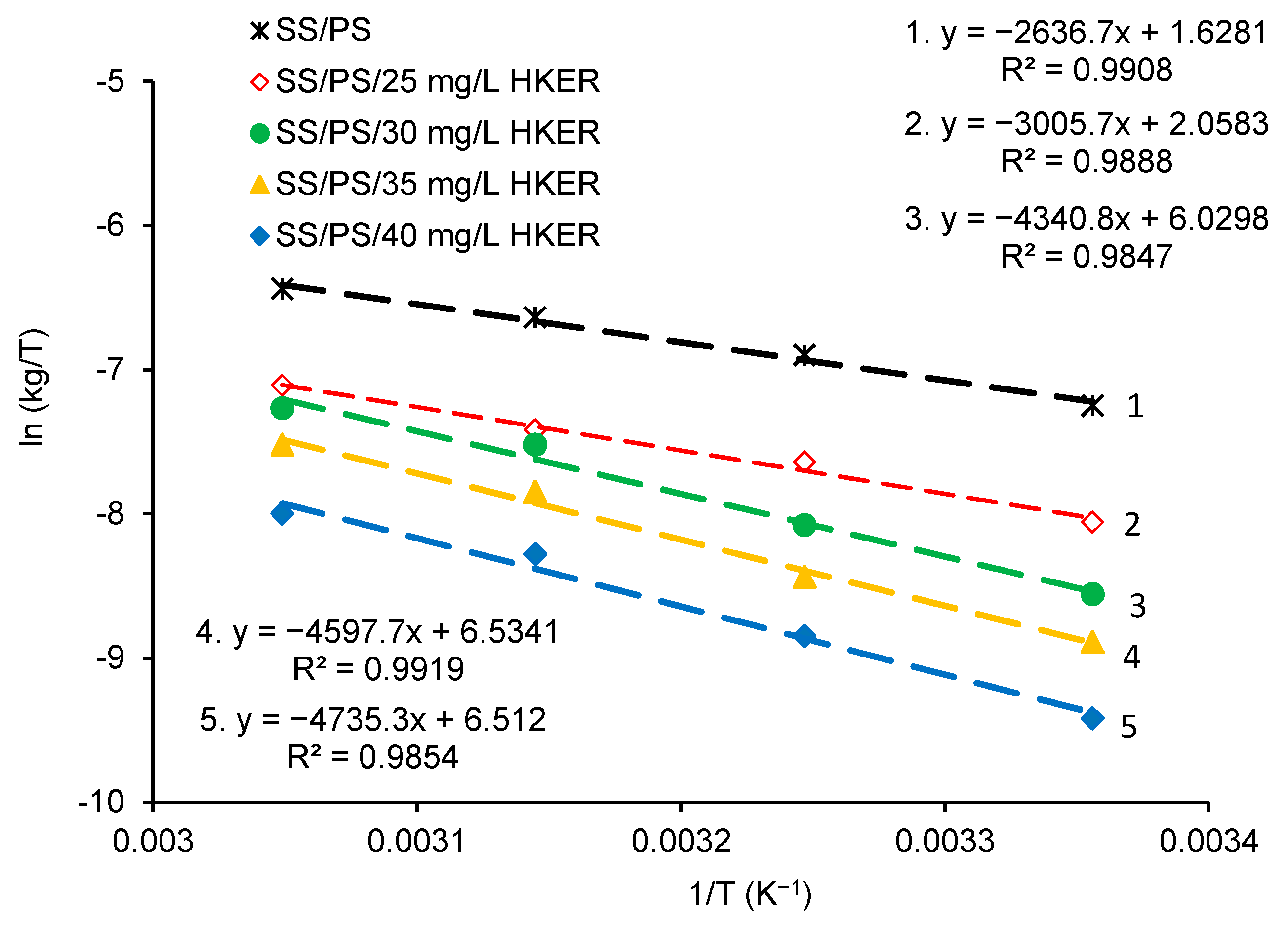
3.4. Activation Energy Calculations: Determination of Thermodynamic Activation Parameters
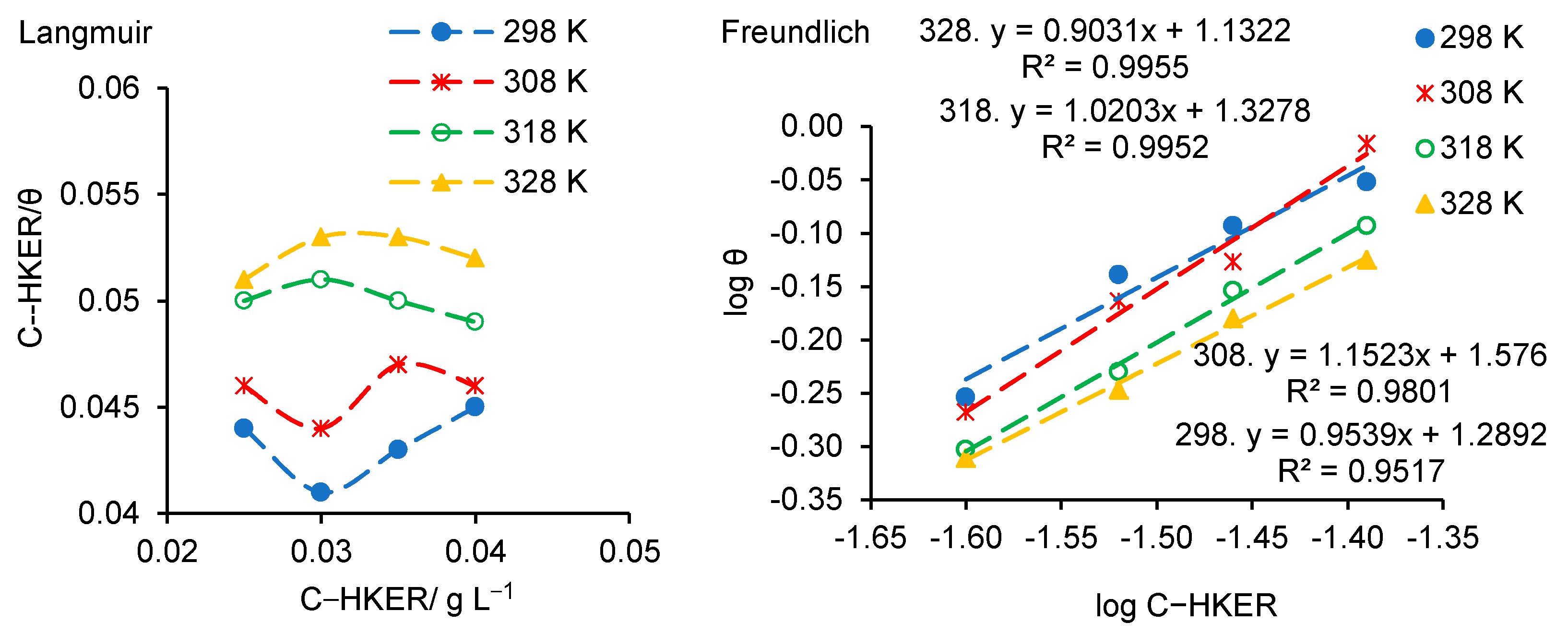
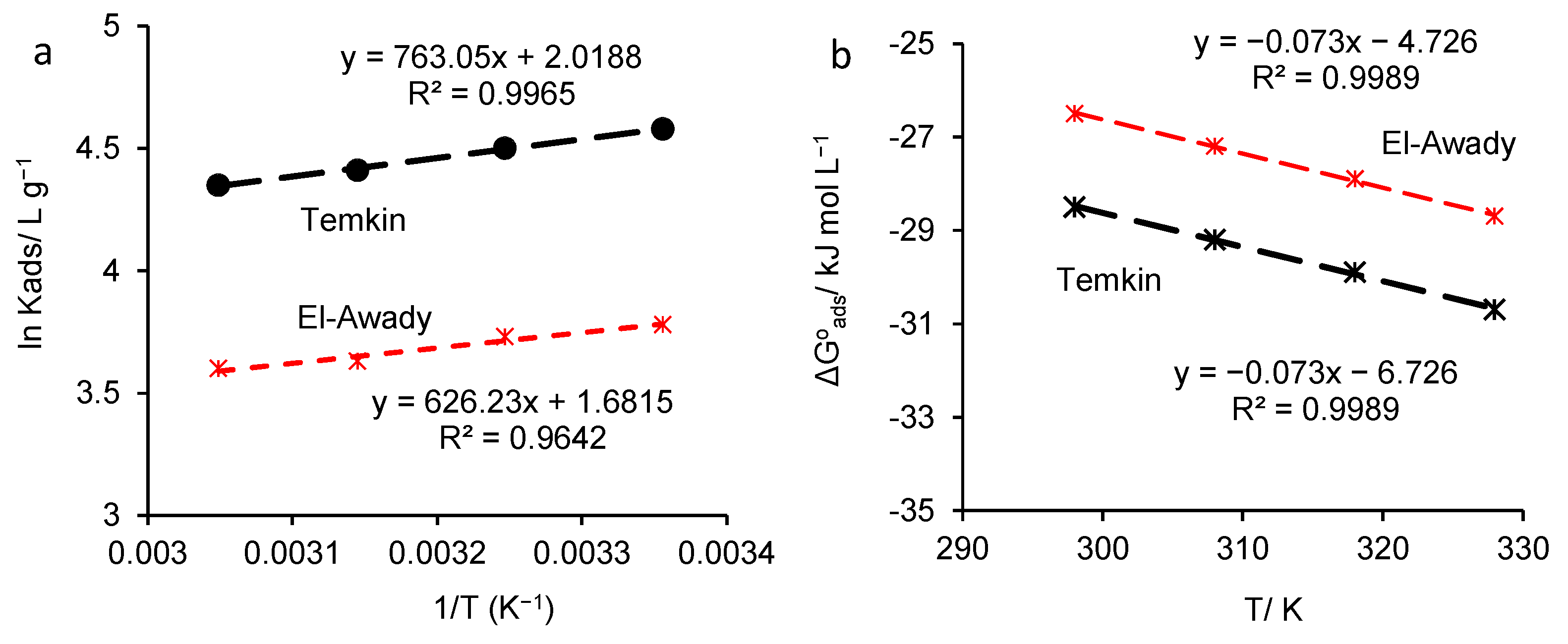
3.5. Adsorption Isotherm Approach. Calculation of Adsorption Parameters
3.6. Surface Characterization
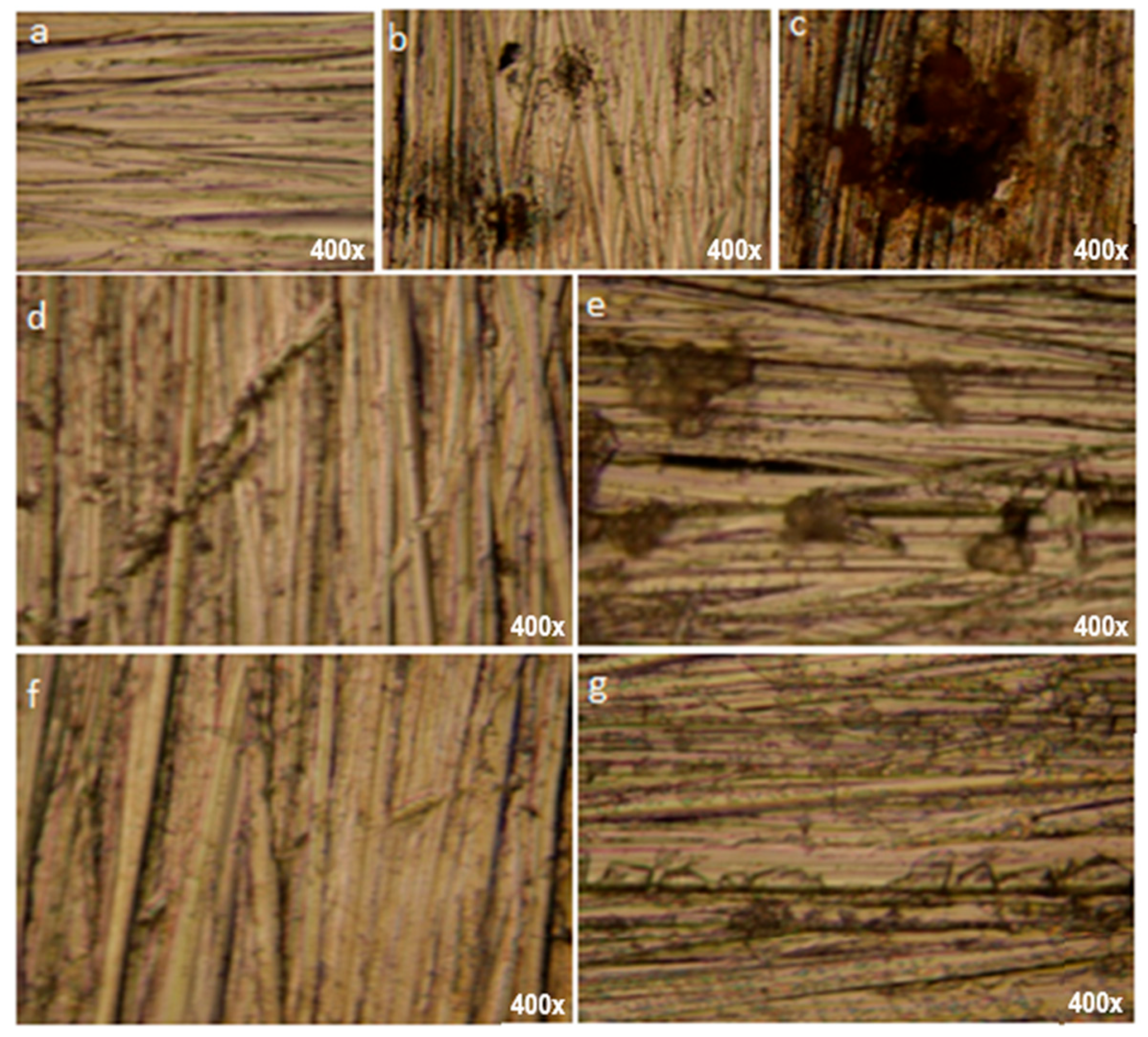
3.6.1. Optical Microscopy
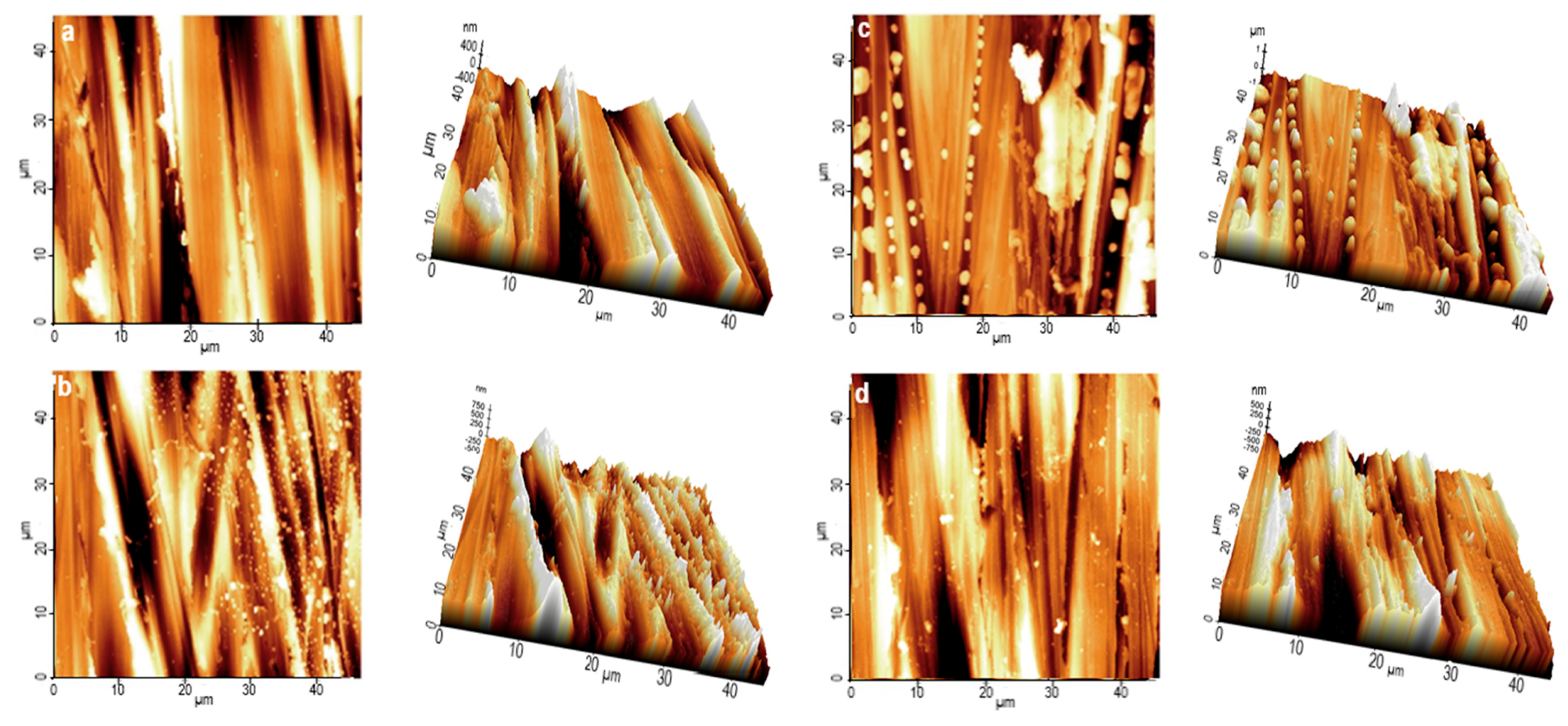
3.6.2. Atomic Force Microscopy (AFM)
3.7. HKER Action Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yihang, Z. Application of water-soluble polymer inhibitor in metal corrosion protection: Progress and challenges. Front. Energy Res. 2022, 10, 997107. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Yan, J.; Zhang, J.; Zhang, Q.; Yan, Y. Recent progress of polymeric corrosion inhibitor: Structure and application. Materials 2023, 16, 2954. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Rhee, K.Y. Aqueous phase polymeric corrosion inhibitors: Recent advancements and future opportunities. J. Mol. Liq. 2022, 348, 118387–118422. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors—Principles, mechanisms and applications. In Developments in Corrosion Protection; IntechOpen Limited: London, UK, 2014; Chapter 16; pp. 365–378. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Merisanu, C.; Cioatera, N. Thermal analysis: An effective characterization method of polyvinyl acetate films applied in corrosion inhibition field. J. Therm. Anal. Calorim. 2020, 142, 1825–1834. [Google Scholar] [CrossRef]
- Samide, A.; Iacobescu, G.I.; Tutunaru, B.; Iordache, S. Silver nanoparticles/polyvinyl alcohol film: Studies of thermal characterization, AFM and corrosion protection by electrodeposition on 304L stainless steel. J. Therm. Anal. Calorim. 2022, 14, 1041–1051. [Google Scholar] [CrossRef]
- Samide, A.; Stoean, C.; Stoean, R. Surface study of inhibitor films formed by polyvinyl alcohol and silver, nanoparticles on stainless steel in hydrochloric using convolutional neural networks. Appl. Surf. Sci. 2019, 475, 1–5. [Google Scholar] [CrossRef]
- Merisanu, C.; Samide, A.; Iacobescu, G.E.; Tutunaru, B.; Tigae, C.; Popescu, A. Anticorrosive performance of vinyl butyral-co-vinyl alcohol-co-vinyl acetate based copolymer adsorbed on steel surfaces. Electrochemical and AFM studies. Int. J. Electrochem. Sci. 2020, 15, 10197–10211. [Google Scholar] [CrossRef]
- Farahati, R.; Ghaffarinejad, A.; Rezania, H.J.; Mousavi-Khoshdel, S.M.; Behzadi, H. Sulfonated aromatic polyamide as water-soluble polymeric corrosion inhibitor of copper in HCl. Colloids Surf. A 2019, 578, 123626. [Google Scholar] [CrossRef]
