Post-Heat Flexural Properties of Siloxane-Modified Epoxy/Phenolic Composites Reinforced by Glass Fiber
Abstract
1. Introduction
2. Experiment
2.1. Experimental Materials
2.2. Material Preparation
2.2.1. Polyphenylpropylsiloxane-Modified Epoxy Resin (EPh)
2.2.2. Polydimethylsiloxane-Modified Epoxy Resin (EM)
2.2.3. Preparation of Resin Blends
2.2.4. Preparation of Glass-Fiber-Reinforced Composites
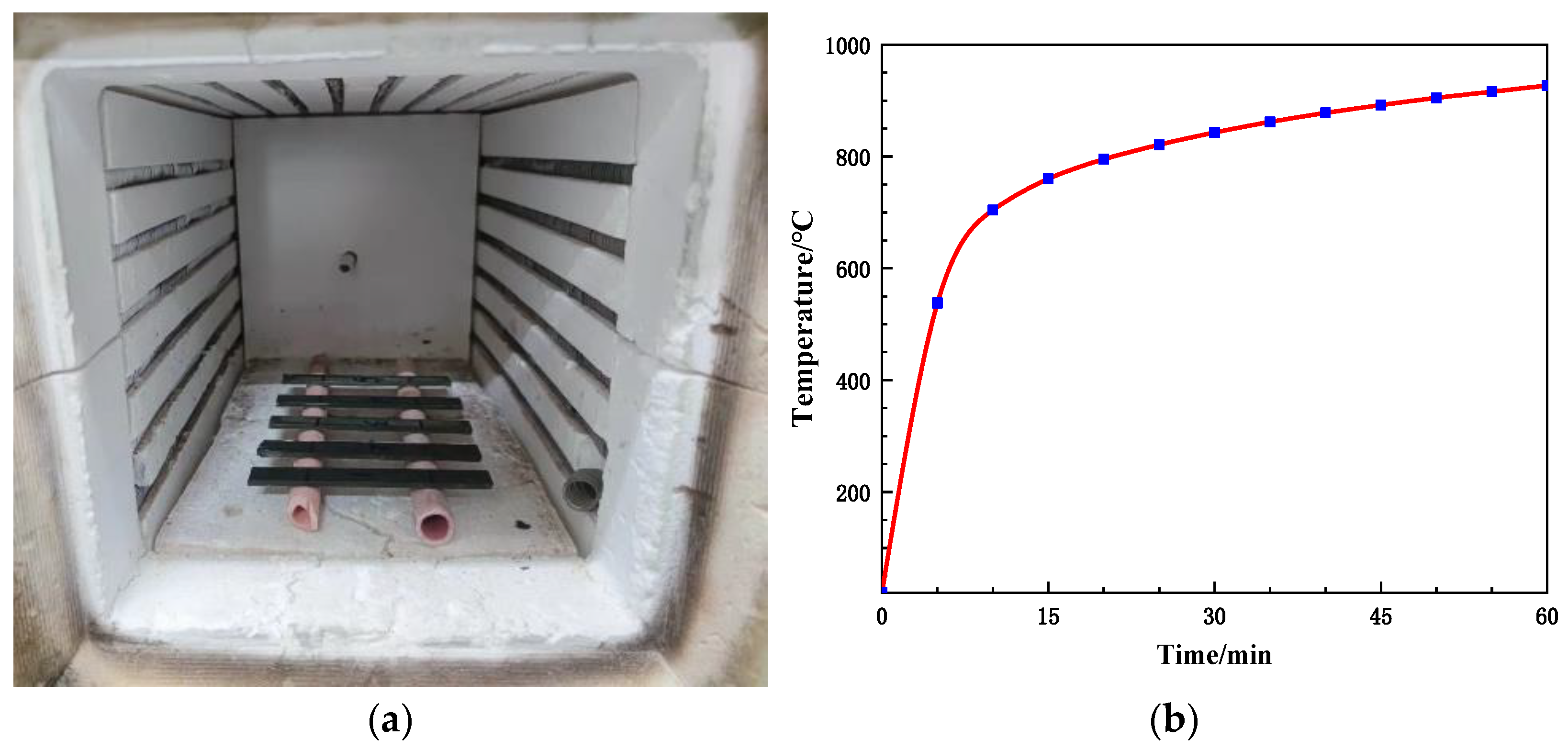
2.3. The Heat Treatment of Flexural Specimens
2.4. Characterization Methods
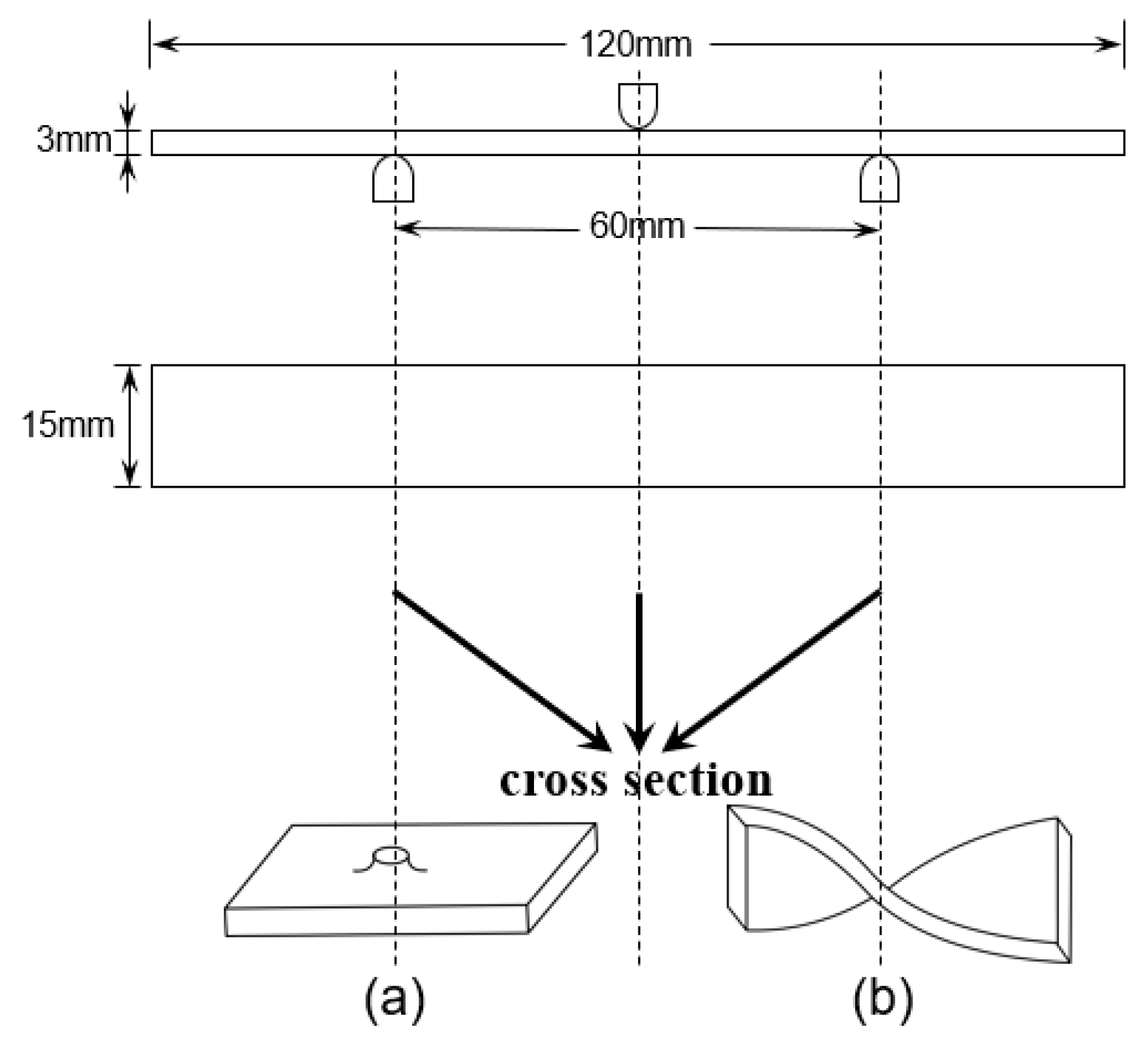
2.4.1. Flexural Property Testing
2.4.2. The Char Yield
2.4.3. Morphology
3. Results and Discussion
3.1. Flexural Properties of Composites
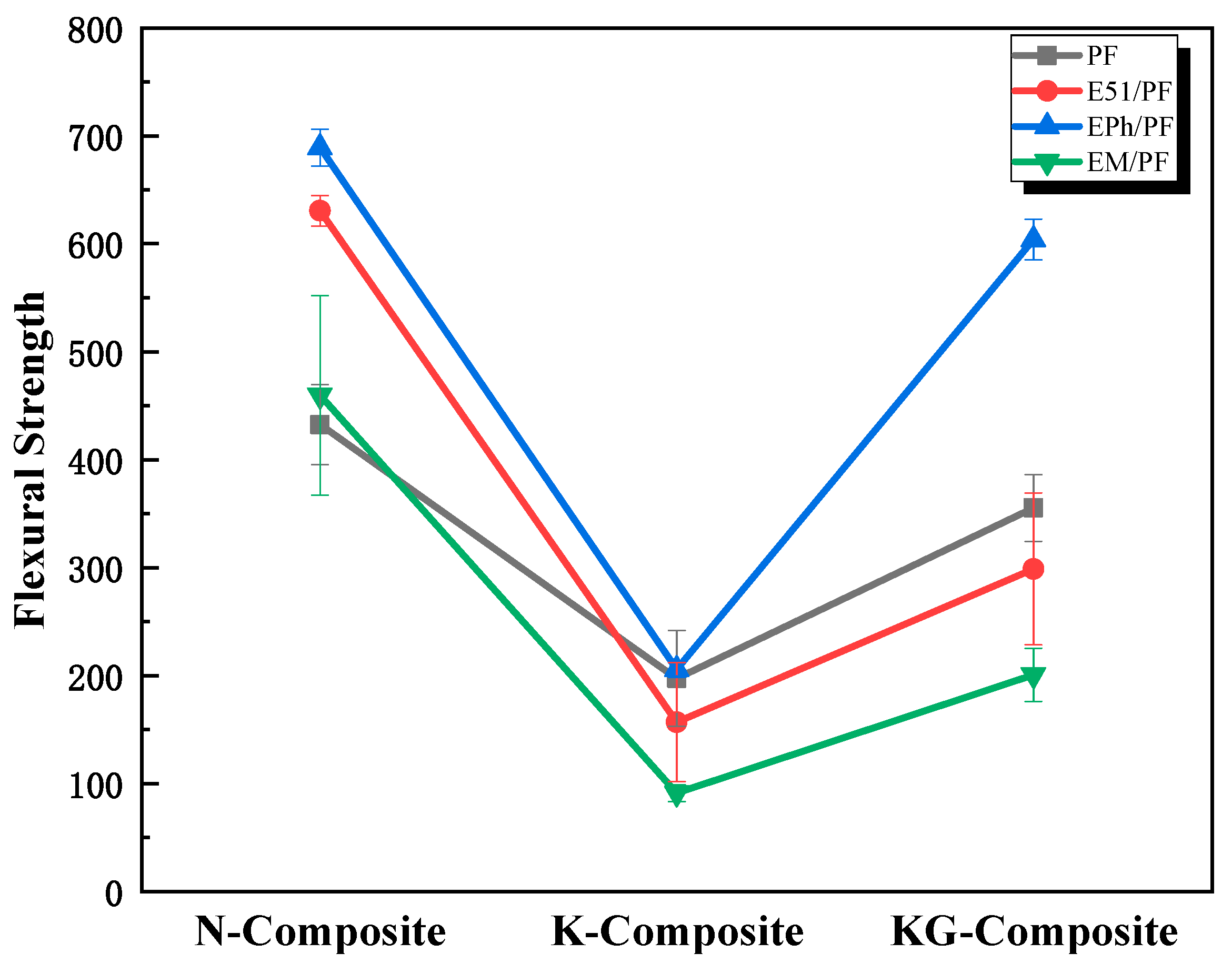
