Two-Level Self-Thickening Mechanism of a Novel Acid Thickener with a Hydrophobic-Associated Structure during High-Temperature Acidification Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Synthesis of ADMC and ADOM
2.4. Characterization and Performance Testing
2.4.1. Characterization
2.4.2. Rheological Properties
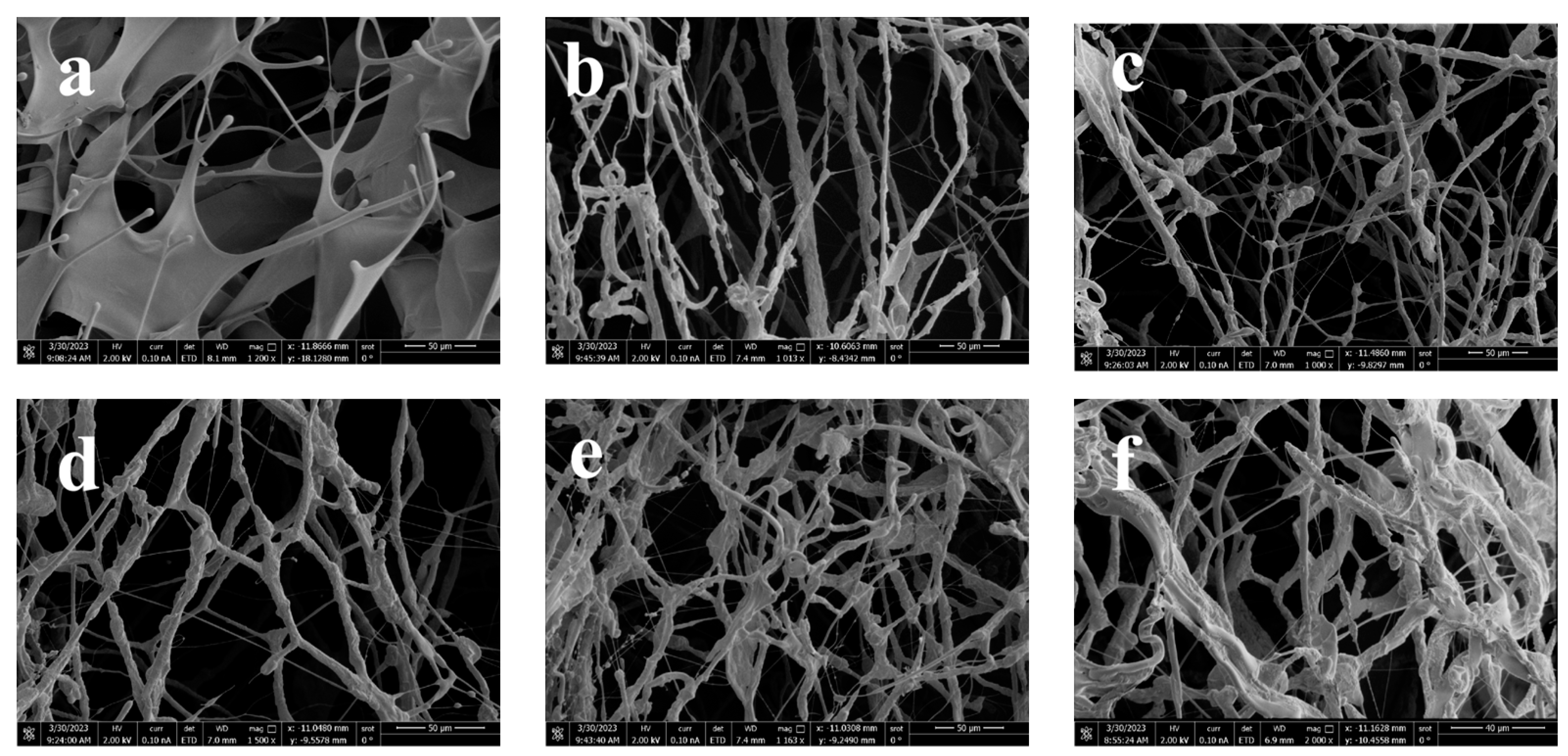
2.4.3. Measurement of Environmental Scanning Electron Microscope
3. Results and Discussion
3.1. Characterization of Polymers
3.1.1. FT-IR
3.1.2. Surface and Interfacial Tension Tests
3.1.3. Fluorescence Spectrum Test of Pyrene
3.1.4. 1H NMR
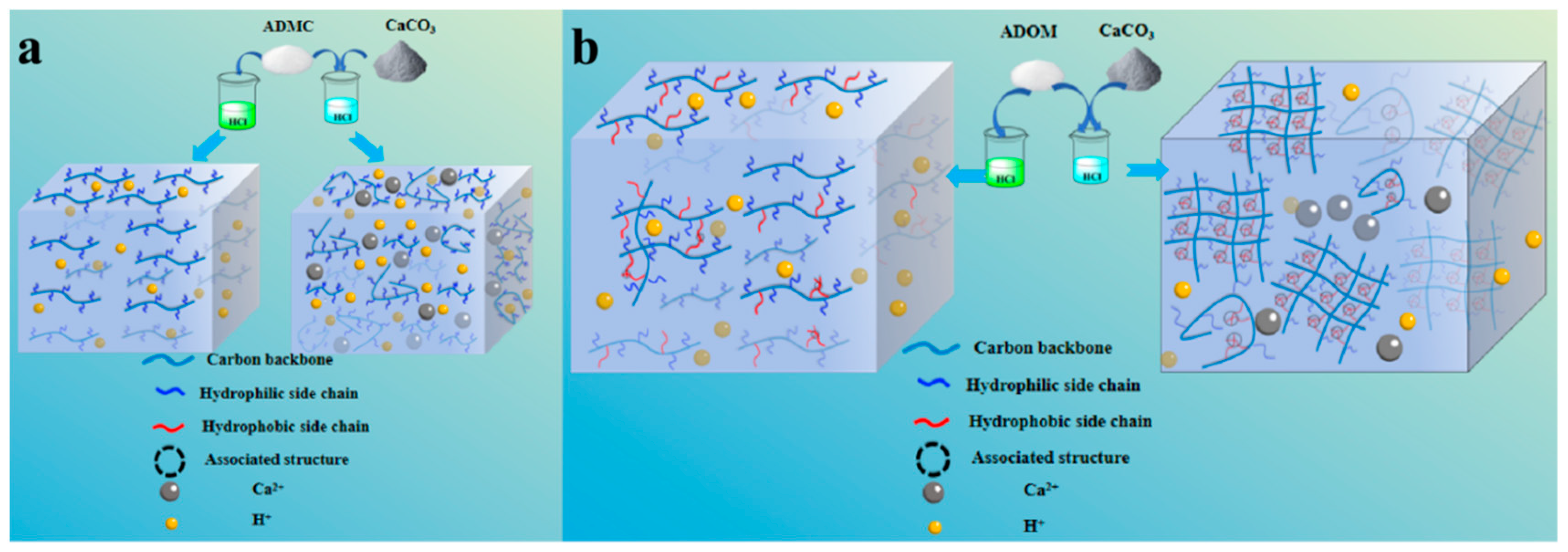
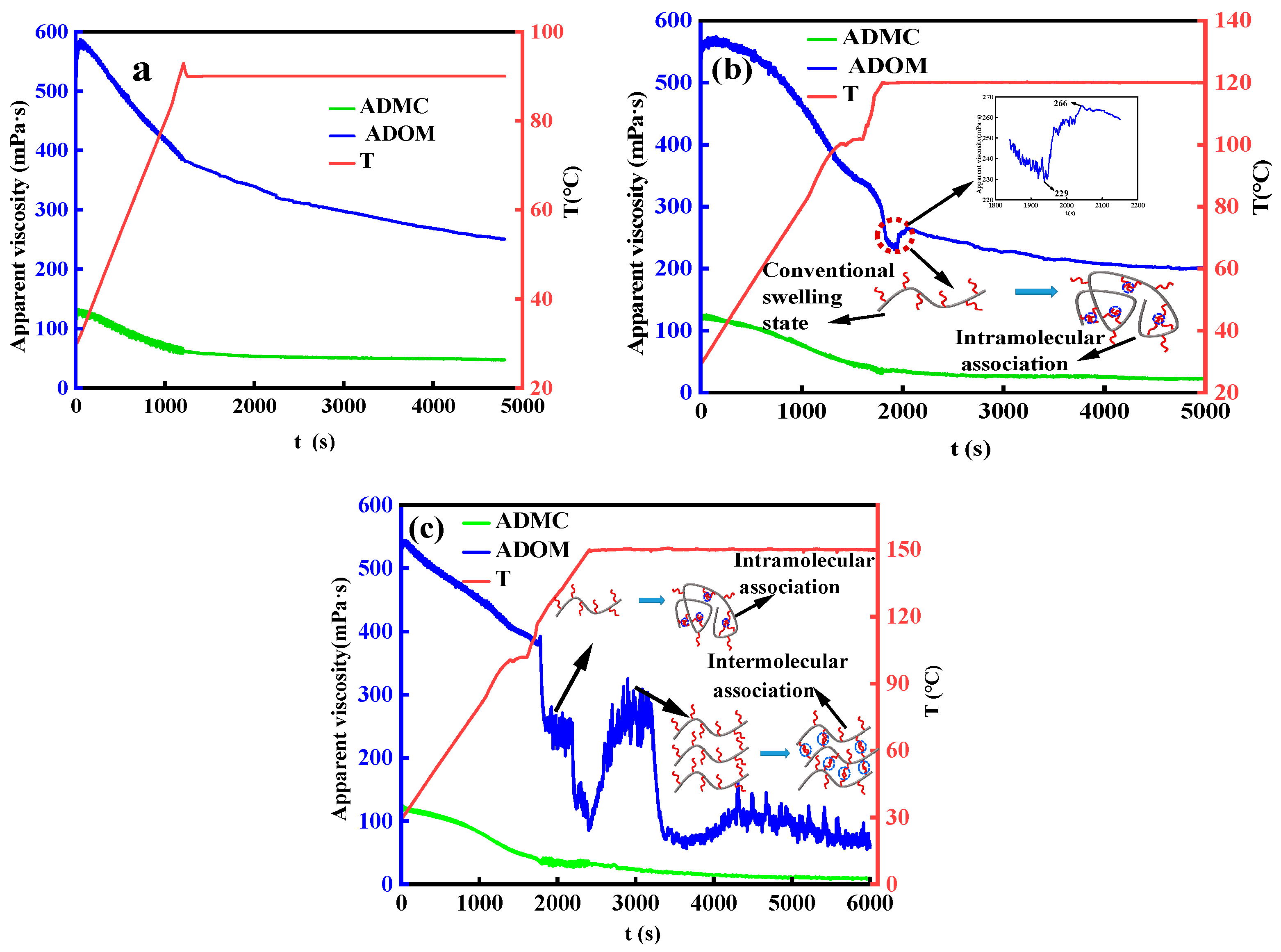
3.2. Study on Thickening Mechanism of ADMC and ADOM Acid Solution
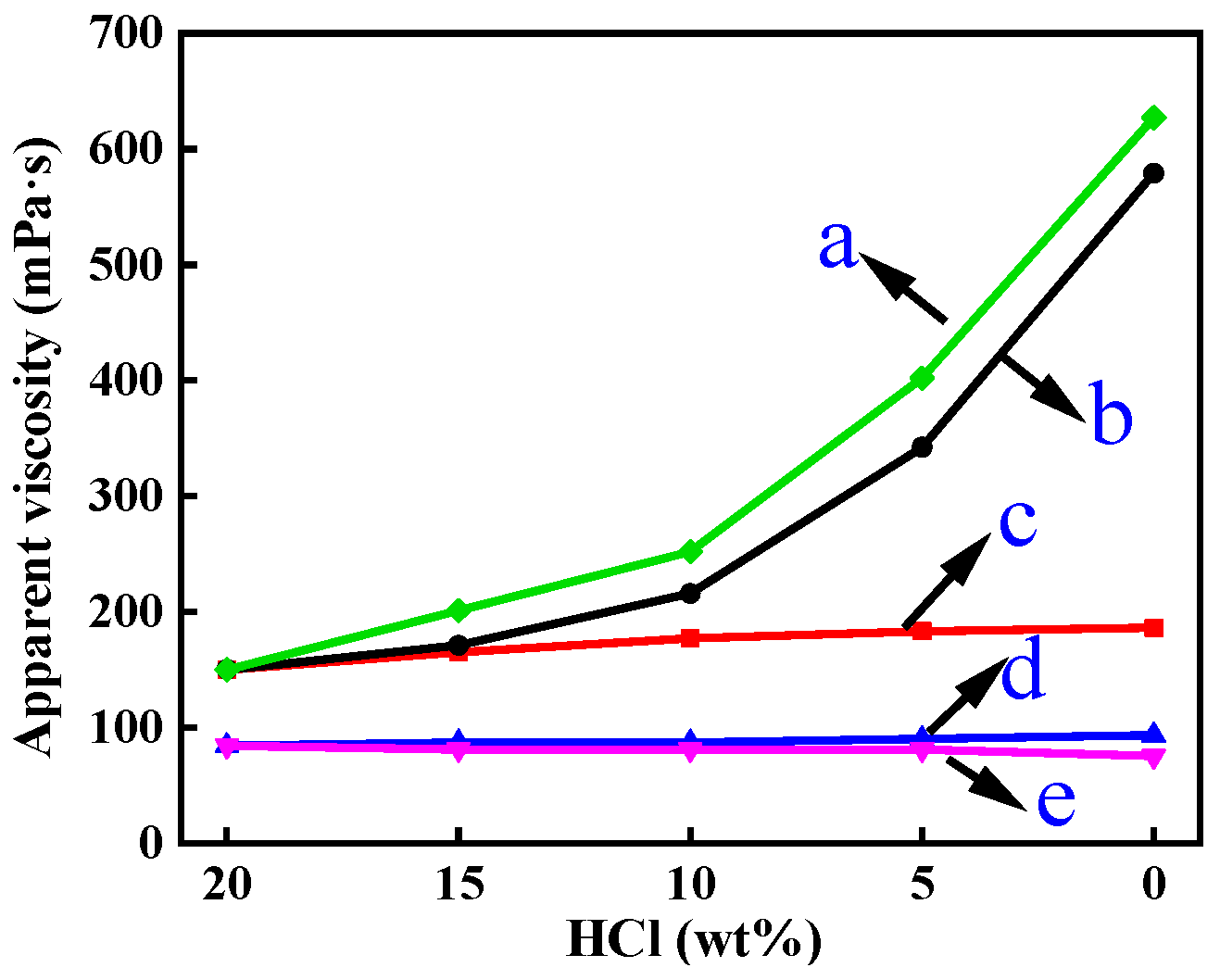
3.2.1. Effect of Salt
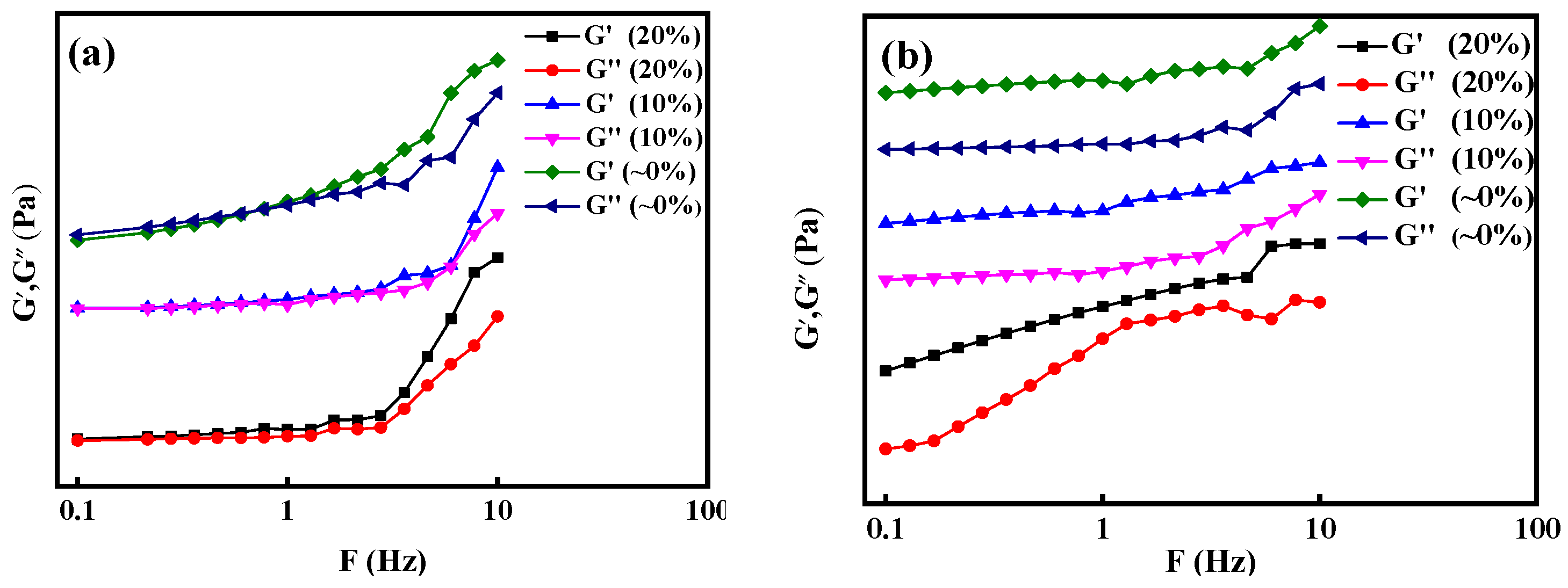
3.2.2. Effect of Temperature
3.2.3. Formula of rheology behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islamov, S.R.; Bondarenko, A.V.; Gabibov, A.F.; Mardashov, D.V. Polymer compositions for well killing operation in fractured reservoirs. In Advances in Raw Material Industries for Sustainable Development Goals; CPC Press: Boca Raton, FL, USA, 2021; pp. 343–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.; Mao, J.C.; Qin, S.-H.; Li, J.; Yang, X.-J.; Lin, C.; Huang, Z.-Y.; Liu, Y.-F. Preparation of a functional fracturing fluid with temperature-and salt-resistance, and low damage using a double crosslinking network. Pet. Sci. 2023, 20, 3223–3230. [Google Scholar] [CrossRef]