- Omrani, A.; Rostami, A.A.; Sharifirad, M. Synthesize, Characterization, and corrosion inhibition of polypyrrole thin films on copper. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 513–521. [Google Scholar] [CrossRef]
- Mouaden, K.E.; Ibrahimi, B.E.; Oukhrib, R.; Bazzi, L.; Hammouti, B.; Jbara, O.; Tara, A.; Chauhan, D.S.; Quraishi, M.A. Chitosan polymer as a green corrosion inhibitor for copper in sulfide-containing synthetic seawater. Int. J. Biol. Macromol. 2018, 119, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Pan, C.; Li, Y.; Wang, F.H. Elucidation of mechanism of corrosion inhibition by polyacrylic acid and synergistic action with iodide ions by in-situ AFM. Adhes. Sci. Technol. 2014, 28, 31–37. [Google Scholar] [CrossRef]
- Yabuki, A.; Tanabe, S.; Fathona, I.W. Self-healing polymer coating with the microfibers of superabsorbent polymers provides corrosion inhibition in carbon steel. Surf. Coat. Technol. 2018, 341, 71–77. [Google Scholar] [CrossRef]
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.A.; Ahmad, Z.; Montemor, M.F.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 2019, 372, 121–133. [Google Scholar] [CrossRef]
- Kamran, M.; Shah, A.U.H.A.; Rahman, G.; Bilal, S. Potential impacts of prunus domestica based natural gum on physicochemical properties of polyaniline for corrosion inhibition of mild and stainless steel. Polymers 2022, 14, 3116. [Google Scholar] [CrossRef]
- Ma, Y.; Talha, M.; Wang, Q.; Li, Z.; Lin, Y. Evaluation of the corrosion behavior of AZ31 magnesium alloy with different protein concentrations. Anti-Corros. Methods Mater. 2021, 69, 47–54. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Asl, S.K. Casein as a natural protein to inhibit the corrosion of mild steel in HCl solution. J. Mol. Liq. 2019, 276, 694–704. [Google Scholar] [CrossRef]
- Zhang, Z.; Ba, H.; Wu, Z. Sustainable corrosion inhibitor for steel in simulated concrete pore solution by maize gluten meal extract: Electrochemical and adsorption behavior studies. Constr. Build. Mater. 2019, 227, 117080. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G. Corrosion inhibition properties of small peptides: DFT and Monte Carlo simulation studies, Corrosion inhibition properties of small peptides: DFT and Monte Carlo simulation studies. J. Mol. Liq. 2021, 331, 115782. [Google Scholar] [CrossRef]