3.1.1. Flexural Properties of Composites at Room Temperature
3.1.2. Post-Heat Flexural Properties of Composites
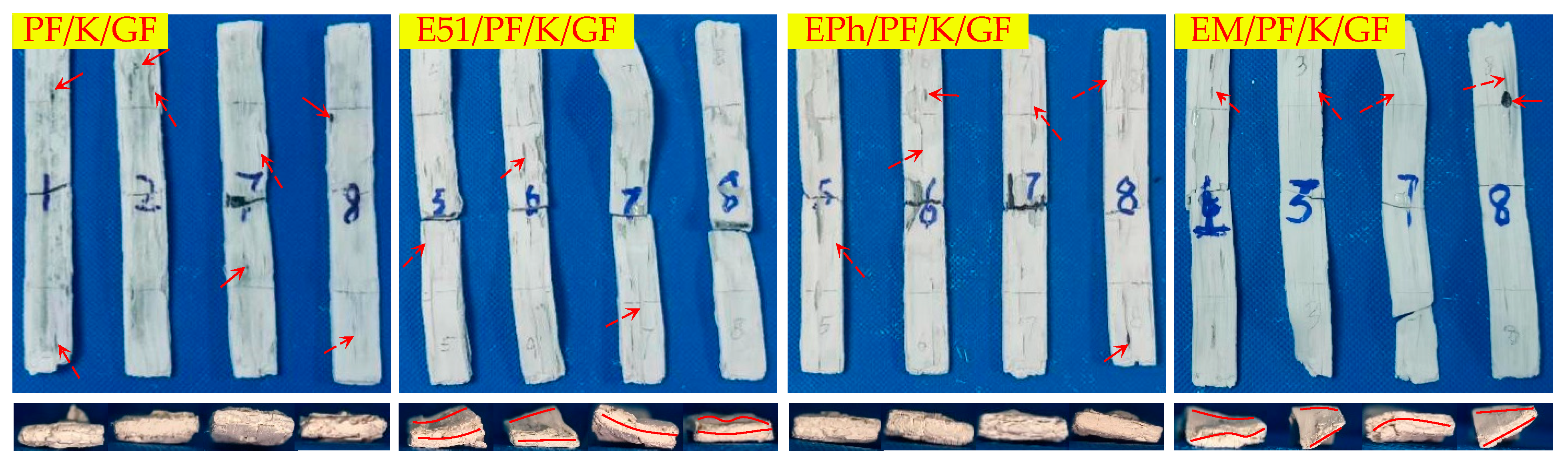
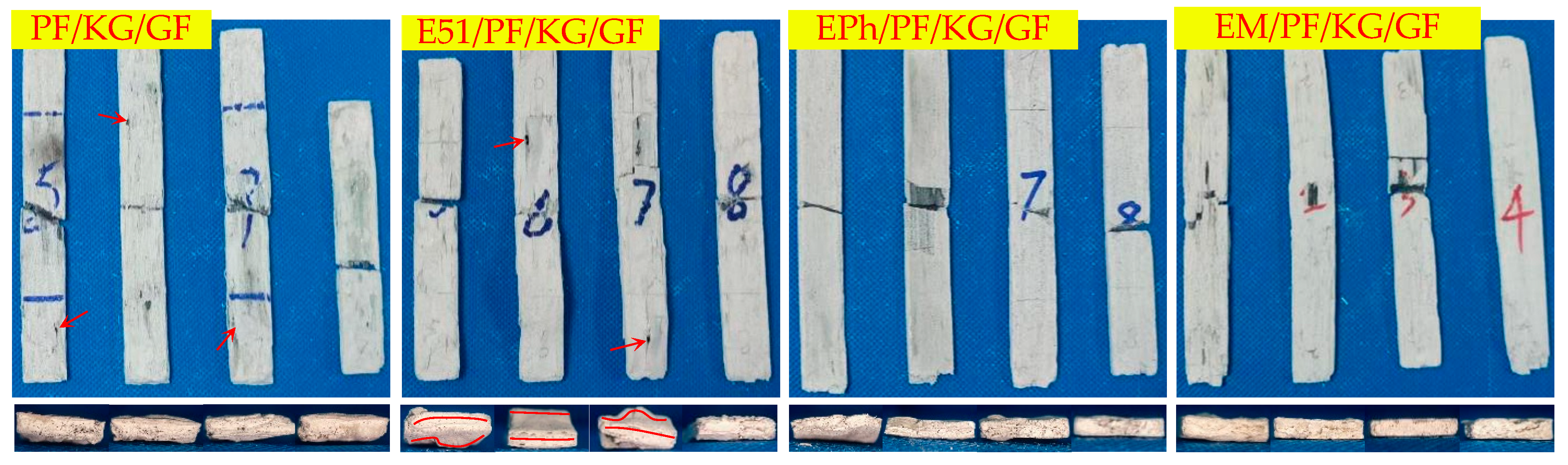
3.2. Macroscopic Morphology of Composites after Heat
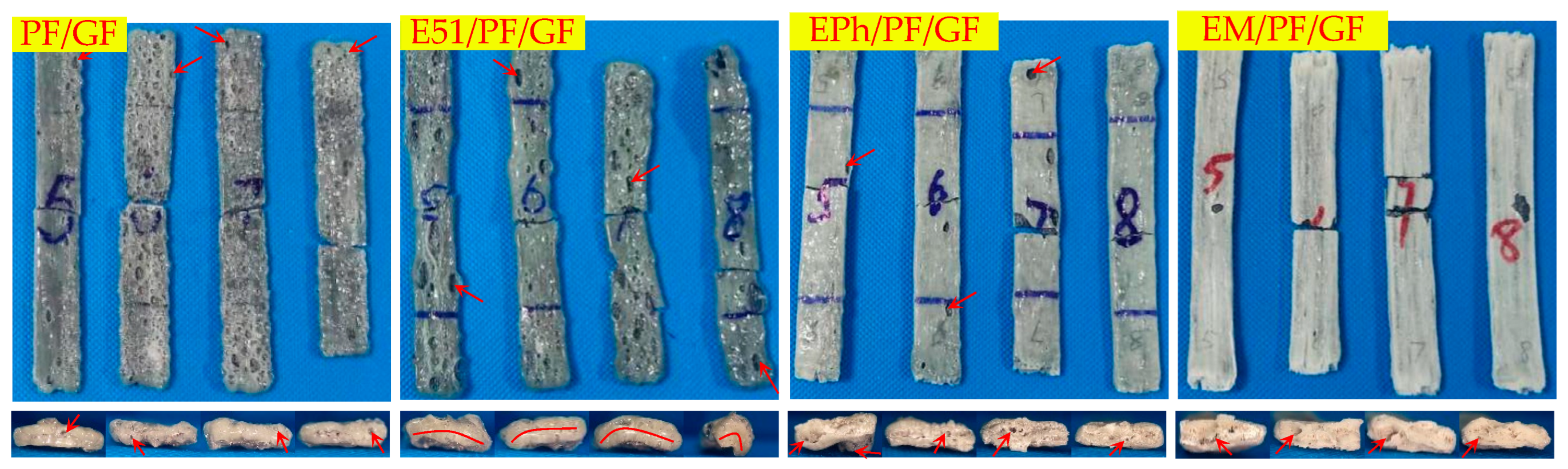
3.2.1. Macroscopic Morphology of Upper Surface of Composites after Heat