- Islamov, S.R.; Bondarenko, A.V.; Mardashov, D.V. Substantiation of a well killing technology for fractured carbonate reservoirs. In Proceedings of the Youth Technical Sessions Proceedings: VI Youth Forum of the World Petroleum Council–Future Leaders Forum, London, UK, 23–28 June 2019; Taylor & Francis: New York, NY, USA, 2019; pp. 256–264. [Google Scholar] [CrossRef]
- Guo, B.; Liu, X.; Tan, X. Petroleum Production Engineering; Gulf Professional Publishing: Woburn, MA, USA, 2017. [Google Scholar]
- Zhao, G.; Dai, C.L.; Chen, A.; Yan, Z.; Zhao, M. Experimental study and application of gels formed by nonionic polyacrylamide and phenolic resin for in-depth profile control. J. Pet. Sci. Eng. 2015, 135, 552–560. [Google Scholar] [CrossRef]
- Jain, R.; Mahto, V. Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J. Pet. Sci. Eng. 2015, 133, 612–621. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, C.J.; Gu, T.; Li, X.; Shi, Y. Cucurbit[7]uril-modified intelligent polymer as acid thickening agent for unconventional reservoir recovery. J. Pet. Sci. Eng. 2017, 149, 65–74. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Kim, K.; Yang, S.-T.; Kima, J.-I.; Jon, S. A rationally designed macrocyclic cavitand that kills bacteria with high efficacy and good selectivity. Chem. Commun. 2007, 11, 1151–1153. [Google Scholar] [CrossRef]