- Simović, A.; Stevanović, S.; Milovanović, B.; Etinski, M.; Bajat, J.B. Green corrosion inhibitors of steel based on peptides and their constituents: A combination of experimental and computational approach. J. Solid State Electrochem. 2023, 27, 1821–1834. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Nazdracheva, T.; Kukharskii, A.; Yavna, V.; Kochur, A. Theoretical evaluation of the corrosion inhibition performance of aliphatic dipeptides. New J. Chem. 2021, 45, 3610–3629. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Hill, P.; Brantley, H.; Van Dyke, M. Some properties of keratin biomaterials: Kerateines. Biomaterials 2010, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.K.; Mija, A. Keratin associations with synthetic, biosynthetic and natural polymers: An extensive review. Polymers 2020, 12, 32. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.B.M.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Zehra, S.; Aslam, J. A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J. Mol. Liq. 2022, 364, 119992. [Google Scholar] [CrossRef]
- Joseph, G.; Mageshwaran, G.; Kumar, R.A.; Raj, R.D.; Jeevahan, J.; Kunjan, M. Characteristics studies of stainless steel (AISI Type 304l) welded by ER310l filler using tig welding. Int. J. Chem. Sci. 2016, 14, 2527–2534. [Google Scholar]
- AlHazaa, A.; Haneklaus, N. Diffusion bonding and transient liquid phase (TLP) bonding of type 304 and 316 austenitic stainless steel—A review of similar and dissimilar material join. Metals 2020, 10, 613. [Google Scholar] [CrossRef]
- Stanca, M.; Gaidau, C.; Constantinescu, R.; Trusca, R.; Stanculescu, I.; Berechet, D.; Alexe, C.; Enascuta, C.; Olariu, L.; Dumitriu, B. Preparation and characterization of bioactive micro and nano keratin particles. In Proceedings of the Innovative Aspects for Leather Industry (IAFLI) 2021, Izmir, Turkiye, 25–26 November 2021. [Google Scholar]
- ASTM-G3-89(2004); Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM-G5-94(2004); Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2004.
- Samide, A.; Dobritescu, A.; Tigae, C.; Spinu, C.I.; Oprea, B. Experimental and computational study on inhibitory effect and adsorption properties of n-acetylcysteine amino acid in acid environment. Molecules 2023, 28, 6799. [Google Scholar] [CrossRef]
- Haruna, K.; Saleh, T.A.; Obot, I.B.; Umoren, S.A. Cyclodextrin-based functionalized graphene oxide as an effective corrosion inhibitor for carbon steel in acidic environment. Prog. Org. Coat. 2019, 128, 157–167. [Google Scholar] [CrossRef]
- Tchoumene, R.; Dedzo, G.K.; Ngameni, E. Intercalation of 1,2,4-triazole in methanol modified-kaolinite: Application for copper corrosion inhibition in concentrated sodium chloride aqueous solution. J. Solid State Chem. 2022, 311, 123103. [Google Scholar] [CrossRef]