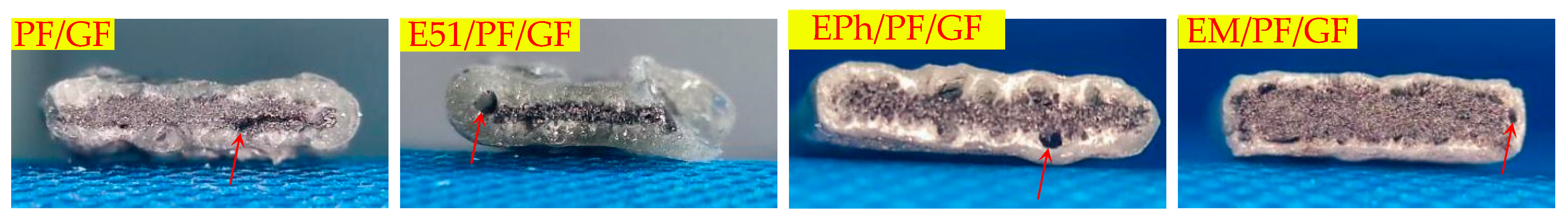
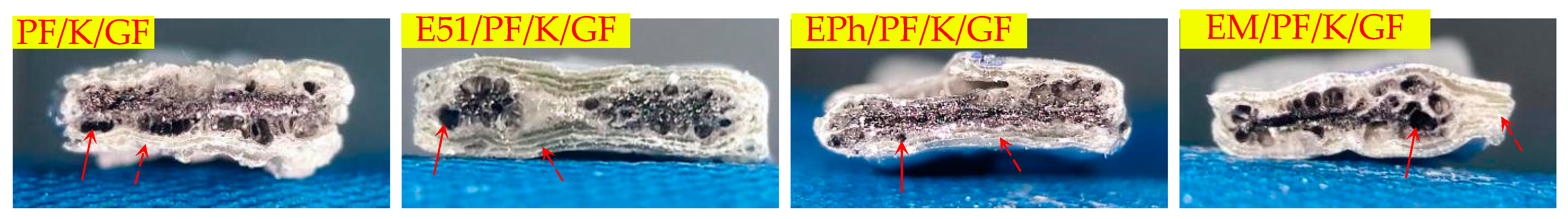
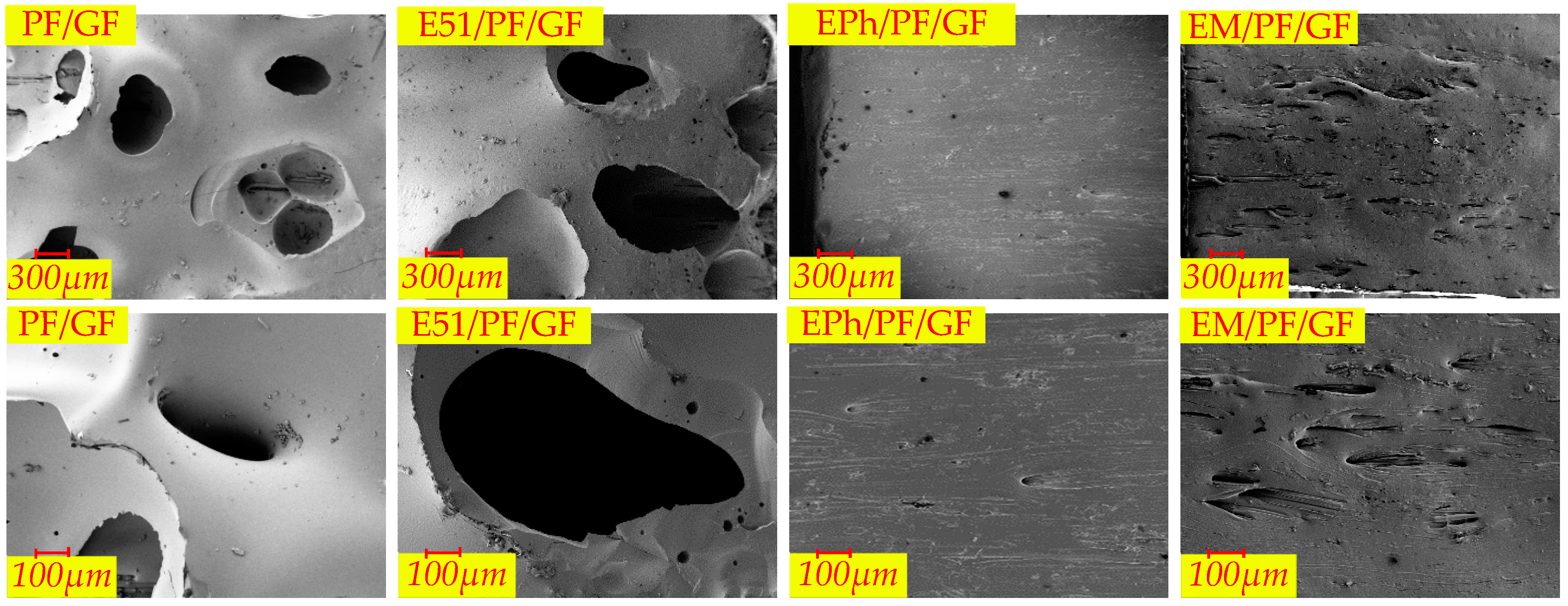
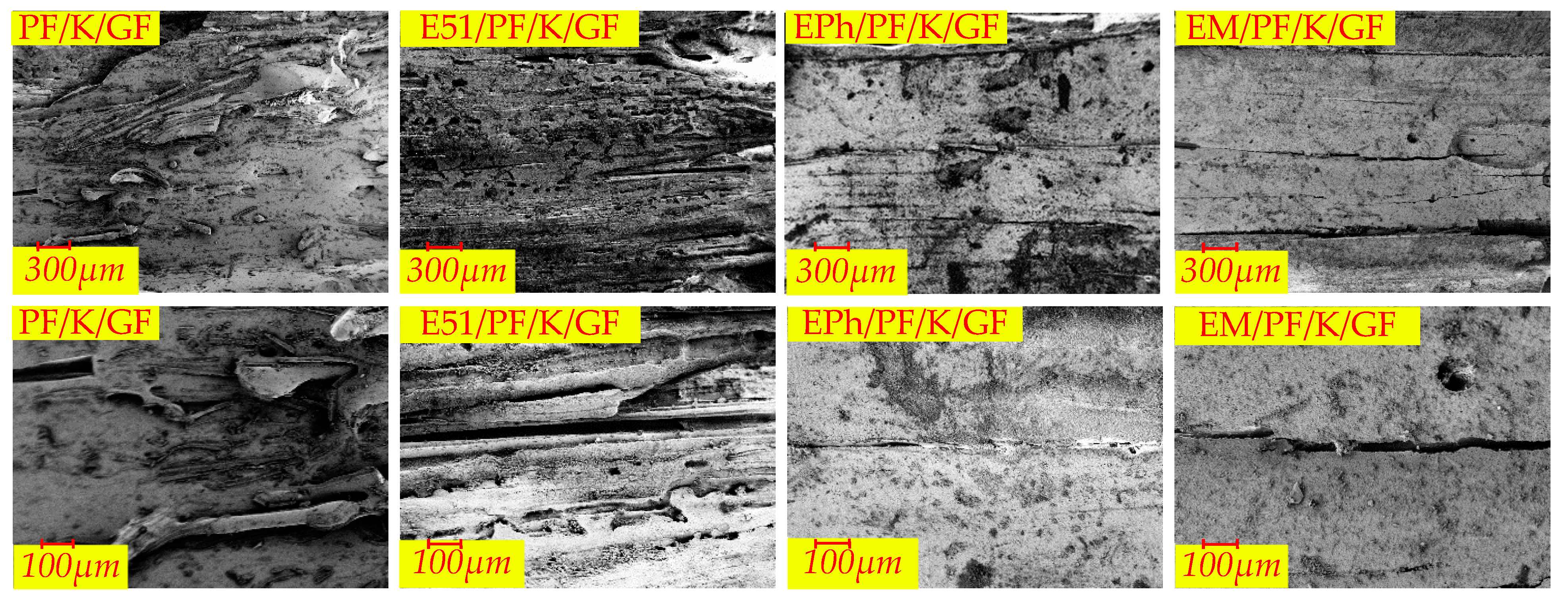
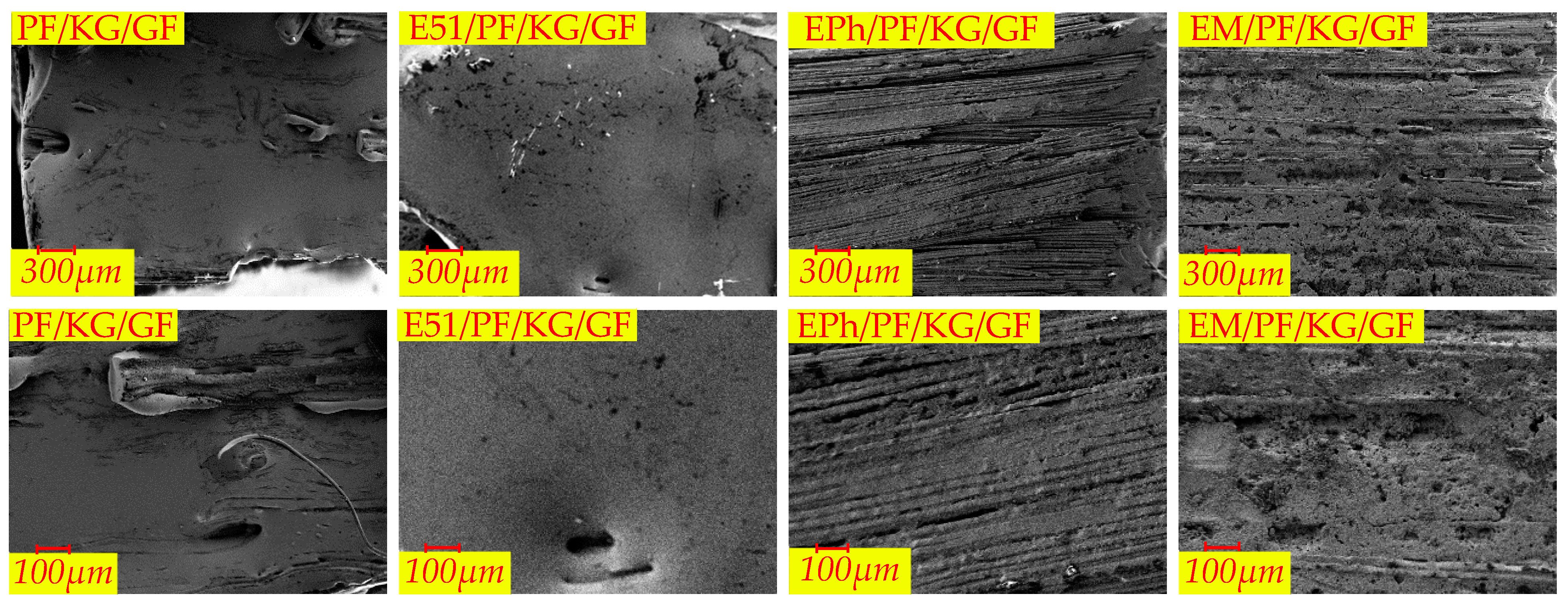
3.2.2. Macroscopic Morphology of Cross-Section of Composites after Heat
- (1)
- There are a large number of volcanic pores for PF/GF and E51/PF/GF, presenting on the upper surfaces.