- Guo, B.; Li, Z.; Guo, J.C.; Zhang, R.; Zhou, C.; Wu, L.; Ye, J.; Zeng, J. Effect of different types of stimulation fluids on fracture propagation behavior in naturally fractured carbonate rock through CT scan. J. Pet. Sci. Eng. 2021, 201, 108529. [Google Scholar] [CrossRef]
- Lynn, J.D.; Nasr-El-Din, H.A. A Core Based Comparison of The Reaction Characteristics of Emulsified And In-Situ Gelled Acids In Low Permeability, High Temperature, Gas Bearing Carbonates. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Mohammad, A.M.; Al-Shurei, A.A.; Merwat, N.K.; Erbil, M.M.; Samuel, M. Restoring the injectivity of water disposal wells using a viscoelastic surfactant-based acid. J. Pet. Sci. Eng. 2006, 54, 10–24. [Google Scholar] [CrossRef]
- Neto, A.; Silva, C.; Torres, R.S.; Farias, R.L.; Prata, F.G.; Souza, L.A.; Pereira, A.Z.; Calderon, A.; Sandes, E.F. Self-Diverting Acid for Effective Carbonate Stimulation Offshore Brazil: A Successful History. In Proceedings of the SPE European Formation Damage Conference & Exhibition, Noordwijk, The Netherlands, 5–7 June 2013. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y. Synthesis and performance of temperature-and acid-resistant ternary-copolymer thickener. Mater. Chem. Phys. 2022, 292, 126866. [Google Scholar] [CrossRef]