- Ma, I.A.W.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Mobin, M.; Basik, M.; Shoeb, M. A novel organic-inorganic hybrid complex based on cissus quadrangularis plant extract and zirconium acetate as a green inhibitor for mild steel in 1 M HCl solution. Appl. Surf. Sci. 2019, 469, 387–403. [Google Scholar] [CrossRef]
- Oubaaqa, M.; Ouakki, M.; Rbaa, M.; Abousalem, A.S.; Maatallah, M.; Benhiba, F.; Jarid, A.; Ebn Touhami, M.; Zarrouk, A. Insight into the corrosion inhibition of new amino-acids as efficient inhibitors for mild steel in HCl solution: Experimental studies and theoretical calculations. J. Mol. Liq. 2021, 334, 116520. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.-P.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Ghada, M.; El-Hafez, A.; Badawy, W.A. The use of cysteine, N-acetyl cysteine and methionine as environmentally friendly corrosion inhibitors for Cu–10Al–5Ni alloy in neutral chloride solutions. Electrochim. Acta 2013, 108, 860–866. [Google Scholar] [CrossRef]
- Mobin, M.; Zehra, S.; Parveen, M. L-Cysteine as corrosion inhibitor for mild steel in 1M HCl and synergistic effect of anionic, cationic and non-ionic surfactants. J. Mol. Liq. 2016, 216, 598–607. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Negrila, C.; Prunaru, I. Surface analysis of inhibitor film formed by 4-amino-N-(1,3-thiazol-2-yl) benzene sulfonamide on carbon steel surface in acidic media. Spectrosc. Lett. 2012, 45, 55–64. [Google Scholar] [CrossRef]
- Mohsenifar, F.; Jafari, H.; Sayin, K. Investigation of thermodynamic parameters for steel corrosion in acidic solution in the presence of n,n′-bis(phloroacetophenone)-1,2 propanediamine. J. Bio Tribo Corros. 2016, 2, 1. [Google Scholar] [CrossRef]
- Ituen, E.B.; Akaranta, O.; Umoren, S.A. N-acetyl cysteine based corrosion inhibitor formulations for steel protection in 15% HCl solution. J. Mol. Liq. 2017, 246, 112–118. [Google Scholar] [CrossRef]
- Samide, A.; Iacobescu, G.E.; Tutunaru, B.; Grecu, R.; Tigae, C.; Spînu, C. Inhibitory properties of neomycin thin film formed on carbon steel in sulfuric acid solution: Electrochemical and AFM investigation. Coatings 2017, 7, 181. [Google Scholar] [CrossRef]
- Hegde, M.; Nayak, S.P. Aqueous extract of Dillenia Pentagyna fruit as green inhibitor for mild steel corrosion in 0.5 M hydrochloric acid solution. J. Mater. Environ. Sci. 2019, 10, 22–31. [Google Scholar]
- Odejobi Oludare, J.; Akinbulumo, O.A. Modeling and optimization of the inhibition efficiency of Euphorbia heterophylla extracts based corrosion inhibitor of mild steel corrosion in HCl media using a response surface methodology. J. Chem. Technol. Metall. 2019, 54, 217–232. [Google Scholar]