- (2)
- Distortion is observed in the specimens with E51.
- (3)
- When considering the K-Composite, it is evident that they exhibit characteristics such as linear cracks on the upper surface, internal and interfacial vent pores, as well as layered composite structures on the outer layer.
- (4)
- The defects of the upper surfaces of the composites with siloxane-modified epoxy are minimal after heat, and the structural integrity is optimal upon the addition of kaolin/graphite.
- (5)
- The addition of inorganic fillers lowers the propensity of fiber fusion.
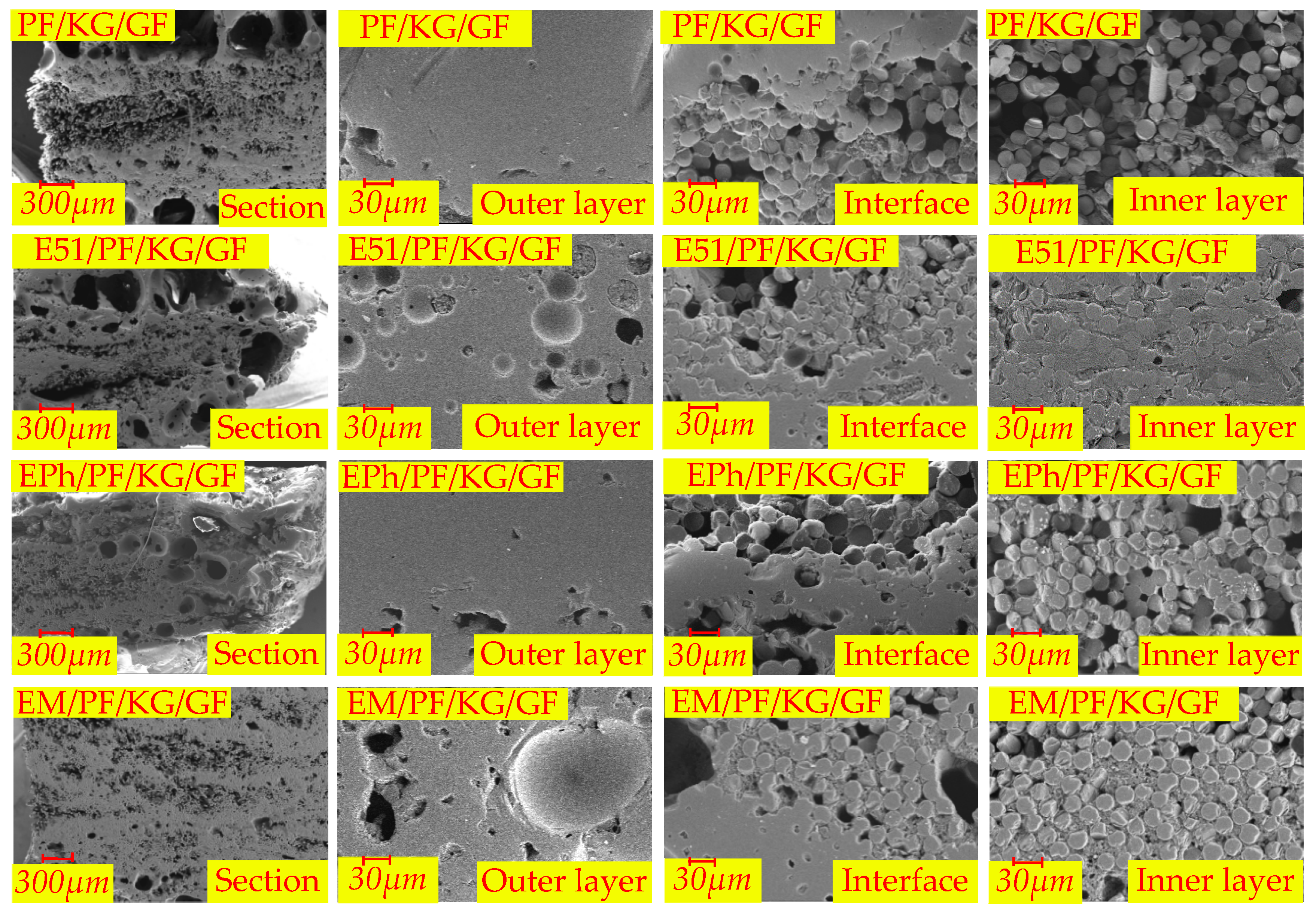
3.3. Microscopic Morphologies of Composites after Heat
3.3.1. Microscopic Morphologies of Composite Surfaces after Heat
3.3.2. Microscopic Morphology of Cross-Section of Composites after Heat
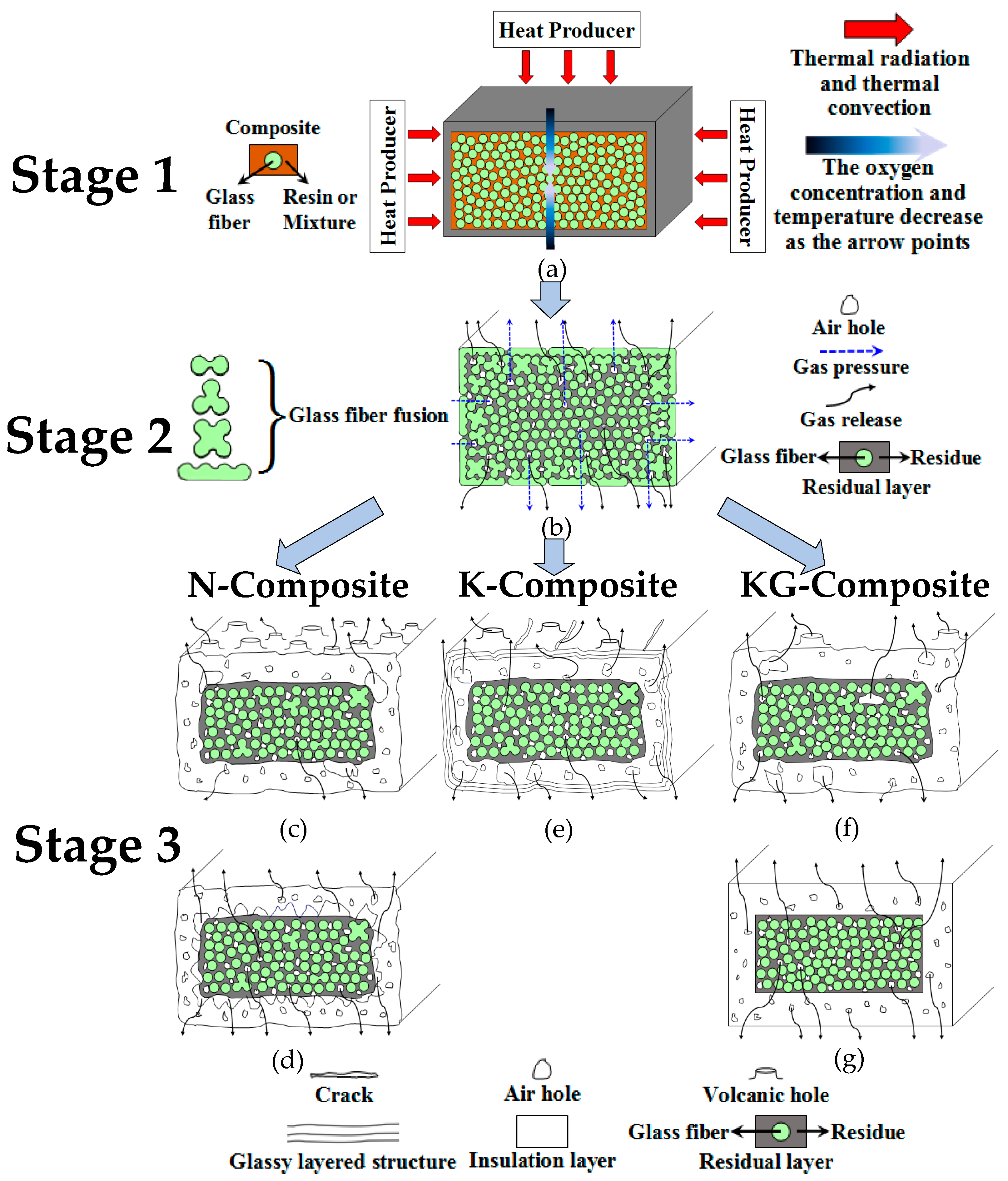
3.4. The Pyrolysis Process and Residual Structure Model of Composites
- (1)
- When exposed to high temperatures, the resins undergo oxidation and pyrolysis, and subsequently the pyrolysis gas releases through the gaps between the fibers.
- (2)
- Over time, the fusion of fibers occurs, which obstructs the outflow of gases. As it penetrates the molten layer formed by fibers fusion, volcanic pores are generated.
- (3)
- The samples, when incorporating siloxane-modified epoxy, yield C/Si/O pyrolysis products at high temperature, resulting in a slow pyrolysis process that obstructs both gas release and fibers fusion. Similarly, the inclusion of inorganic fillers into the composites has greater difficulties in gas escape and impedes fibers fusion. Therefore, these two types of composites do not generate volcanic pores. But they operate through different mechanisms. In the former case, the C/Si/O pyrolysis products are generated in situ and subsequently interconnect to form homogeneous materials, while in the latter case, the uneven distribution of inorganic fillers brings a limited number of pores.
- (4)
- Sintered fillers, such as kaolin, obstruct fibers fusion and induce sintering shrinkage of materials, causing linear cracks on the surface.
- (5)
- Non-sintered fillers, such as graphite, retard sintering shrinkage of materials, prevent linear cracks on the surface, and consume oxygen to preserve the char.
- (6)
- Graphite also has strong compatibility with the C/Si/O hybrid pyrolysis products of siloxane-modified epoxy. This compatibility mitigates the number of large vent pores at the interface and in the inner layer.
3.4.1. Three-Stage Pyrolysis Process of Composites
3.4.2. Residual Structure Model of Composites after Heat
4. Conclusions
- (1)
- Initially, the introduction of epoxy into the phenolic composites resulted in an increase in room-temperature strength, a decrease in the post-heat flexural strength, and the appearance of abundant volcanic pores on the surface, along with the distortion of structure. The second thing is that the addition of phenylpropylsiloxane-modified epoxy leads to an increase in room-temperature strength, a decrease in the post-heat flexural strength, the absence of surface defects, and the presence of numerous vent pores in the