- Guo, B.; Zeng, M.Y.; Wang, K.J.; Li, X.; Guo, J. Effect of fiber on the rheological properties of gelled acid. J. Pet. Explor. Prod. Technol. 2021, 11, 2207–2215. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, C.; Li, W.; Yan, Z.; Zhao, M. Research on a temporary plugging agent based on polymer gel for reservoir acidification. J. Pet. Explor. Prod. Technol. 2016, 6, 465–472. [Google Scholar] [CrossRef]
- Qiao, M.J.; Cao, G.S.; Gao, Y.J.; Ge, L. The Research and Application of Injection Wells Acidification with Temporary Plugging Agent. Appl. Mech. Mater. 2014, 535, 807–810. [Google Scholar] [CrossRef]
- Tian, H.; Quan, H.; Huang, Z.; Duan, W.; Deng, S. Polymeric and non-crosslinked acid self-thickening agent based on hydrophobically associating water-soluble polymer during the acid rock reaction. J. Appl. Polym. Sci. 2019, 136, 47907. [Google Scholar] [CrossRef]
- Quan, H.; Zhen, X.; Wu, Y.; Duan, W. Effect of non-crosslinked polymer (ADDA) on acid-rock reactions: Synthesis and thickening laws. J. Polym. Res. 2022, 29, 160. [Google Scholar] [CrossRef]
- Ge, Y.R.; Zhao, Z.C.; Cheng, X.L.; Chen, T.; Liu, T.; Guo, X. Research of a novel double cross-linking fracturing fluid. J. Pet. Explor. Prod. Technol. 2021, 11, 2191–2197. [Google Scholar] [CrossRef]
- Tang, W.Y.; Zou, C.J.; Peng, H.; Wang, Y.; Shi, L. Influence of Nanoparticles and Surfactants on Stability and Rheological Behavior of Polymeric Nanofluids and the Potential Applications in Fracturing Fluids. Energy Fuels 2021, 35, 8657–8671. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Yang, B.; Mao, J.C.; Zhang, Y.; Yang, X.; Zhang, Z.; Shao, Y. A Novel Hydrophobic Associative Polymer by RAFT-MADIX Copolymerization for Fracturing Fluids with High Thermal Stability. Energy Fuels 2018, 32, 3039–3051. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, S.; Chen, Y.; Chen, H.; Wang, M.; Tan, Y. Salt endurable and shear resistant polymer systems based on dynamically reversible acyl hydrazone bond. J. Mol. Liq. 2022, 346, 117083. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, H.; Liu, M.; Grage, S.L.; Hoffmann, M.; Sedghamiz, E.; Wenzel, W.; Levkin, P.A. Tough, transparent, 3D-printable, and self-healing poly (ethylene glycol)-gel (PEGgel). Adv. Mater. 2022, 34, 2107791. [Google Scholar] [CrossRef]
- Patel, V.; Trivedi, J.; Sharma, T. Influence of hydrophobic association in the aqueous media on the rheology and polymer conformation of associative polymers. Polym. Bull. 2023, 80, 8939–8959. [Google Scholar] [CrossRef]
- Yang, E. The Synthesis of Associative Copolymers with Both Amphoteric and Hydrophobic Groups and the Effect of the Degree of Association on the Instability of Emulsions. Polymers 2021, 13, 4041. [Google Scholar] [CrossRef]
- Feng, Y.; Billon, L.; Grassl, B.; Khoukh, A.; François, J. Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification. 1. Synthesis and characterization. Polymers 2002, 43, 2055–2064. [Google Scholar] [CrossRef]
- Abdollahipour, A.; Marji, M.F. A thermo-hydromechanical displacement discontinuity method to model fractures in high-pressure, high-temperature environments. Renew. Energy 2020, 153, 1488–1503. [Google Scholar] [CrossRef]