- Hamdy, A.; El-Gen, N.S. Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt. J. Pet. 2013, 22, 17–25. [Google Scholar] [CrossRef]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Ntibi, J.E.; Okafor, P.C.; Ita, B.I.; Agobi, A.U.; Asogwa, F.C.; Omang, B.J.; Eno, E.A.; Loius, H.; Adalikwu, S.A.; et al. Kinetic and thermodynamic evaluation of azithromycin as a green corrosion inhibitor during acid cleaning process of mild steel using an experimental and theoretical approach. Results Chem. 2023, 5, 100909. [Google Scholar] [CrossRef]
- Valbon, A.; Neubi, F.; Xavier, N.F., Jr.; Carlos, M.F.L.P.; Bauerfeldt, G.F.; Francisco, W.Q.; Almeida-Neto, F.W.Q.; de Lima-Neto, P.; Neves, M.A.; Rodrigues-Santos, C.E.; et al. The corrosion inhibition performance of eco-friendly bis-schiff bases on carbon steel in a hydrochloric solution. Surfaces 2023, 6, 509–532. [Google Scholar] [CrossRef]
- Samide, A.; Ilea, P.; Vladu, A.C. Metronidazole performance as corrosion inhibitor for carbon steel, 304l stainless steel and aluminum in hydrochloric acid solution. Int. J. Electrochem. Sci. 2017, 12, 5964–5983. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.O.; Obot, I.B. Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corrosion in H2SO4. Corros. Sci. 2011, 53, 263–275. [Google Scholar] [CrossRef]
- Gobara, M.; Saleh, A.; Naeem, I. Synthesis, characterization and application of acrylate-based poly ionic liquid for corrosion protection of C1020 steel In hydrochloric acid solution. Mater. Res. Express 2020, 7, 016517. [Google Scholar] [CrossRef]
- Kokalj, A. On the use of the Langmuir and other adsorption isotherms in corrosion inhibition. Corros. Sci. 2023, 217, 111112. [Google Scholar] [CrossRef]
- Habibullah, M.I.; Veawab, A. Cysteine as an alternative eco-friendly corrosion inhibitor for absorption-based carbon capture plants. Materials 2023, 16, 3496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, A.; Zhou, Z.; Wang, O.; Wang, X.; Ma, H.; Zhou, C. Study of okra pectin prepared by sweeping frequency ultrasound/freeze-thaw pretreatment on corrosion inhibition of ANSI 304 stainless steel in acidic environment. Int. J. Biol. Macromol. 2023, 253 Pt 1, 126587. [Google Scholar] [CrossRef] [PubMed]
- Satpati, S.; Suhasaria, A.; Ghosal, S.; Dey, S.; Sukul, D. Interaction of newly synthesized dipeptide Schiff bases with mild steel surface in aqueous HCl: Experimental and theoretical study on thermodynamics, adsorption and anti-corrosion characteristics. Mater. Chem. Phys. 2023, 296, 127200. [Google Scholar] [CrossRef]