cross-section. Lastly, the incorporation of the dimethylsiloxane-modified epoxy brings about a slight increase in room-temperature strength, an increase in post-heat flexural strength, the absence of surface defects, and an increase in cross-sectional porosity. The chain flexibility of siloxane-modified epoxy improves the brittleness of phenolic composites at room temperature. Furthermore, at elevated temperatures, the C/Si/O hybrid pyrolysis products impede gas escape, forming a uniform surface without defects. The diminished post-heat flexural strength observed in samples with epoxy modified by phenylpropylsiloxane may be attributed to the fragility and low strength of the generated SiO2.
- (2)
- The addition of kaolin into phenolic composites led to a reduction in room-temperature strength, an increase in post-heat flexural strength, the formation of a loose and powdery surface, the emergence of layered composite structures, and large vent pores in the cross-section, as well as the distortion of overall structure; meanwhile, the number of volcanic pores on the surface declined. Additionally, after adding kaolin/graphite, the phenolic composites exhibited reduced strength loss at room temperature and improved post-heat flexural strength, dense surface with minimal powder peeling, and no discernible defects. Although vent pores are present in its cross-section, it was denser than the K-Composite. These phenomena are a result of the fact that kaolin decreases resin curing, while the reduction in kaolin mass improves this weakening effect at room temperature. At high temperatures, kaolin not only hinders the gas escape and sintering of glass fibers, but also retains the char. However, this property of preventing sintering causes material deformation. Moreover, graphite serves to consume oxygen and protect the char.
- (3)
- There is an interactive effect between resin and inorganic fillers on the post-heat flexural strength. The in situ C/Si/O hybrid pyrolysis products of epoxy modified with siloxane and kaolin both blocked gas releasing and glass fiber sintering, and promoted carbon retention, as well as enhancing the post-heat flexural strength of the samples. Due to the poor compatibility between these two substances, after heat the structure underwent the occurrence of distortion and defects. In contrast, the great compatibility and even certain interactions between graphite and C/Si/O hybrid pyrolysis products occurred, causing the formation of micro/nanoscale vent pores and the densification of the material after heat.
- (4)
- The pyrolysis process of phenolic composites reinforced by glass fiber correlates with the oxidation as well as decomposition of matrix and the fusion among glass fibers. In addition, the residual structure model of composites after heat involves structural characteristics such as surface defects, vent pores at the interface of the cross-section, glass fiber fusion, and material deformation. These findings uncover the influence of variables like resin, kaolin, and graphite on the morphology, structure, and mechanical properties of polymer composites after heat.