- Fan, M.; Wang, L.; Li, J.; He, P.; Lai, X.; Gao, J.; Liu, G.; Wen, X. Preparation of supramolecular viscoelastic polymers with shear, temperature, and salt resistance/sensitivity by amphiphilic functional monomer modification. Polym. Test. 2022, 116, 107799. [Google Scholar] [CrossRef]
- Feng, Y.J.; Billon, L.; Grassl, B.; Bastiat, G.; Borisov, O.; François, J. Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification. 2. Properties of non-hydrolyzed polymers in pure water and brine. Polymer 2005, 46, 9283–9295. [Google Scholar] [CrossRef]
- Selb, J.; Biggs, S.; Renoux, D.; Candau, F. Hydrophobic and Electrostatic Interactions in Water-Soluble Associating Copolymers; Chemical Society: Washington, DC, USA, 1996; pp. 251–278. [Google Scholar]
- Ma, X.; Huang, Q.; Zhou, Z.; Mu, Y. Synthesis and evaluation of water-soluble fracturing fluid thickener based on hydrophobic association. Mater. Lett. 2022, 325, 132857. [Google Scholar] [CrossRef]
- Seymour, R.B.; Carraher, C.E., Jr. Structure—Property Relationships in Polymers; Plenum Press: New York, NY, USA, 1984; p. 104. [Google Scholar]
- Amis, E.J.; Hu, N.; Seery, T.A.P.; Hogen-Esch, T.E.; Yassini, M.; Hwang, F. Associating Polymers Containing Fluorocarbon Hydrophobic Units; American Chemical Society: Washington, DC, USA, 1996; pp. 279–302. [Google Scholar]