- Vashishth, P.; Bairagi, H.; Narang, R.; Shukla, S.K.; Olasunkanmi, L.O.; Ebenso, E.E.; Mangla, B. Experimental investigation of sustainable Corrosion Inhibitor Albumin on low-carbon steel in 1N HCl and 1N H2SO4. Results Surf. Interfaces 2023, 13, 100155. [Google Scholar] [CrossRef]
- Geetha, M.B.; Rajendran, B.S. Synergistic inhibition of corrosion of mild steel in sulphuric acid by new ternary system. Der Pharma Chem. 2016, 8, 194–201. [Google Scholar]
- Jafari, H.; Akbarzade, K.; Danaee, I. Corrosion inhibition of carbon steel immersed in a 1 M HCl solution using benzothiazole derivatives. Arab. J. Chem. 2019, 12, 1387–1394. [Google Scholar] [CrossRef]
- Jasim, E.Q.; Mohammed-Ali, M.A.; Hussain, A.A. Investigation of Salvadora persica roots extract as corrosion inhibitor for mild steel in 1 M HCl and in cooling water. Chem. Mater. Res. 2015, 7, 147–158. Available online: https://iiste.org/Journals/index.php/CMR/article/view/21642/21872 (accessed on 30 November 2023).

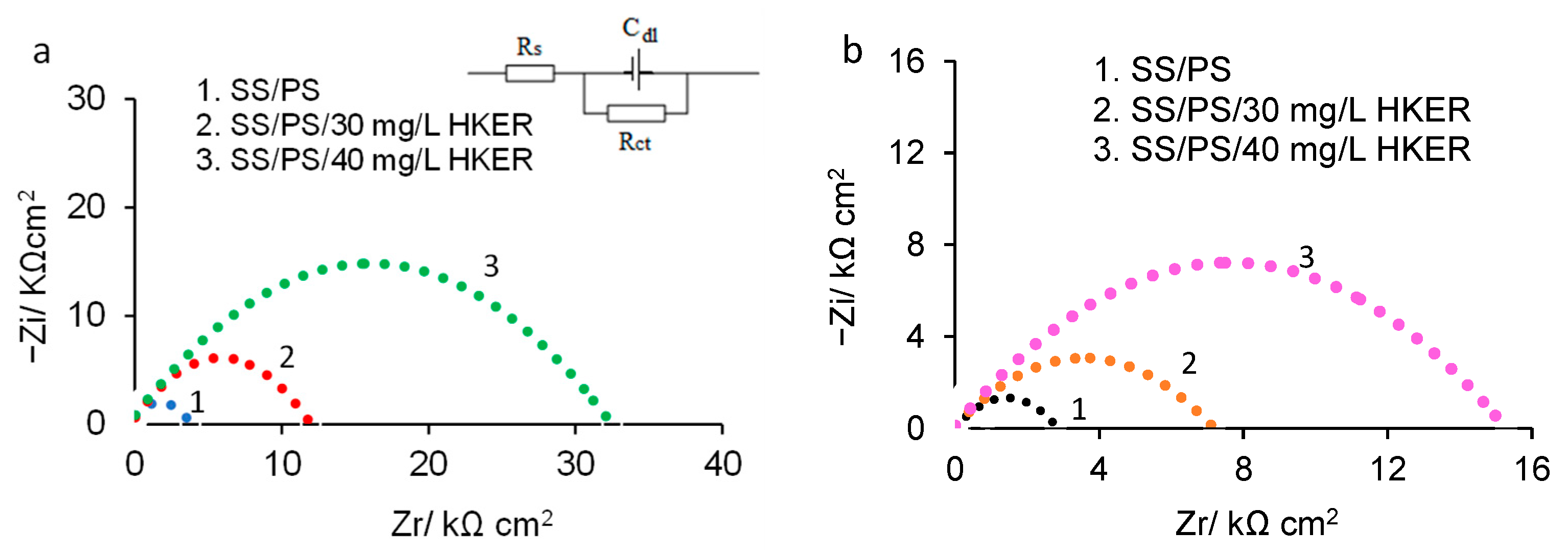

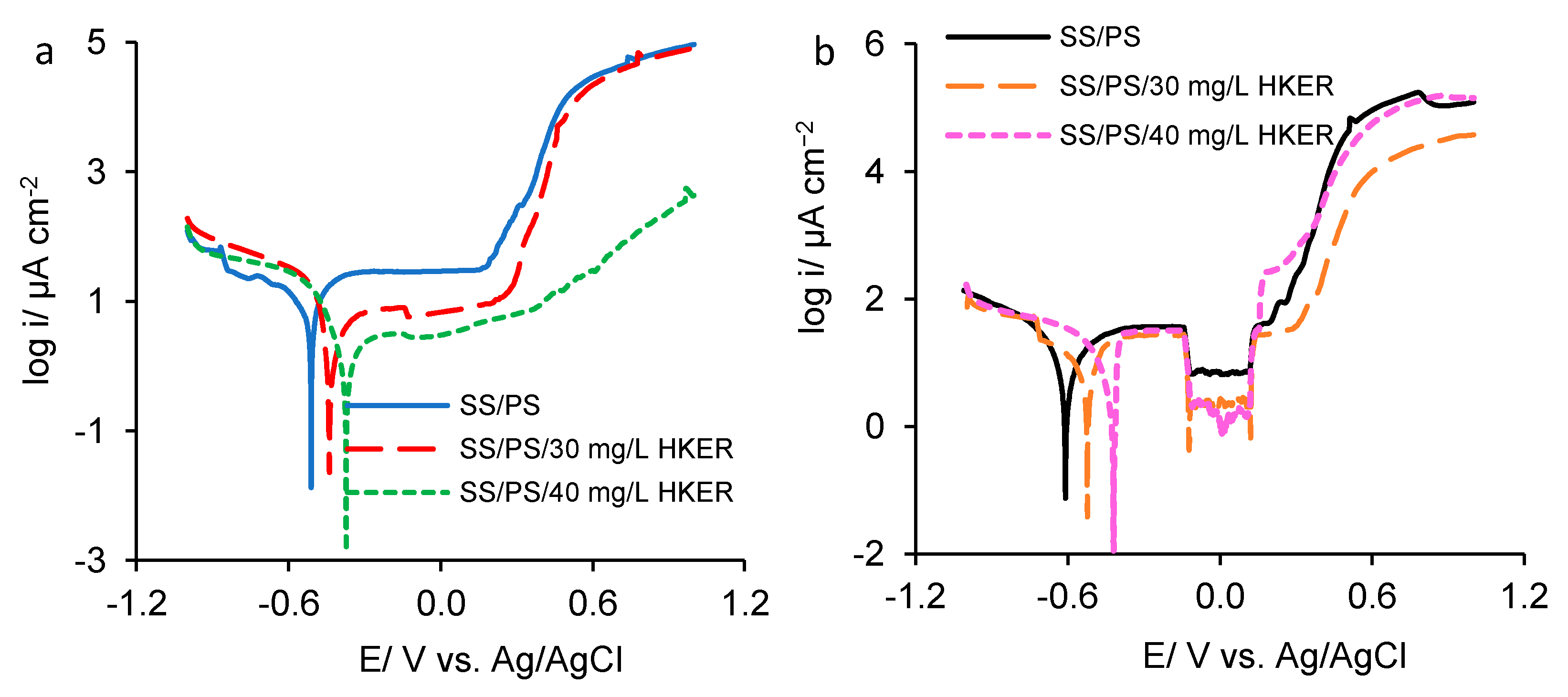
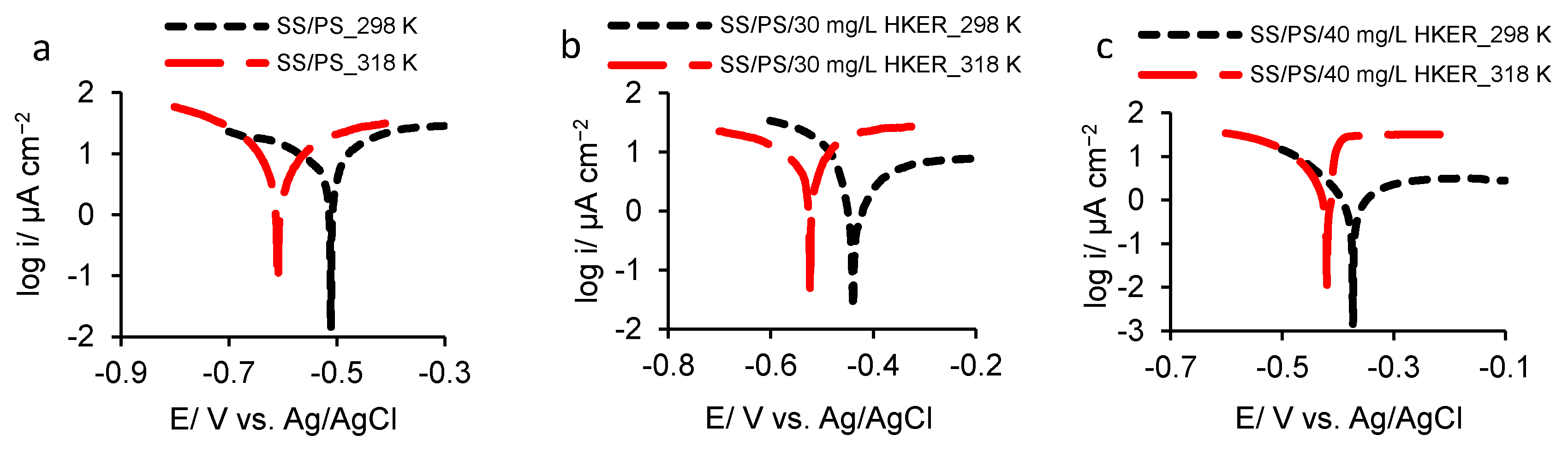
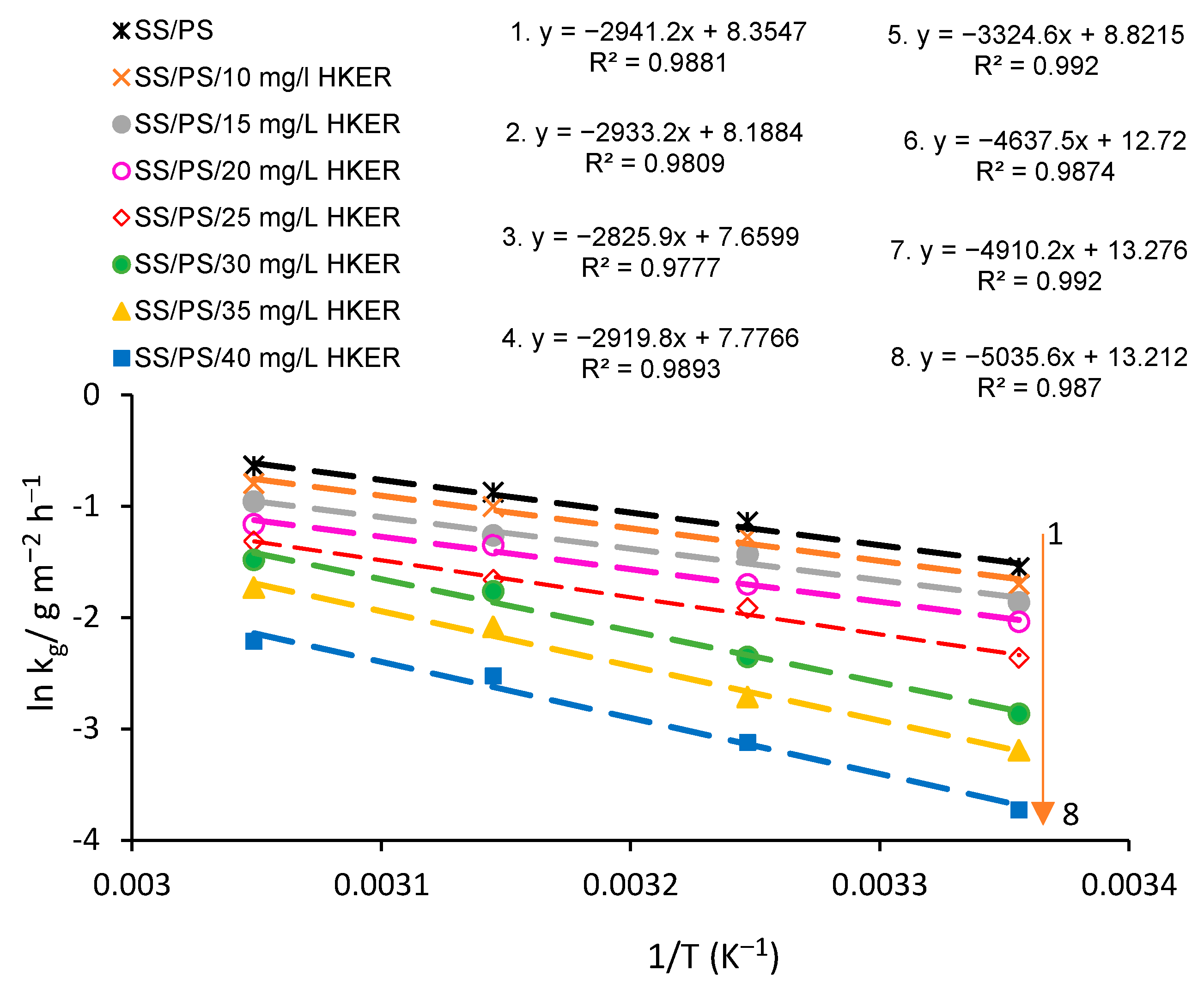

| Sample | OCP/mV vs. Ag/AgCl | 25 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| Nyquist Parameters | Bode Parameters | IE/ % | ||||||
| Rs/ Ω cm2 | Rct/ kΩ cm2 | Cdl/ μF cm−2 | log Z/ Ω cm2 | Z/ kΩ cm2 | Phase/ Degrees | |||
| SS/PS | −209.3 ± 15.6 | 256.7 ± 25.3 | 4.1 ± 1.2 | 363.7 ± 19.3 | 3.63 ± 0.01 | 4.27 ± 0.1 | −79.2 ± 2.3 | - |
| SS/PS/30 mg L−1 HKER | −177.1 ± 12.2 | 234.5 ± 24.6 | 13.5 ± 3.6 | 247.2 ± 13.6 | 4.15 ± 0.01 | 14.12 ± 0.32 | −79.03 ± 1.9 | 69.6 ± 0.9 |
| SS/PS/40 mg L−1 HKER | −89.8 ± 10.8 | 222.9 ± 23.8 | 32.1 ± 6.8 | 213.8 ± 13.2 | 4.53 ± 0.01 | 33.8 ± 0.78 | −79.64 ± 1.9 | 87.2 ± 0.8 |
| Sample | OCP/mV vs. Ag/AgCl | 45 °C | ||||||
| Nyquist Parameters | Bode Parameters | IE/ % | ||||||
| Rs/ Ω cm2 | Rct/ kΩ cm2 | Cdl/ μF cm−2 | log Z/ Ω cm2 | Z/ kΩ cm2 | Phase/ Degrees | |||
| SS/PS | −232 ± 22 | 326.4 ± 28.1 | 2.8 ± 0.8 | 394.5 ± 37.1 | 3.47 ± 0.01 | 2.95 ± 0.07 | −72.69 ± 4.1 | - |
| SS/PS/30 mg L−1 HKER | −201 ± 18 | 296.7 ± 25.4 | 7.2 ± 2.0 | 312.8 ± 16.1 | 3.87 ± 0.01 | 7.41 ± 0.18 | −80.19 ± 1.8 | 61.1 ± 0.4 |
| SS/PS/40 mg L−1 HKER | −120 ± 13 | 267.2 ± 24.2 | 15.3 ± 3.8 | 292.6 ± 15.7 | 4.19 ± 0.01 | 15.48 ± 0.36 | −79.76 ± 1.8 | 81.7 ± 0.6 |
| Sample | 25 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrochemical Parameters | Corrosion Rate | IE/% | IEm/ % | ||||||
| Ecorr/mV vs./AgCl | icorr/ μA cm−2 | Rp/ kΩ cm2 | kg/ g m−2 h−1 | CR/ μm Year−1 | From Equation (12) | From Equation (13) | From Equation (14) | ||
| SS/PS | −511 ± 15 | 19.95 ± 3.2 | 3.3 ± 0.5 | 0.211 | 237 ± 37 | - | - | - | |