- (5)
- Based on the investigation of the morphology, structure, and post-heat flexural strength of four diverse resin systems and two various filler systems, it is observed that siloxane-modified epoxy and inorganic fillers share similar effects on the post-heat flexural strengths and morphologies of composites after heat. These materials can improve surface morphology and prevent the loss of char. Nevertheless, the former is more effective, yielding uniformly distributed “in situ” C/Si/O hybrid pyrolysis products, which exhibits the interactive effects with fillers at high temperature. Hence, in order to adapt to the pyrolysis characteristics of siloxane-modified epoxy, optimizing the content and altering the type of inorganic fillers are crucial for the structural fire retardancy of materials. This facilitates the research of the pyrolysis mechanism and the high-temperature utilization of phenolic composites.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mouritz, A.P. Post-fire flexural properties of fibre-reinforced polyester, epoxy and phenolic composites. J. Mater. Sci. 2002, 37, 1377–1386. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Mathys, Z. Post-fire mechanical properties of marine polymer composites. Compos. Struct. 1999, 47, 643–653. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Mathys, Z. Post-fire mechanical properties of glass-reinforced polyester composites. Compos. Sci. Technol. 2001, 61, 475–490. [Google Scholar] [CrossRef]
- Chen, R.Y.; Lu, S.X.; Li, C.H.; Lo, S.M. Experimental Study on Ignition and Combustion Characteristics of Fibre-Reinforced Phenolic Composite. Key Eng. Mater. 2016, 707, 13–22. [Google Scholar] [CrossRef]
- ASTM F3059-18; Standard Specification for Fiber-Reinforced Polymer (FRP) Gratings Used in Marine Construction and Shipbuilding. ASTM: West Conshohocken, PA, USA, 2018.
- Gardiner, C.; Mathys, Z.; Mouritz, A. Post-fire structural properties of burnt GRP plates. Mar. Struct. 2004, 17, 53–73. [Google Scholar] [CrossRef]
- Gibson, A.; Humphrey, J.; Di-Modica, P.; Christke, S.; Kotsikos, G.; Holliday, R. Post-fire integrity of composite gratings for offshore platforms. J. Reinf. Plast. Compos. 2014, 33, 543–555. [Google Scholar] [CrossRef]
- Shi, S.; Liang, J.; Gu, L.; Gong, C.; Wen, L.; Wang, Y. Degradation in compressive strength of silica/phenolic composites subjected to thermal and mechanical loading. J. Reinf. Plast. Compos. 2016, 35, 579–588. [Google Scholar] [CrossRef]
- Asaro, L.; Villanueva, S.; Alvarez, V.; Manfredi, L.B.; Rodriguez, E.S. Fire performance of composites made from carbon/phenolic prepregs with nanoclays. J. Compos. Mater. 2017, 51, 3515–3524. [Google Scholar] [CrossRef]
- Gaafar, M.; Afifi, H.; Al Zenidy, K. Structural Investigation of Semi Crystalline LDPE Nano-polymer. J. Eng. Appl. Sci. 2016, 3, 24. [Google Scholar] [CrossRef]
- Ganjaee Sari, M.; Rostami, M.; Khamseh, S. Poly(amidoamine)-grafted Graphene oxide/epoxy nanocomposite: Thermal/mechanical characteristics and viscoelastic properties. Prog. Color Color. Coat. 2022, 15, 157–174. [Google Scholar]
- Hayatu, U.S. Methane Adsorption on Hybrid and Non Hybrid Activated Carbon Synthesized from Coconut Shells and Poly Ether Ether Ketone. J. Eng. Appl. Sci. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Rallini, M.; Torre, L.; Kenny, J.M.; Natali, M. Effect of boron carbide nanoparticles on the thermal stability of carbon/phenolic composites. Polym. Compos. 2017, 38, 1819–1827. [Google Scholar] [CrossRef]
- Rallini, M.; Wu, H.; Natali, M.; Koo, J.; Torre, L. Nanostructured phenolic matrices: Effect of different nanofillers on the thermal degradation properties and reaction to fire of a resol. Fire Mater. 2017, 41, 817–825. [Google Scholar] [CrossRef]
- Mouritz, A.; Mathys, Z. Mechanical properties of fire-damaged glass-reinforced phenolic composites. Fire Mater. 2000, 24, 67–75. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, C.; Chen, P. The effects of zirconium diboride particles on the ablation performance of carbon–phenolic composites under an oxyacetylene flame. RSC Adv. 2013, 3, 13734–13739. [Google Scholar] [CrossRef]
- Asaro, L.; Manfredi, L.B.; Pellice, S.; Procaccini, R.; Rodriguez, E.S. Innovative ablative fire resistant composites based on phenolic resins modified with mesoporous silica particles. Polym. Degrad. Stab. 2017, 144, 7–16. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, H.; Zhang, B.; Zhao, H.; Wu, Y.; Ba, X. Halloysite/phenol-formaldehyde nanocomposites with enhanced mechanical properties and lowered fire hazard. Appl. Clay Sci. 2023, 231, 106743. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Z.; Luo, H.; Qin, Y.; Shi, M. Preparation and thermal stability of boron-containing phenolic resin/microcrystalline muscovite composites. Mater. Res. Innov. 2015, 19, S8-440–S8-444. [Google Scholar] [CrossRef]
- Ibrahem, R.A. Improvement of surface properties of carbon fiber reinforced epoxy composites using solid lubricants during machining processes. J. Eng. Appl. Sci. 2023, 10, 100. [Google Scholar] [CrossRef]
- Najafabadi, E.P.; Khaneghahi, M.H.; Amiri, H.A.; Estekanchi, H.E.; Ozbakkaloglu, T. Experimental investigation and probabilistic models for residual mechanical properties of GFRP pultruded profiles exposed to elevated temperatures. Compos. Struct. 2019, 211, 610–629. [Google Scholar] [CrossRef]
- Peng, Y.L.; Zeng, L. Study on the phenolic-epoxy resin system. Adv. Mater. Res. 2015, 1088, 439–443. [Google Scholar] [CrossRef]
- Tyberg, C.S.; Bergeron, K.; Sankarapandian, M.; Shih, P.; Loos, A.C.; Dillard, D.A.; McGrath, J.E.; Riffle, J.S.; Sorathia, U. Structure–property relationships of void-free phenolic–epoxy matrix materials. Polymer 2000, 41, 5053–5062. [Google Scholar] [CrossRef]