- Taylor, K.C.; Nasr-EI-Din, H.A. Water-soluble hydrophobically associating polymers for improved oil recovery: A literature review. In Proceedings of the SPE International Conference on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995; SPE: Kuala Lumpur, Malaysia, 1995. [Google Scholar]
- Uhl, J.T.; Ching, T.Y.; Bae, J.H. A Laboratory Study of New, Surfactant-Containing Polymers for High Salinity Reservoirs. SPE Adv. Technol. Ser. 1995, 3, 113–119. [Google Scholar] [CrossRef]
- Salami, O.T.; Plank, J. Synthesis, effectiveness, and working mechanism of humic acid-sodium 2-acrylamido-2-methylpropane sulfonate-co-N,N-dimethyl acrylamide-co-acrylic acid graft copolymer as high-temperature fluid loss additive in oil well cementing. J. Appl. Polym. Sci. 2012, 126, 1449–1460. [Google Scholar] [CrossRef]



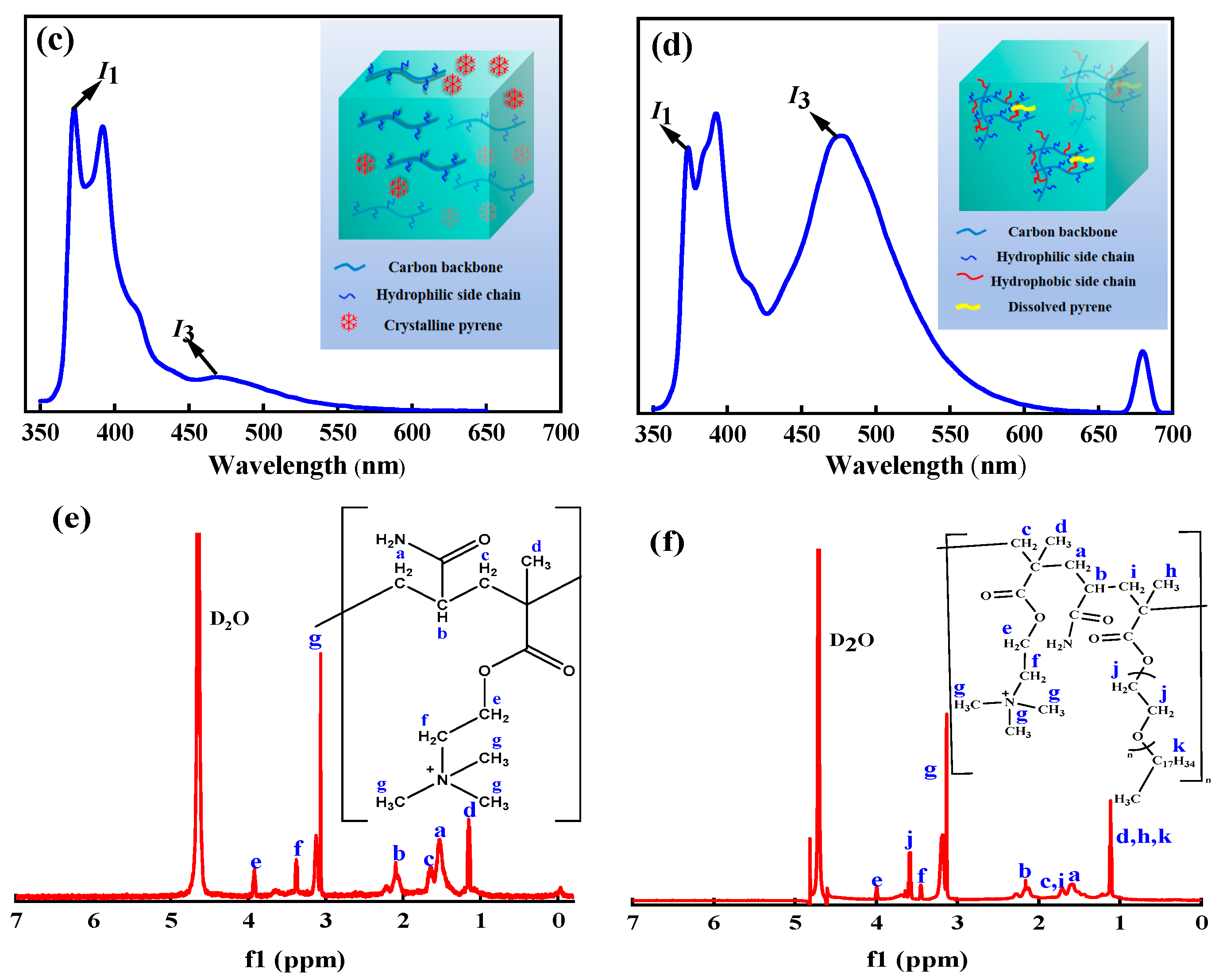
| δ, ppm | Protons from ADMC | Protons from ADOM |
|---|---|---|
| 1.2 | d, -C-CH3, (DMC) | d, h, k, -C-CH3, (DMC), -C-CH3, (-CH2-)17, (OEMA) |
| 1.5 | a, -CH2-, (AM) | a, -CH2-, (AM) |
| 1.7 | c, -CH2-C-, (DMC) | c, i, -CH2-C-, (DMC), -CH2-C-, (OEMA) |
| 2.2 | b, -CH-, (AM) | b, -CH-, (AM) |
| 3.1 | g, N-(CH3)3, (DMC) | g, N-(CH3)3, (DMC) |
| 3.3 | f, -CH2-N-, (DMC) | f, -CH2-N-, (DMC) |
| 3.9 | e, O-CH2-, (DMC) | e, O-CH2-, (DMC) |
| 3.5 | / | j, (-CH2CH2-O-)n, (OEMA) |
| Simulated Concentration of Hydrochloric Acid | 20% | 15% | 10% | 5% | ~0% |
|---|---|---|---|---|---|