| SS/PS/30 mg L−1 HKER | −439 ± 15 | 5.37 ± 0.9 | 11.9 ± 2.1 | 0.057 | 65 ± 10.5 | 73.1 ± 0.2 | 72.6 ± 0.2 | 72.3 ± 0.6 | 72.7 ± 0.3 |
| SS/PS/40 mg L−1 HKER | −373 ± 15 | 2.24 ± 0.7 | 27.8 ± 2.3 | 0.024 | 27 ± 8.2 | 88.7 ± 0.3 | 88.7 ± 0.5 | 88.1 ± 0.8 | 88.5 ± 0.5 |
| Sample | 45 °C | ||||||||
| Electrochemical Parameters | Corrosion Rate | IE/% | IEm/ % | ||||||
| Ecorr/mV vs./AgCl | icorr/ μA cm−2 | Rp/ kΩ cm2 | kg/ g m−2 h−1 | CR/ μmY−1 | From Equation (12) | From Equation (13) | From Equation (14) | ||
| SS/PS | −609 ± 28 | 39.8 ± 5.4 | 2.4 ± 0.4 | 0.415 | 462 ± 62.9 | - | - | - | - |
| SS/PS/30 mg L−1 HKER | −523 ± 28 | 16.4 ± 2.8 | 5.8 ± 0.9 | 0.171 | 190 ± 32.6 | 58.8 ± 1.3 | 58.9 ± 1.4 | 58.6 ± 0.6 | 58.8 ± 1.1 |
| SS/PS/40 mg L−1 HKER | −420 ± 28 | 8.1 ± 1.3 | 12.9 ± 1.8 | 0.08 | 89 ± 15.1 | 79.6 ± 0.5 | 80.7 ± 0.6 | 81.4 ± 0.5 | 80.6 ± 0.5 |
| Sample | CR/μm Year−1 | kg/g m−2 h−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 55 °C | 25 °C | 35 °C | 45 °C | 55 °C | |
| SS/PS/ | 237 | 356 | 462 | 583 | 0.211 | 0.319 | 0.415 | 0.523 |
| SS/PS/10 mg L−1 HKER | 203 | 311 | 408 | 501 | 0.182 | 0.279 | 0.366 | 0.450 |
| SS/PS/15 mg L−1 HKER | 173 | 265 | 315 | 426 | 0.155 | 0.238 | 0.282 | 0.383 |
| SS/PS/20 mg L−1 HKER | 145 | 203 | 289 | 347 | 0.130 | 0.182 | 0.259 | 0.312 |
| SS/PS/25 mg L−1 HKER | 105 | 164 | 232 | 298 | 0.094 | 0.147 | 0.190 | 0.268 |
| SS/PS/30 mg L−1 HKER | 65 | 106 | 190 | 253 | 0.057 | 0.095 | 0.171 | 0.227 |
| SS/PS/35 mg L−1 HKER | 46 | 73 | 138 | 197 | 0.041 | 0.066 | 0.124 | 0.177 |
| SS/PS/40 mg L−1 HKER | 27 | 49 | 89 | 141 | 0.024 | 0.044 | 0.08 | 0.109 |
| Sample | Ea/kJ mol−1 | lnA = y (x = 0) | A/g m−2 h−1 A = ey(x=0) | ΔHa/kJ mol−1 | Ea − ΔHa | ΔSa/J mol−1 K−1 |
|---|---|---|---|---|---|---|
| SS/PS | 24.44 | 8.3547 | 4250.1 | 21.91 | 2.53 | −183.91 |
| SS/PS/10 mg L−1 HKER | 24.37 | 8.1884 | 3598.9 | - | - | - |
| SS/PS/15 mg L−1 HKER | 24.31 | 7.6599 | 2121.5 | - | - | - |
| SS/PS/20 mg L−1 HKER | 24.26 | 7.7766 | 2384.2 | - | - | - |
| SS/PS/25 mg L−1 HKER | 27.62 | 8.8215 | 6778.4 | 24.97 | 2.65 | −180.34 |
| SS/PS/30 mg L−1 HKER | 38.53 | 12.720 | 334,368.8 | 36.07 | 2.46 | −147.34 |
| SS/PS/35 mg L−1 HKER | 40.80 | 13.276 | 583,033.5 | 38.21 | 2.59 | −143.15 |
| SS/PS/40 mg L−1 HKER | 41.84 | 13.212 | 546,888.4 | 39.35 | 2.49 | −143.33 |
| Sample | Surface Coverage Degree (θ) | |||
|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 55 °C | |
| SS/PS | - | - | - | - |
| SS/PS/10 mg L−1 HKER | 0.143 | 0.126 | 0.116 | 0.140 |
| SS/PS/15 mg L−1 HKER | 0.270 | 0.255 | 0.318 | 0.269 |
| SS/PS/20 mg L−1 HKER | 0.388 | 0.429 | 0.374 | 0.404 |
| SS/PS/25 mg L−1 HKER | 0.557 | 0.539 | 0.497 | 0.488 |