- Kaynak, C.; Cagatay, O. Rubber toughening of phenolic resin by using nitrile rubber and amino silane. Polym. Test. 2006, 25, 296–305. [Google Scholar] [CrossRef]
- Song, W.; Jia, X.; Ma, C.; Wang, J.; Qiao, W.; Ling, L. Facile fabrication of lightweight carbon fiber/phenolic ablator with improved flexibility via natural rubber modification. Compos. Commun. 2022, 31, 101119. [Google Scholar] [CrossRef]
- Xu, X.F.; Wu, Z.P.; Wu, D.Z.; Jin, R.G. Studies on modification of phenolic resin by CTBN. Compos. Sci. Eng. 2009, 26, 47–50. [Google Scholar]
- Chen, Y.; Zhou, C.; Chang, J.; Zou, H.; Liang, M. The effect of epoxy–silicone copolymer content on the thermal and mechanical properties of cured epoxy resin modified with siloxane. RSC Adv. 2014, 4, 60685–60693. [Google Scholar] [CrossRef]
- Lin, S.-T.; Huang, S.K. Thermal degradation study of siloxane-DGEBA epoxy copolymers. Eur. Polym. J. 1997, 33, 365–373. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Q.; Zhang, H.; Lei, X.; Yin, D.; Fan, X.; Zhou, L. Preparation of diamine-POSS/Ag hybrid microspheres and its application in epoxy resin. J. Polym. Res. 2012, 19, 1–8. [Google Scholar] [CrossRef]
- Shen, Z.Q. Modification of Epoxy Resin with Halloysite nanotubes Grafted by Siloxane Benzoxazine. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2021. [Google Scholar]
- Gupta, P.; Bajpai, M. Development of Siliconized Epoxy Resins and Their Application as Anticorrosive Coatings. Adv. Chem. Eng. Sci. 2011, 1, 133–139. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, S.X.; Ji, Y.D.; Cao, D.F.; Hu, H.X.; Chen, Z. Effect of polysiloxane modified epoxy on high temperature residual strength of glass fiber/phenolic composites. Acta Mater. Compos. Sin. 2023, 40, 6619–6629. [Google Scholar]
- Li, Z.Q.; Jiang, Y.Y.; Ji, Y.D.; Lu, X.C. Study on polysiloxane modified epoxy resin and its pyrolysis residue. Thermosetting Resins 2022, 37, 1–8. [Google Scholar]
- Wen, Q.; Liu, B.W.; Ji, Y.D. Study on the hydroxyl-terminated polydimethylsiloxane modified epoxy resins. Thermosetting Resins 2020, 35, 25–28. [Google Scholar]
- Ji, Y.D.; Jiang, Y.Y.; Cao, D.F.; Li, S.X. Structure and Composition Evolution of Polysiloxane Modified Epoxy/Phenolic Blends Under the Condition of Hot Oxygen. Polym. Mater. Sci. Eng. 2022, 38, 90–97. [Google Scholar]
- Cao, D.F.; Liu, J.B.; Ji, Y.D.; Hu, H.X.; Chen, X.C.; Li, C.Y.; Chen, Z.; Li, S.X. Synthesis and Flame Retardancy of Silicon and Phosphorus Modified Epoxy Resin. Polym. Mater. Sci. Eng. 2023, 39, 40–50. [Google Scholar]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- ISO 14125:1998; Fibre-Reinforced Plastic Composites—Determination of Flexural Properties. International Organization for Standardization: Geneva, Switzerland, 1998.








| Composite System | PF-Composite | E51/PF-Composite | EPh/PF-Composite | EM/PF-Composite |
|---|---|---|---|---|
| N-Composite (No filler) | PF/GF (60 g/270 g) | E51/PF/GF (18 g/42 g/270 g) | EPh/PF/GF (18 g/42 g/270 g) | EM/PF/GF (18 g/42 g/270 g) |
| K-Composite (Containing kaolin) | PF/K/GF (60 g/30 g/270 g) | E51/PF/K/GF (18 g/42 g/30 g/270 g) | EPh/PF/K/GF (18 g/42 g/30 g/270 g) | EM/PF/K/GF (18 g/42 g/30 g/270 g) |
| KG-Composite (Containing kaolin/graphite, 24 g and 6 g, respectively) | PF/KG/GF (60 g/30 g/270 g) | E51/PF/KG/GF (18 g/42 g/30 g/270 g) | EPh/PF/KG/GF (18 g/42 g/30 g/270 g) | EM/PF/KG/GF (18 g/42 g/30 g/270 g) |
| Data Type | PF/GF | PF/K/GF | PF/KG/GF | E51/PF/GF | E51/PF/K/GF | E51/PF/KG/GF | EPh/PF/GF | EPh/PF/K/GF | EPh/PF/KG/GF | EM/PF/GF | EM/PF/K/GF | EM/PF/KG/GF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ML/N | 32 | 47 | 50 | 28 | 51 | 32 | 25 | 46 | 65 | 61 | 42 | 93 |
| bh2/mm3 | 220 | 189 | 206 | 291 | 321 | 212 | 190 | 212 | 125 | 262 | 235 | 179 |
| Data Type | PF/KG/GF | E51/PF/KG/GF | EPh/PF/KG/GF | EM/PF/KG/GF |
|---|---|---|---|---|
| Flexural strength before heat (MPa) | 355.3 | 298.9 | 604 | 200.7 |
| Flexural strength after heat (MPa) | 20.6 | 13.6 | 46.7 | 47.1 |
| Flexural strength residual rate (%) | 5.8 | 4.6 | 7.7 | 23.5 |
| Macroscopic Morphology | Composites | |
|---|---|---|
| Upper surface | Volcanic pores | PF/GF, E51/PF/GF, |
| Linear cracks | PF/K/GF, E51/PF/K/GF | |
| Both flat and straight | EM/PF/GF, EM/PF/GF, EPh/PF/KG/GF, EM/PF/KG/GF | |
| Smooth | EPh/PF/GF, EM/PF/GF | |
| Side | Distortion | E51/PF/GF, E51/PF/K/GF, E51/PF/KG/GF, EM/PF/K/GF |
| Pores | PF/GF, EPh/PF/GF, EM/PF/GF | |
| Cross-section | Vent pores in the inner layer | PF/K/GF, E51/PF/K/GF, EPh/PF/K/GF, EM/PF/K/GF |
| Vent pores at the interface | PF/GF, E51/PF/GF, EPh/PF/GF, EM/PF/GF, PF/K/GF, E51/PF/K/GF, EPh/PF/K/GF, EM/PF/K/GF, PF/KG/GF, E51/PF/KG/GF | |
| No obvious defects | EPh/PF/KG/GF, EM/PF/KG/GF | |
| Outer glassy state | PF/GF, E51/PF/GF, EPh/PF/GF, EM/PF/GF | |
| Outer layered composite structure | PF/K/GF, E51/PF/K/GF, EPh/PF/K/GF, EM/PF/K/GF | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhang, X.; Wang, C.; Li, S.; Cao, D. Post-Heat Flexural Properties of Siloxane-Modified Epoxy/Phenolic Composites Reinforced by Glass Fiber. Polymers 2024, 16, 708. https://doi.org/10.3390/polym16050708
Ji Y, Zhang X, Wang C, Li S, Cao D. Post-Heat Flexural Properties of Siloxane-Modified Epoxy/Phenolic Composites Reinforced by Glass Fiber. Polymers. 2024; 16(5):708. https://doi.org/10.3390/polym16050708
Chicago/Turabian StyleJi, Yundong, Xinchen Zhang, Changzeng Wang, Shuxin Li, and Dongfeng Cao. 2024. "Post-Heat Flexural Properties of Siloxane-Modified Epoxy/Phenolic Composites Reinforced by Glass Fiber" Polymers 16, no. 5: 708. https://doi.org/10.3390/polym16050708
APA StyleJi, Y., Zhang, X., Wang, C., Li, S., & Cao, D. (2024). Post-Heat Flexural Properties of Siloxane-Modified Epoxy/Phenolic Composites Reinforced by Glass Fiber. Polymers, 16(5), 708. https://doi.org/10.3390/polym16050708