| Addition of hydrochloric acid | 100 g of 20% hydrochloric acid | 92.47 g of 15% hydrochloric acid | 84.94 g of 10% hydrochloric acid | 77.41 g of 5% hydrochloric acid | 69.88 g of water |
| Addition of CaCl2 | 0 g | 7.53 g | 15.06 g | 22.59 g | 30.12 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, L.; Lai, X.; Gao, J.; Dang, Z.; Wang, R.; Mao, F.; Li, Y.; Jia, G. Two-Level Self-Thickening Mechanism of a Novel Acid Thickener with a Hydrophobic-Associated Structure during High-Temperature Acidification Processes. Polymers 2024, 16, 679. https://doi.org/10.3390/polym16050679
Li P, Wang L, Lai X, Gao J, Dang Z, Wang R, Mao F, Li Y, Jia G. Two-Level Self-Thickening Mechanism of a Novel Acid Thickener with a Hydrophobic-Associated Structure during High-Temperature Acidification Processes. Polymers. 2024; 16(5):679. https://doi.org/10.3390/polym16050679
Chicago/Turabian StyleLi, Peng, Lei Wang, Xiaojuan Lai, Jinhao Gao, Zhiqiang Dang, Rong Wang, Fan Mao, Yemin Li, and Guangliang Jia. 2024. "Two-Level Self-Thickening Mechanism of a Novel Acid Thickener with a Hydrophobic-Associated Structure during High-Temperature Acidification Processes" Polymers 16, no. 5: 679. https://doi.org/10.3390/polym16050679
APA StyleLi, P., Wang, L., Lai, X., Gao, J., Dang, Z., Wang, R., Mao, F., Li, Y., & Jia, G. (2024). Two-Level Self-Thickening Mechanism of a Novel Acid Thickener with a Hydrophobic-Associated Structure during High-Temperature Acidification Processes. Polymers, 16(5), 679. https://doi.org/10.3390/polym16050679