| SS/PS/30 mg L−1 HKER | 0.725 | 0.685 | 0.588 | 0.566 |
| SS/PS/35 mg L−1 HKER | 0.806 | 0.745 | 0.701 | 0.660 |
| SS/PS/40 mg L−1 HKER | 0.886 | 0.862 | 0.807 | 0.758 |
| T/K | From Temkin Adsorption Isotherm | |||||||
|---|---|---|---|---|---|---|---|---|
| f | R2 | Kads/ L g−1 | / kJ mol−1 | /kJ mol−1 | /J mol−1K−1 | |||
| From Figure 10a | From Figure 10b | From Figure 10a | From Figure 10b | |||||
| 298 | 1.53 | 0.988 | 97.51 | −28.5 | −6.34 | −6.726 | 74.2 | 73 |
| 308 | 1.45 | 0.9792 | 89.12 | −29.2 | −6.34 | −6.726 | 74.2 | 73 |
| 318 | 1.5 | 0.9904 | 82.27 | −29.9 | −6.34 | −6.726 | 74.2 | 73 |
| 328 | 1.51 | 0.9996 | 77.48 | −30.7 | −6.34 | −6.726 | 74.2 | 73 |
| T/K | From Awady’s Adsorption Model | |||||||
| y | R2 | Kads/ L g−1 | / kJ mol−1 | /kJ mol−1 | /J mol−1K−1 | |||
| From Figure 10a | From Figure 10b | From Figure 10a | From Figure 10b | |||||
| 298 | 3.38 | 0.9986 | 43.71 | −26.5 | −5.2 | −4.726 | 71.4 | 73 |
| 308 | 3.63 | 0.9995 | 41.71 | −27.2 | −5.2 | −4.726 | 71.4 | 73 |
| 318 | 3.39 | 0.9889 | 37.57 | −27.9 | −5.2 | −4.726 | 71.4 | 73 |
| 328 | 2.93 | 0.996 | 36.58 | −28.7 | −5.2 | −4.726 | 71.4 | 73 |
| Sample | Rp−v/nm | Rq/nm | Ra/nm |
|---|---|---|---|
| SS standard | 845 | 125 | 90 |
| SS/PS blank | 1318 | 281 | 198 |
| SS/PS/30 mg L−1 KERH | 1047 | 156 | 117 |
| SS/PS/40 mg L−1 KERH | 948 | 149 | 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samide, A.; Iacobescu, G.E.; Tutunaru, B.; Tigae, C.; Spînu, C.I.; Oprea, B. New Inhibitor Based on Hydrolyzed Keratin Peptides for Stainless Steel Corrosion in Physiological Serum: An Electrochemical and Thermodynamic Study. Polymers 2024, 16, 669. https://doi.org/10.3390/polym16050669
Samide A, Iacobescu GE, Tutunaru B, Tigae C, Spînu CI, Oprea B. New Inhibitor Based on Hydrolyzed Keratin Peptides for Stainless Steel Corrosion in Physiological Serum: An Electrochemical and Thermodynamic Study. Polymers. 2024; 16(5):669. https://doi.org/10.3390/polym16050669
Chicago/Turabian StyleSamide, Adriana, Gabriela Eugenia Iacobescu, Bogdan Tutunaru, Cristian Tigae, Cezar Ionuţ Spînu, and Bogdan Oprea. 2024. "New Inhibitor Based on Hydrolyzed Keratin Peptides for Stainless Steel Corrosion in Physiological Serum: An Electrochemical and Thermodynamic Study" Polymers 16, no. 5: 669. https://doi.org/10.3390/polym16050669
APA StyleSamide, A., Iacobescu, G. E., Tutunaru, B., Tigae, C., Spînu, C. I., & Oprea, B. (2024). New Inhibitor Based on Hydrolyzed Keratin Peptides for Stainless Steel Corrosion in Physiological Serum: An Electrochemical and Thermodynamic Study. Polymers, 16(5), 669. https://doi.org/10.3390/polym16050669






